Abstract
Objectives
Lung deflation and inflation during cardiac surgery with cardiopulmonary bypass contributes to pulmonary dysfunction postoperatively. Theophylline treatment for lung diseases has traditionally been thought to act by phosphodiesterase inhibition; however, increasing evidence has suggested other plausible mechanisms. We investigated the effects of deflation and reinflation on signaling pathways (p38-mitogen-activated protein kinase [MAPK], extracellular signal-regulated kinase 1 and 2 [ERK1/2], and Akt) and whether theophylline influences the deflation-induced lung injury and associated signaling.
Methods
Isolated rat lungs were perfused (15 mL/min) with deoxygenated rat blood in bicarbonate buffer and ventilated. After 20 minutes' equilibration, the lungs were deflated (60 minutes, aerobic perfusion 1.5 mL/min), followed by reinflation (60 minutes, anaerobic reperfusion 15 mL/min). Compliance, vascular resistance, and kinase phosphorylation were assessed during deflation and reinflation. The effects of SB203580 (50 μM), a p38-MAPK inhibitor, and theophylline (0.083 mM [therapeutic] or 3 mM [supratherapeutic]) on physiology and signaling were studied.
Results
Deflation reduced compliance by 44% compared with continuously ventilated lungs. p38-MAPK and Akt phosphorylation increased (three to fivefold) during deflation and reinflation, and ERK1/2 phosphorylation increased (approximately twofold) during reinflation. SB203580 had no effect on lung physiology or ERK1/2 and Akt activation. Both theophylline doses increased cyclic adenosine monophosphate, but only 3 mM theophylline improved compliance. p38-MAPK phosphorylation was not affected by theophylline; 0.083 mM theophylline inhibited reinflation-induced ERK1/2 phosphorylation (72% ± 3%); and 3 mM theophylline inhibited Akt phosphorylation during deflation (75% ± 5%) and reinflation (87% ± 4%).
Conclusions
Lung deflation and reinflation stimulates differential p38-MAPK, ERK1/2, and Akt activation, suggesting a role in lung injury during cardiopulmonary bypass. However, p38-MAPK was not involved in the compromised compliance. A supratherapeutic theophylline dose protected lungs against deflation-induced injury and was associated with inhibition of phosphoinositide 3-kinase/Akt rather than phosphodiesterase.
Abbreviations and Acronyms: BB, bicarbonate buffer; cAMP, cyclic adenosine monophosphate; CPB, cardiopulmonary bypass; ERK1/2, extracellular signal-regulated kinase 1 and 2; MAPK, mitogen-activated protein kinase; PDE, phosphodiesterase; PI3K, phosphoinositide 3-kinase; SBB, sanguineous BB; TBST, Tris-buffered saline with Tween 20
Pulmonary dysfunction after cardiac surgery with cardiopulmonary bypass (CPB) affects a significant number of patients, with clinical manifestations ranging from fever and unproductive cough to acute respiratory distress syndrome.1 During CPB, the lungs are deflated and excluded from the pulmonary circulation, with the blood supply provided only through the bronchial arteries (∼10% normal perfusion).2 Deflation, combined with the reduced blood flow and the systemic inflammatory response to the extracorporeal circuit, leads to pulmonary injury.2 Although strategies to reduce post-CPB lung injury have been examined,3 few have been applied in clinical practice, and the ideal management for the lungs during CPB remains controversial.
Our previous experimental studies of the long-term preservation of the rat lung have shown that theophylline (clinically, aminophylline) improved lung function after prolonged hypothermic storage.4 Theophylline is an inexpensive, orally administered drug that has historically been used for asthma and chronic obstructive pulmonary disease; however, it has a narrow therapeutic window (recommended dose, 5-15 μg/mL), and its mechanism of action remains uncertain.5 Traditionally, its clinical effects have been attributed to nonselective phosphodiesterase (PDE) inhibition and cyclic adenosine monophosphate (cAMP) elevation.6 However, evidence has been increasing for other plausible mechanisms, including adenosine receptor antagonism,7 interleukin-10 release,8 inhibition of phosphoinositide 3-kinase (PI3K) activity,9 and activation of histone deacetylases.10 Intraoperative administration of theophylline attenuated the post-CPB systemic inflammatory response in patients undergoing valve replacement.11 However, that small study was limited by the use of aprotinin, which might interact with theophylline.11 Thus, the effect of theophylline on CPB-induced lung injury requires further investigation.
Little is known about the cell types and signaling cascades activated in lungs during CPB. Studies in animal models of CPB have reported activation of pulmonary extracellular signal-regulated kinase 1 and 2 (ERK1/2)12 and p38-mitogen-activated protein kinase (MAPK),12, 13 localized in pulmonary epithelial cells, bronchial smooth muscle, and vascular endothelial cells.12 Their histologic localization suggests they could be involved in vascular permeability,14 neutrophil adhesion,15 and cytokine production.16 Accumulating evidence has indicated that the PI3K/Akt pathway is involved in various models of lung injury.17, 18 PI3K is a family of lipid-modifying enzymes involved in cell survival, proliferation, secretion, and glucose transport.19 However, the downstream targets of the PI3K pathway and its role in CPB-induced lung injury remain unknown.
The etiology of post-CPB pulmonary dysfunction is multifactorial. Although the contribution of each factor and the underlying molecular mechanisms remain unknown, pulmonary abnormalities result, at least in part, by atelectasis.20 Thus, we hypothesized that deflation and reinflation, such as occurs during CPB, will stimulate the MAPK/PI3K pathways and promote lung injury that can be ameliorated by theophylline. To test this hypothesis, we used isolated perfused rat lungs to model the clinical scenario of CPB and established the kinetic effects of deflation and reinflation on the MAPK/PI3K pathways. We also determined the influence of MAPK and PDE inhibition on physiology and signaling.
Methods
Lung Preparation
All rats received humane care in compliance with the “Guidance on the Operation of the Animals (Scientific Procedures) Act 1986” (Her Majesty's Stationary Office, London, UK). Lungs from male Wistar rats (weight, 250-330 g) were isolated and perfused as described previously.4 In brief, the rats were anesthetized with pentobarbitone, at a dose (160 mg/kg intraperitoneally) that induced a surgical level of general anesthesia, established by the absence of corneal reflexes. The rats were then ventilated by positive pressure (80 breaths/min, room air, Harvard Small Animal Ventilator; Harvard Apparatus, Harvard Bioscience, Holliston, Mass), heparinized (500 IU into the inferior vena cava), and exsanguinated. After the thorax was opened, the pulmonary artery and left atrium were cannulated, and the lungs were removed and suspended in a sealed chamber. Perfusion was begun with modified bicarbonate buffer (BB, composition, NaCl 118.5 mmol/L, KCl 3.8 mmol/L, KH2PO4 1.2 mmol/L, NaHCO3 25.0 mmol/L, CaCl2 2.0 mmol/L, MgSO4 1.2 mmol/L, glucose 10.0 mmol/L) mixed with whole rat blood (4:1) to produce sanguineous BB (SBB, approximately 6% hematocrit and 23 g/L hemoglobin). The apparatus included the lung chamber, a perfusate reservoir, and a membrane deoxygenator (gassed with 100% nitrogen); all components were water-jacketed (37°C) and connected with noncoated silicone tubing. The SBB was gassed with 100% carbon dioxide intermittently to maintain a pH at 7.35 to 7.45. Perfusion was nonpulsatile, at a flow rate of 15 mL/min and pressure of approximately 14 cmH2O. Perfusate was passed through the deoxygenator before entering the lung; buffer leaving the lungs was returned to the reservoir and recycled. Thus, oxygenation of the perfusate was by the isolated lungs. The peak tracheal pressure applied by the ventilator was set to give a tidal volume of 2.0 to 2.3 mL (0.7 mL/100 g animal weight), and a positive end-expiratory pressure of 1 to 2 cmH2O was applied. Every 10 minutes, a deep breath (sigh) was initiated to avoid atelectasis. Pressure transducers were connected to the tracheal, arterial, and venous cannulas. The peak tracheal pressure and tidal volume, calculated by integration of the flow through a pneumotachograph connecting the trachea to the ventilator, were measured. The ratio of the tidal volume to tracheal pressure was taken as a measure of dynamic lung compliance. The difference in pressures between the pulmonary artery and venous cannulas divided by the perfusate flow rate measured the vascular resistance. All outputs from the pressure transducers and pneumotachograph were recorded using PowerLab analogue–digital converter and PowerLab Chart software (ADInstruments Ltd, Oxford, UK). The parameters were sampled at 30 points in each breath and dynamic compliance and vascular resistance calculated during a 10-breath period for each point examined.
Experimental Protocol
The lungs were perfused at 15 mL/min (deoxygenated SBB) for an initial 20-minute period to establish control lung function. During clinical CPB, bronchial artery lung perfusion, with a flow of ∼10% that of the normal pulmonary artery flow rate,2 is maintained. To mimic this clinical scenario, ventilation was stopped, and the lungs were allowed to deflate while perfusion continued with oxygenated SBB (gassed with 95% oxygen/5% carbon dioxide) at a rate of 1.5 mL/min. After deflation, the lungs were reperfused with deoxygenated SBB at 15 mL/min (for the period indicated) and ventilated. Each experimental group consisted of the lungs from 6 rats. The physiologic parameters were measured during equilibration and reinflation for the deflated lungs and throughout the experiment for the control lungs. After each experimental period, the lungs were washed with BB for 2 minutes, frozen in liquid nitrogen, and stored at −80°C.
Study 1
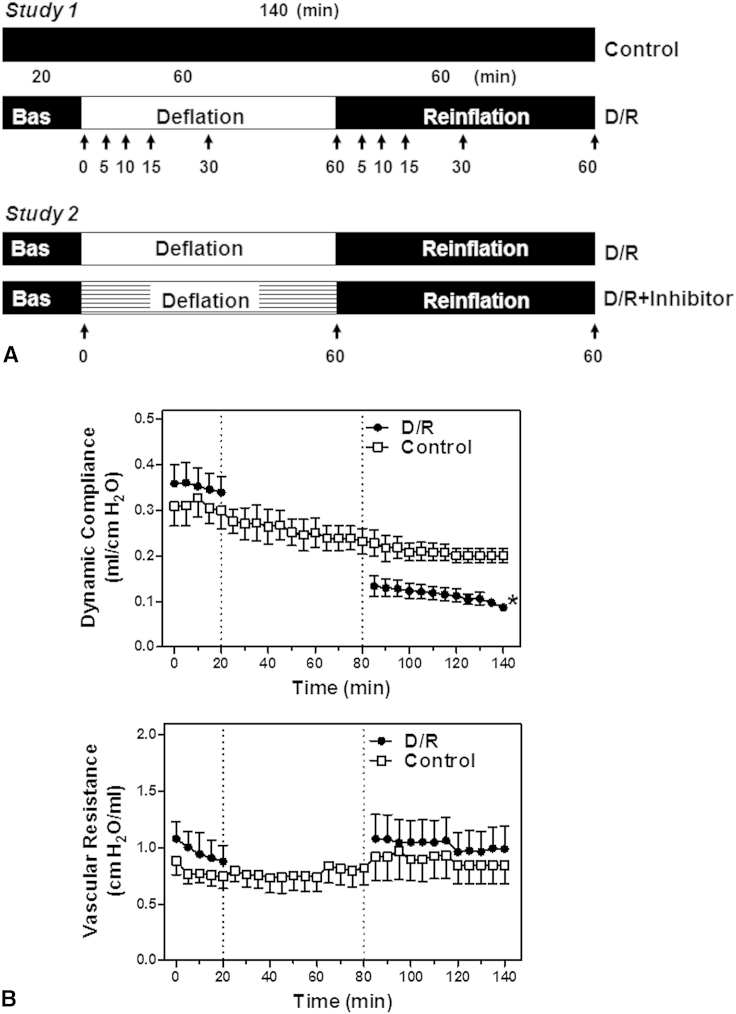
The effect of deflation on lung physiology was studied in lungs deflated for 60 minutes and reinflated for 60 minutes (Figure 1, A). This duration of deflation and reinflation had been established in preliminary studies (data not shown) as sufficient to induce significant lung injury (compliance at 60 minutes of reinflation, 60% ± 4%, 49% ± 8%, 47% ± 3%, 32% ± 5% of baseline after 15, 30, 60, and 90 minutes of deflation, respectively) and also to observe any acute effects on recovery. In time-matched controls, the lungs were continuously ventilated and perfused (15 mL/min, deoxygenated SBB) for 140 minutes (Figure 1, A). The course of protein kinase activation during deflation was assessed in lungs deflated for 5, 10, 15, 30, or 60 minutes. The course of kinase activation during reinflation was studied in lungs deflated for 60 minutes and reinflated for 5, 10, 15, 30, or 60 minutes (Figure 1, A).
Figure 1.
A, Experimental protocols. The lungs were either ventilated and perfused for 140 minutes (control) or subjected to 20 minutes of equilibration, followed by deflation (60 minutes) and reinflation (60 minutes) in the absence (D/R) or presence of inhibitors (D/R+inhibitor). The samples were collected at the points indicated. B, Effects of lung deflation and reinflation on dynamic compliance and vascular resistance. Dynamic compliance and vascular resistance of the control lungs or lungs subjected to deflation and reinflation (D/R) were measured. Data are expressed as the mean ± standard error of the mean of 6 lungs per group. *P < .05 compared with ventilated lungs (area under the curve, unpaired t test). Bas, Baseline.
Study 2
We have previously shown that a high (supratherapeutic) dose of theophylline (3 mM) protects lungs against injury during prolonged hypothermic storage.4 Therefore, we tested this theophylline dose against deflation-induced lung injury and compared it with a dose within the therapeutic range (0.083 mM, corresponding to 15 μg/mL).21 Theophylline (0.083 or 3 mM, dissolved in BB) or the p38-MAPK inhibitor SB203580 (50 μM) was added to the perfusate from the start of the experiment, and the lungs were subjected to deflation for 60 minutes, followed by reperfusion and reinflation for 60 minutes. Kinase activation was assessed in lung samples at the end of equilibration (baseline), deflation, and reinflation (Figure 1 A).
Immunoblotting
The lung tissue was homogenized with 5 mL/g of buffer (20 mM β-glycerophosphate, pH 7.5, 20 mM sodium fluoride, 2 mM ethylenediaminetetraacetic acid, 0.2 mM Na3VO4, 10 mM benzamidine, 5 mM dithiothreitol, and 1% [vol/vol] Triton X100) containing complete protease inhibitor cocktail (Roche, Diagnostics Limited, Burgess Hill, UK). The samples were centrifuged (4°C, 10 minutes, 10,000g), and the protein concentration was determined in the supernatant using the Bradford method. The samples were boiled with 0.33 volume of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer (10% [wt/vol] sodium dodecyl sulfate, 13% [vol/vol] glycerol, 300 mM Tris-HCl, pH 6.8, 130 mM dithiothreitol, and 0.2% [wt/vol] bromophenol blue). The proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% acrylamide, 0.275% [wt/vol] bis-acrylamide slab gels and transferred electrophoretically onto a polyvinyldine diflouride membrane. Nonspecific binding sites were blocked (20 minutes) with 5% (wt/vol) nonfat milk powder in Tris-buffered saline with Tween 20 (TBST) buffer (20 mM Tris-HCl, pH 7.5, 137 mM NaCl, 0.1% [vol/vol] Tween 20). The membranes were incubated overnight with primary antibodies in TBST buffer containing 1% (wt/vol) bovine serum albumin and then washed in TBST buffer (3 × 5 minutes). Primary antibodies (Cell Signaling Technology, Inc, Beverly, Mass) against phospho(Thr180/Tyr182)-p38-MAPK, phospho(Thr202/Tyr204)-ERK1/2, phospho(Thr308)-Akt, phospho(Ser82)-hsp27, total p38-MAPK, and total ERK1/2 were diluted 1/1000. The membranes were incubated for 60 minutes with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology Inc., Dallas, Tex), diluted 1/5000 in TBST buffer containing 1% (wt/vol) nonfat milk powder, and washed in TBST buffer (3 × 5 minutes). The bands were detected using enhanced chemiluminescence, and the blots were quantified using scanning densitometry.
Determination of Lung cAMP
cAMP was measured in lung tissue powders using a commercially available immunoassay kit (Sigma-Aldrich, Inc, St Louis, Mo) according to the manufacturer's instructions.
Statistical Analysis
Data are presented as the mean ± standard error of the mean. The number of independent experiments has been indicated in the relevant sections. To compare the effects of the various treatments on lung function during the course of reperfusion, trapezoid integration was used to calculate the area under the curve for each parameter for each rat. These individual values were then used for statistical comparisons of the various groups. Statistical analyses (1-way analysis of variance with post hoc Dunnett's t test for multiple comparisons or 2-tailed Student's t test, as appropriate) were performed using GraphPad Prism (GraphPad Software, San Diego, Calif).
Results
Study 1
Deflation-induced lung injury
We studied the effect of deflation in isolated perfused rat lungs mimicking clinical CPB. If unchallenged, the preparations were stable for ≥4 hours. Stability of the control perfusions of 140 minutes' duration was achieved for compliance and vascular resistance (Figure 1, B). Lung deflation with aerobic perfusion for 60 minutes reduced compliance by 44.5% compared with ventilated controls (Figure 1, B, area under the curve analysis, 85-140 minutes). In contrast, vascular resistance was not affected by deflation (Figure 1, B).
Differential phosphorylation of p38-MAPK, Akt, and ERK1/2 by deflation and reinflation
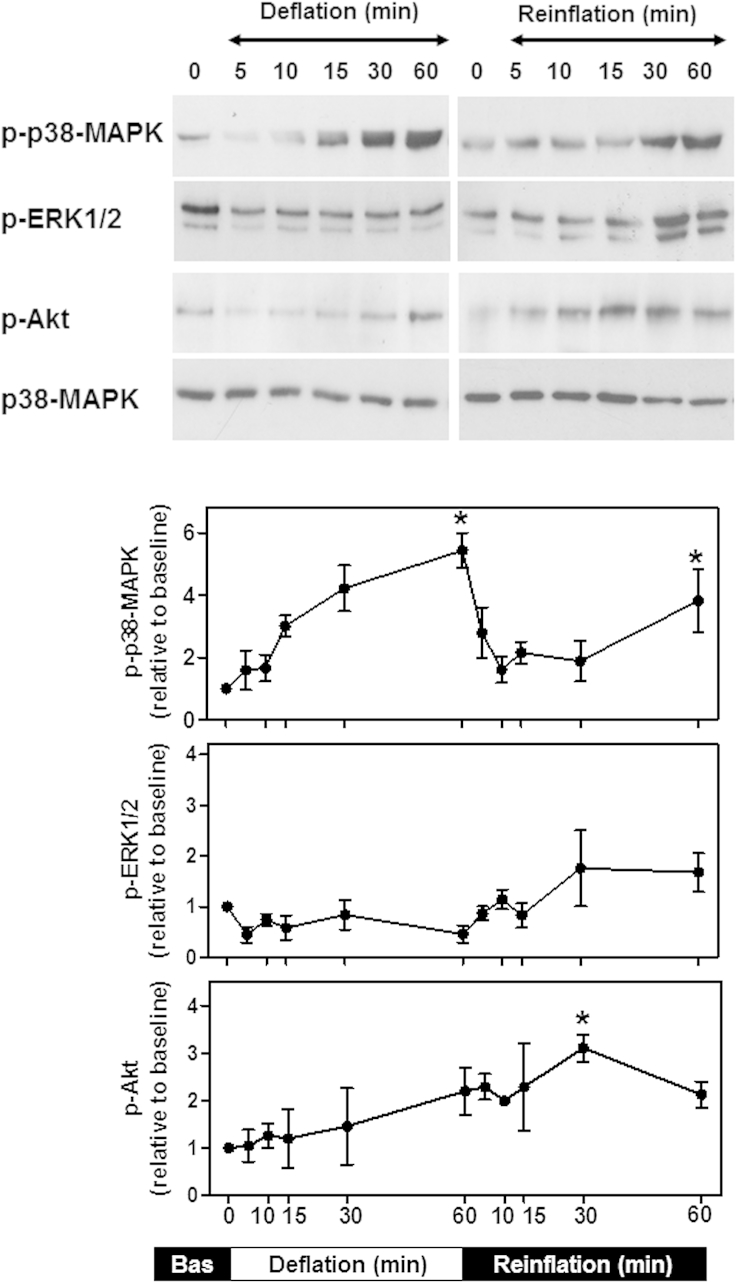
The time course of activation of signaling pathways in lungs during CPB has not been fully elucidated. Thus, the kinetics of p38-MAPK, Akt, and ERK1/2 phosphorylation in deflated and reinflated isolated lungs was examined. p38-MAPK phosphorylation increased throughout deflation, and the maximal levels (∼5.5-fold compared with baseline) were attained after 60 minutes (Figure 2). The p38-MAPK phosphorylation levels declined during early reinflation but peaked again (∼4-fold compared with baseline) after 60 minutes. Akt was also phosphorylated during deflation, but only moderately (approximately twofold compared with baseline), and this was delayed (60 minutes; Figure 2). On reinflation, Akt phosphorylation increased further (∼3-fold compared with baseline, 30 minutes) and remained elevated. In contrast, the ERK1/2 basal levels of phosphorylation were decreased during deflation (∼0.5-fold compared with baseline) and were only weakly increased at the end of reinflation (Figure 2). Equal protein loading was verified by immunoblotting for total p38-MAPK. Thus, lung deflation and reinflation, such as would occur during cardiac surgery with CPB, promotes activation of p38-MAPK, Akt, and ERK1/2 with different time courses.
Figure 2.
Lung deflation and reinflation promoted differential phosphorylation of p38-mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase 1 and 2 (ERK1/2), and Akt. The lungs were subjected to 20 minutes of equilibration (baseline, 0 minutes), followed by deflation (60 minutes) and reinflation and reperfusion (60 minutes). The tissue samples were collected at the points indicated. Whole cell extracts were immunoblotted for phosphorylated (p)-p38-MAPK, p-ERK, or p-Akt. Equal protein loading was verified by immunoblotting for total p38-MAPK. The blots were quantified using scanning densitometry. The results were normalized to the baseline measurements and are expressed as the mean ± standard error of the mean of 4 lungs per group. *P < .05 compared with baseline (1-way analysis of variance with Dunnett's test). Bas, Baseline.
Study 2
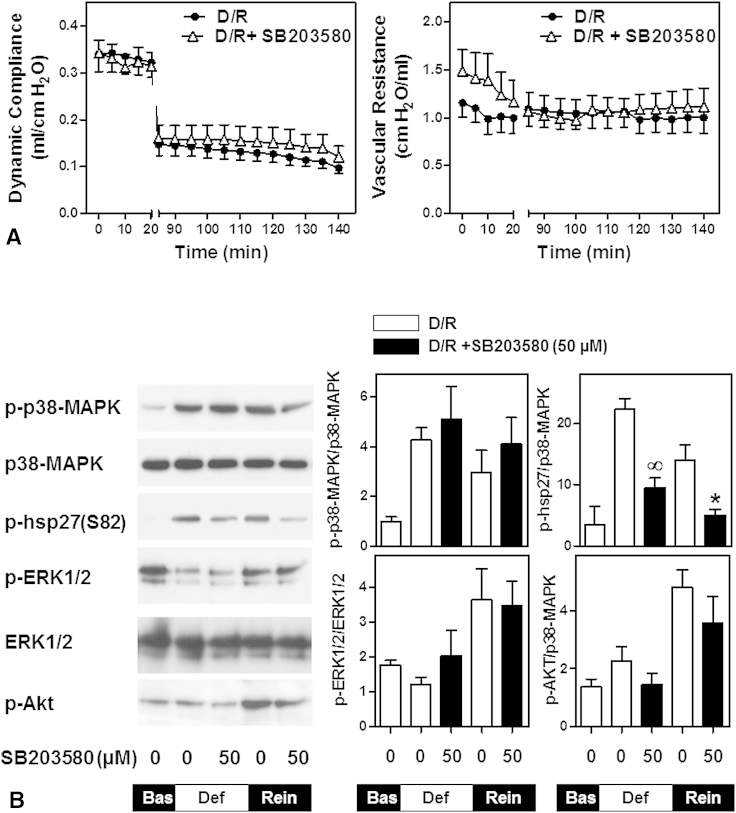
Effect of SB203580 on lung physiology and signaling
Because p38-MAPK was potently activated during deflation and reinflation, we examined its role in deflation-induced lung injury using the p38-MAPK catalytic site inhibitor SB203580. No significant difference was seen in compliance or vascular resistance after deflation in the presence of SB203580 compared with the control deflated lungs (Figure 3, A). p38-MAPK, ERK1/2, and Akt phosphorylation by deflation and reinflation was not reduced by SB203580. To confirm inhibition of p38-MAPK activity by SB203580 in our system, we examined the phosphorylation of hsp27(Ser82), a downstream target of p38-MAPK. SB203580 significantly reduced hsp27 phosphorylation by deflation and reinflation (Figure 3, B).
Figure 3.
Effect of p38-mitogen-activated protein kinase (MAPK) inhibition on lung physiology and signaling. The lungs were subjected to deflation and reinflation in the absence of SB203580 (D/R) or in the presence of 50 μM of SB203580 (D/R +SB203580). A, Dynamic compliance and vascular resistance were measured throughout the experiment. Data are expressed as the mean ± standard error of the mean of 6 lungs per group. B, Tissue samples were collected at the end of equilibration (baseline), deflation, or reperfusion. Whole cell extracts were immunoblotted for phosphorylated (p)-p38-MAPK, p-hsp27, p-ERK, or p-Akt. Equal protein loading was verified by immunoblotting for total p38-MAPK or total ERK1/2. The blots were quantified using scanning densitometry. The results were normalized to total p38-MAPK or total ERK1/2 and are expressed as the mean ± standard error of the mean of 4 lungs per group. ∞P < .05 compared with deflation without SB203580; *P < .05 compared with reinflation without SB203580 (unpaired t test). ERK1/2, Extracellular signal-regulated kinase 1 and 2; Bas, baseline.
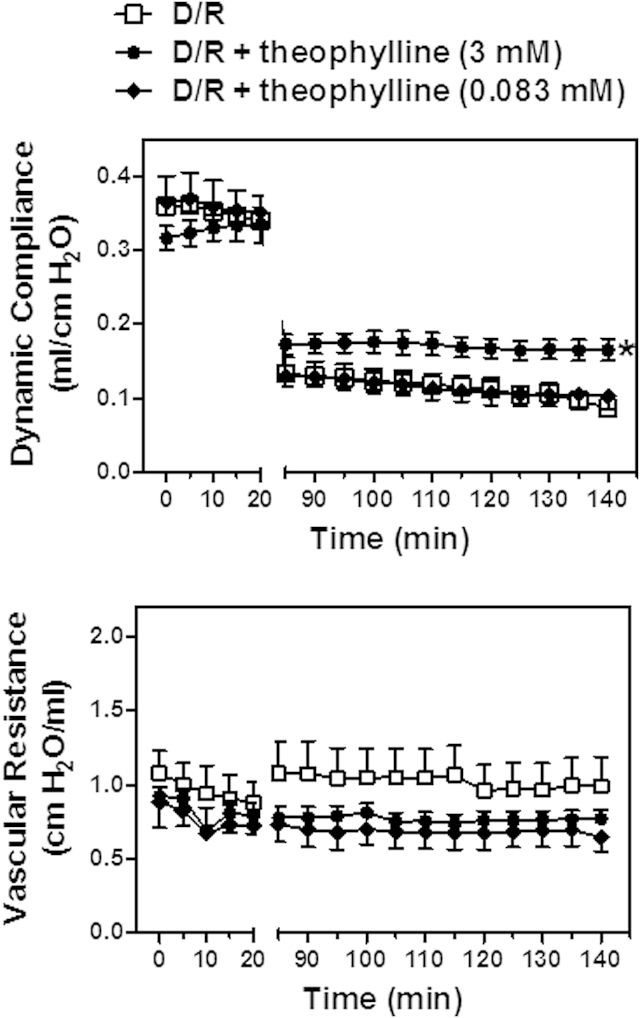
Effect of theophylline on deflation-induced lung injury and signaling
Compliance was markedly improved after deflation in the presence of 3 mM theophylline. In contrast, the therapeutic dose of theophylline (0.083 mM) had no effect (Figure 4). Theophylline at both concentrations appeared to reduce vascular resistance during lung reinflation, but this effect was not significant (Figure 4).
Figure 4.
A high dose of theophylline improved lung function after deflation. After equilibration, the lungs were subjected to deflation/reinflation in the absence of theophylline (D/R) or in the presence of theophylline (D/R+theophylline 0.083 or 3 mM). Dynamic compliance and vascular resistance were measured throughout the experiment. Data are expressed as the mean ± standard error of the mean of 6 lungs per group. *P < .05 compared with no treatment (area under the curve analysis and 1-way analysis of variance with Dunnett's test).
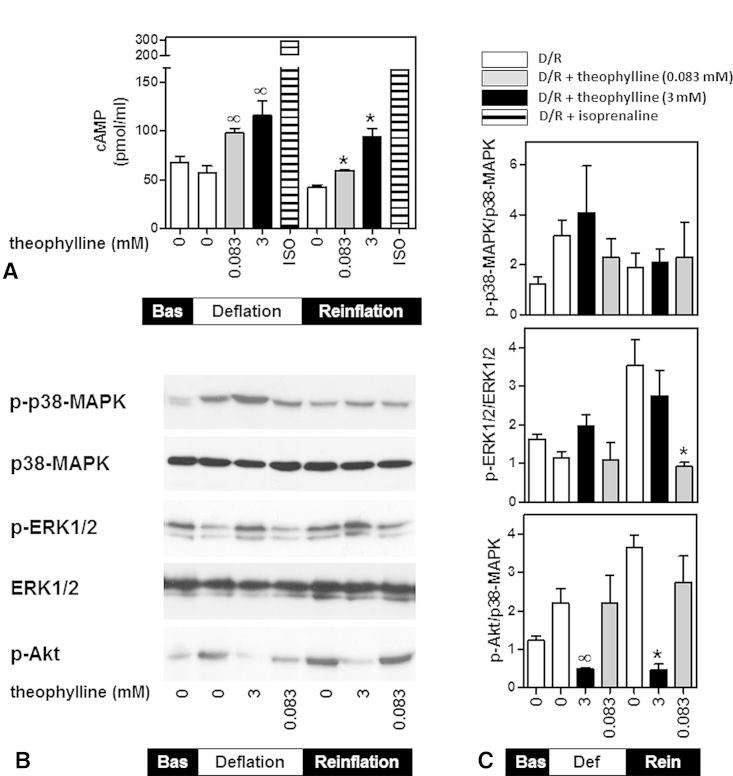
Theophylline is generally accepted as a nonspecific PDE inhibitor21; therefore, we assessed its effect on the intracellular cAMP levels. Lungs treated with isoprenaline (10−5 M) were used as positive controls.22 Deflation and reinflation decreased the lung cAMP content (57 ± 6.9 and 42.2 ± 2.3 pmol/mL, respectively, compared with baseline, 67.6 ± 6.0 pmol/mL), but the difference failed to reach statistical significance (Figure 5, A). Theophylline at 0.083 and 3 mM reversed the decrease in cAMP content by deflation and further increased the level to 97.8 ± 5.3 and 115.8 ± 14.9 pmol/mL, respectively (Figure 5, A). Similarly, theophylline increased cAMP during reinflation to 59.5 ± 0.9 pmol/mL (0.083 mM) and 94.0 ± 8.8 pmol/mL (3 mM).
Figure 5.
Effect of theophylline on lung cyclic adenosine monophosphate (cAMP) content and signaling. A, The lungs were either perfused with isoprenaline (10−5 M, positive controls) or were deflated and reinflated in the absence of theophylline (D/R) or in the presence of theophylline (D/R+theophylline 0.083 or 3 mM). The tissue samples were collected at the end of equilibration (baseline [Bas]), deflation, or reinflation, and the cAMP levels were determined. Data are expressed as the mean ± standard error of the mean of 4 lungs per group. ∞P < .05 compared with deflation without theophylline; *P < .05 compared with reinflation without theophylline (1-way analysis of variance with Dunnett's test). B and C, Cell extracts were immunoblotted for phosphorylated (p)-p38-MAPK, p-ERK, p-Akt, total p38-MAPK, or total ERK1/2. The results were normalized to total p38-MAPK or total ERK1/2 and were expressed as the mean ± standard error of the mean of 3 lungs per group. ∞P < .05 compared with deflation without theophylline; *P < .05 compared with reinflation without theophylline (unpaired t test). ERK1/2, Extracellular signal-regulated kinase 1 and 2; MAPK, mitogen-activated protein kinase.
p38-MAPK phosphorylation by deflation and reinflation was not affected by theophylline (Figure 5, B and C). The reinflation-induced phosphorylation of ERK1/2 was only inhibited by 0.083 mM theophylline (by 72.33% ± 3.6% compared with control reinflated lungs). In contrast, 3 mM theophylline markedly reduced Akt phosphorylation during deflation (by 75.6% ± 5.4%) and reinflation (by 87.3% ± 4.4%); however, the lower dose had no effect (Figure 5, B and C). These results suggest that the 2 doses of theophylline have different effects on deflation-induced lung injury and the underlying signaling pathways.
Discussion
The present study is, to our knowledge, the first to demonstrate the lung injury promoted by deflation and reinflation, together with the activation of MAPK/Akt signaling pathways in isolated perfused rat lungs. Although similar observations have been previously shown in pig and rat CPB models,12, 13 our ex vivo model of CPB has the advantage of separation from confounding whole body complications and overcomes sampling limitations. Thus, we established a detailed time course of p38-MAPK, ERK1/2, and Akt activation under conditions mimicking deflation and reinflation during cardiac surgery with CPB. Furthermore, we showed that theophylline protects against the deflation and reinflation-induced lung injury, and we have provided evidence for its potential mechanism of action.
Most cardiac surgery units maintain deflation during CPB to ensure exposure and stability of the surgical field. Nonetheless, pulmonary complications postoperatively result, at least in part, from lung collapse rather than increased edema.20 In agreement with this, deflation markedly reduced compliance in our isolated perfused lungs but had no effect on vascular resistance. Other studies of isolated lungs4, 23 have also reported compromised breathing mechanics (ie, tidal volume, compliance), with vascular resistance remaining unaffected, suggesting that compliance is a sensitive indicator of lung physiology.23
The pulmonary signaling pathways activated during cardiac surgery with CPB remain uncertain. In a pig CPB model, pulmonary p38-MAPK activation was seen during CPB and reperfusion12; however, the time course of this activation was limited. In our isolated rat lungs, we also observed p38-MAPK activation, with a biphasic pattern during deflation and reinflation. The role of p38-MAPK in deflation-induced injury was studied using SB203580, which inhibits p38-MAPK activity by occupying the adenosine triphosphate–binding pocket within the kinase cleft.24 Thus, SB203580 inhibited phosphorylation of hsp27 downstream of p38-MAPK but had no effect on p38-MAPK phosphorylation itself. Phosphorylation of Akt or ERK1/2 by deflation and reinflation was not affected by SB203580, confirming the specificity of the inhibitor for p38-MAPK. In a rat CPB model, SB203580 reduced pulmonary tissue levels of tumor necrosis factor-α and interleukin-1β, decreased lung water content and inflammatory cell infiltration, and improved lung histologic changes.13 However, it did not improve lung compliance after deflation. Thus, the p38-MAPK signaling pathway seems to be involved in the CPB-induced proinflammatory response rather than the compromised breathing mechanics. Oscillations in ERK1/2 activation during institution and weaning of CPB have been illustrated in myocardial tissue25, 26 and skeletal muscle and mesenteric vessels in pigs.25 These systemic changes in ERK1/2 activity have been attributed to shear stress.25 In the same model, CPB produced a sustained activation of pulmonary ERK1/2 that continued during post-CPB reperfusion.12 In contrast, deflation in the isolated rat lungs induced dephosphorylation of ERK1/2, but the total protein levels of the kinases were unchanged. The discrepancy between our results and those from the previous study might reflect differences in anesthetic, surgical, and/or perfusion techniques resulting from the different CPB models. ERK1/2 phosphorylation increased as soon as ventilation and perfusion was restored. Hence, the alterations in ERK1/2 activity might be associated with the mechanical forces of ventilation or the changes in perfusion. Dephosphorylation of ERK1/2 was also reported after CPB in myocardial tissue and was accompanied by upregulation of the MAPK phosphatase-1.25, 26 However, MAPK phosphatase-1 is not solely responsible for ERK1/2 dephosphorylation after CPB. MAPK/ERK kinase 1/2, the kinase that phosphorylates ERK1/2, was also decreased in the activated form after CPB. The course of ERK1/2 phosphorylation in our model suggested the involvement of upstream inhibitory signals such as MAPK/ERK kinase 1/2, rather than upregulation of inhibitory molecules such as MAPK phosphatase-1. The role of the ERK1/2 pathway in lung injury is controversial. Thus, pharmacologic inhibition of ERK1/2 with U0126 attenuated the lipopolysaccharide-induced lung injury and cytokine release,27 although the same inhibitor had no effect on ventilation-induced cytokine release.28 In our model, reinflation-induced ERK1/2 phosphorylation was inhibited by the therapeutic dose of theophylline; however, this was not associated with improved lung compliance after deflation. Additional studies are required to clarify the regulation of ERK1/2 activation by deflation and reinflation and its role in the development of CPB-induced lung injury.
The PI3K pathway has been shown to be involved in lung injury in various models.17, 18, 29 Local delivery of PI3K inhibitors prevented endotoxin- and elastase-induced lung injury through direct inhibitory effects on airway epithelial cells, neutrophils, and macrophages.29 Furthermore, inactivation of phosphatase and tension homolog, the phosphatase that negatively regulates the PI3K/Akt pathway, resulted in exacerbated acute lung injury.30 The present study is, to our knowledge, the first to demonstrate that Akt, a physiologic readout of PI3K activation, is phosphorylated in lungs by deflation and reinflation during CPB. The downstream targets of the PI3K/Akt pathway in CPB-induced lung injury are unknown. In patients with acute respiratory distress syndrome, overventilation increased proinflammatory cytokine release by PI3K/Akt-mediated activation of the transcription factor nuclear factor-kappaB.17, 28 Moreover, silencing of the PI3Kγ gene attenuated ventilator-induced lung injury independently of cytokine release; the proposed mechanism involves enhancement of pulmonary apoptosis,31 potentially through activation of the proapoptotic protein Bax.32
The severity of post-CPB lung injury could be reduced pharmacologically without compromising the exposure of the surgical field. Our previous studies of experimental long-term lung preservation have shown that a high supratherapeutic concentration of theophylline (3 mM) improved compliance and gas exchange after prolonged hypothermic storage.4 In agreement with these results, theophylline increased compliance after deflation at this high concentration (3 mM) but not at a lower dose within the therapeutic range (0.083 mM). This protective effect might have resulted from theophylline's bronchodilator action, which is minimized at therapeutic concentrations.5 PDE inhibition has been proposed as the major molecular mechanism of bronchodilatation.6 In our previous comparative studies, theophylline was more effective in improving lung function after prolonged hypothermic storage than other specific PDE isoenzyme inhibitors.4 Nevertheless, both high and low doses of theophylline increased cAMP levels in isolated perfused lungs; however, only the high dose improved compliance after deflation. Thus, the protective effect of theophylline against deflation-induced injury is likely to be independent of its PDE inhibitory properties. Theophylline in high doses could also act by adenosine receptor antagonism,7 a mechanism likely to account for its cardiac side effects, such as arrhythmias.21 However, a comparison of the effects of theophylline, xanthine amine congener (adenosine A1 receptor antagonist), and enprofylline (selective PDE inhibitor) on lung function after storage suggested that adenosine antagonism was not involved in theophylline's mechanism of action.33 The improvement in lung function by theophylline was associated with inhibition of the deflation- and reinflation-induced Akt phosphorylation. Although often overlooked, theophylline has been identified as a direct inhibitor of PI3K lipid and serine kinase activity in vitro.9 Thus, theophylline treatment of hamster ovary cells or rat soleus muscle blocked insulin-stimulated Akt phosphorylation, with a half maximal inhibitory concentration in the vicinity of 1 mM.9 In agreement with this, theophylline inhibited Akt phosphorylation by deflation and reinflation in isolated lungs at 3 mM. Furthermore, a low concentration of theophylline, used as an add-on therapy, restored corticosteroid sensitivity in patients with chronic obstructive pulmonary disease by inhibition of oxidant-activated PI3Kδ.34 The major side effects of theophylline result from PDE inhibition35; hence, its PI3K inhibitory properties provide a novel approach for the development of therapeutic strategies against CPB-induced lung injury. Theophylline's mechanism of action in the pathophysiologic conditions of CPB requires further investigation.
Study Limitations
The main limitation of our model was that the lungs were only perfused by the pulmonary artery and not also by the bronchial arteries, such as occurs in patients undergoing CPB. We attempted to compensate for this by perfusing the lungs at ∼10% of the normal flow (1.5 mL/min) to mimic the bronchial artery perfusion with oxygenated perfusate. We also acknowledge that hypothermia, routinely applied during CPB, was not included in our model. Additionally, the degree of hemodilution of our perfusate was greater than would be used in patients receiving CBP; however, it eliminated the need for blood donor animals. These differences to the clinical situation could have influenced the clinical relevance of our findings. It would be important in the future to examine these strategies in a more relevant CPB model in either rats or pigs.
Conclusions
Deflation and reinflation, such as occurs during cardiac surgery with CPB, activates the p38-MAPK, Akt, and ERK1/2 signaling pathways and induces lung injury. Understanding CPB pathophysiology is critical to improving patient outcomes, and information on potential interventions should also be applicable to emerging extracorporeal membrane oxygenation technologies. Theophylline protects against CPB-induced lung injury but at a high dose that is not clinically suitable, and this effect was associated with Akt inhibition. Studies using alternative theophylline-like drugs with a wider therapeutic range are required.
Footnotes
This study was supported by a project grant (PG/07/035) from the British Heart Foundation, United Kingdom.
Disclosures: Authors have nothing to disclose with regard to commercial support.
References
- 1.Stephens R.S., Shah A.S., Whitman G.J. Lung injury and acute respiratory distress syndrome after cardiac surgery. Ann Thorac Surg. 2013;95:1122–1129. doi: 10.1016/j.athoracsur.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Apostolakis E., Filos K.S., Koletsis E., Dougenis D. Lung dysfunction following cardiopulmonary bypass. J Card Surg. 2010;25:47–55. doi: 10.1111/j.1540-8191.2009.00823.x. [DOI] [PubMed] [Google Scholar]
- 3.Imura H., Caputo M., Lim K., Ochi M., Suleiman M.S., Shimizu K. Pulmonary injury after cardiopulmonary bypass: beneficial effects of low-frequency mechanical ventilation. J Thorac Cardiovasc Surg. 2009;137:1530–1537. doi: 10.1016/j.jtcvs.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Featherstone R.L., Chambers D.J., Kelly F.J. Comparison of phosphodiesterase inhibitors of differing isoenzyme selectivity added to St. Thomas' Hospital cardioplegic solution used for hypothermic preservation of rat lungs. Am J Respir Crit Care Med. 2000;162:850–856. doi: 10.1164/ajrccm.162.3.9910038. [DOI] [PubMed] [Google Scholar]
- 5.Barnes P.J. Theophylline: new perspectives for an old drug. Am J Respir Crit Care Med. 2003;167:813–818. doi: 10.1164/rccm.200210-1142PP. [DOI] [PubMed] [Google Scholar]
- 6.Rabe K.F., Magnussen H., Dent G. Theophylline and selective PDE inhibitors as bronchodilators and smooth muscle relaxants. Eur Respir J. 1995;8:637–642. [PubMed] [Google Scholar]
- 7.Bjorck T., Gustafsson L.E., Dahlen S.E. Isolated bronchi from asthmatics are hyperresponsive to adenosine, which apparently acts indirectly by liberation of leukotrienes and histamine. Am Rev Respir Dis. 1992;145:1087–1091. doi: 10.1164/ajrccm/145.5.1087. [DOI] [PubMed] [Google Scholar]
- 8.Mascali J.J., Cvietusa P., Negri J., Borish L. Anti-inflammatory effects of theophylline: modulation of cytokine production. Ann Allergy Asthma Immunol. 1996;77:34–38. doi: 10.1016/S1081-1206(10)63476-X. [DOI] [PubMed] [Google Scholar]
- 9.Foukas L.C., Daniele N., Ktori C., Anderson K.E., Jensen J., Shepherd P.R. Direct effects of caffeine and theophylline on p110 delta and other phosphoinositide 3-kinases: differential effects on lipid kinase and protein kinase activities. J Biol Chem. 2002;277:37124–37130. doi: 10.1074/jbc.M202101200. [DOI] [PubMed] [Google Scholar]
- 10.Ito K., Lim S., Caramori G., Cosio B., Chung K.F., Adcock I.M. A molecular mechanism of action of theophylline: induction of histone deacetylase activity to decrease inflammatory gene expression. Proc Natl Acad Sci U S A. 2002;99:8921–8926. doi: 10.1073/pnas.132556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo W.J., Ling X., Huang R.M. Effects of aminophylline on cytokines and pulmonary function in patients undergoing valve replacement. Eur J Cardiothorac Surg. 2004;25:766–771. doi: 10.1016/j.ejcts.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Khan T.A., Bianchi C., Araujo E.G., Ruel M., Voisine P., Sellke F.W. Activation of pulmonary mitogen-activated protein kinases during cardiopulmonary bypass. J Surg Res. 2003;115:56–62. doi: 10.1016/s0022-4804(03)00236-1. [DOI] [PubMed] [Google Scholar]
- 13.Dong X., Liu Y., Du M., Wang Q., Yu C.T., Fan X. p38 Mitogen-activated protein kinase inhibition attenuates pulmonary inflammatory response in a rat cardiopulmonary bypass model. Eur J Cardiothorac Surg. 2006;30:77–84. doi: 10.1016/j.ejcts.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg P.L., MacNaughton D.E., Clements R.T., Minnear F.L., Vincent P.A. p38 MAPK activation by TGF-beta1 increases MLC phosphorylation and endothelial monolayer permeability. Am J Physiol Lung Cell Mol Physiol. 2002;282:L146–L154. doi: 10.1152/ajplung.2002.282.1.L146. [DOI] [PubMed] [Google Scholar]
- 15.Tamura D.Y., Moore E.E., Johnson J.L., Zallen G., Aiboshi J., Silliman C.C. p38 Mitogen-activated protein kinase inhibition attenuates intercellular adhesion molecule-1 up-regulation on human pulmonary microvascular endothelial cells. Surgery. 1998;124:403–407. [PubMed] [Google Scholar]
- 16.Hashimoto S., Gon Y., Matsumoto K., Takeshita I., Horie T. N-acetylcysteine attenuates TNF-alpha-induced p38 MAP kinase activation and p38 MAP kinase-mediated IL-8 production by human pulmonary vascular endothelial cells. Br J Pharmacol. 2001;132:270–276. doi: 10.1038/sj.bjp.0703787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlig U., Fehrenbach H., Lachmann R.A., Goldmann T., Lachmann B., Vollmer E. Phosphoinositide 3-OH kinase inhibition prevents ventilation-induced lung cell activation. Am J Respir Crit Care Med. 2004;169:201–208. doi: 10.1164/rccm.200303-343OC. [DOI] [PubMed] [Google Scholar]
- 18.Kim D.I., Kim S.R., Kim H.J., Lee S.J., Lee H.B., Park S.J. PI3K-gamma inhibition ameliorates acute lung injury through regulation of IkappaBalpha/NF-kappaB pathway and innate immune responses. J Clin Immunol. 2012;32:340–351. doi: 10.1007/s10875-011-9628-1. [DOI] [PubMed] [Google Scholar]
- 19.Medina-Tato D.A., Ward S.G., Watson M.L. Phosphoinositide 3-kinase signalling in lung disease: leucocytes and beyond. Immunology. 2007;121:448–461. doi: 10.1111/j.1365-2567.2007.02663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verheij J., van Lingen A., Raijmakers P.G., Spijkstra J.J., Girbes A.R., Jansen E.K. Pulmonary abnormalities after cardiac surgery are better explained by atelectasis than by increased permeability oedema. Acta Anaesthesiol Scand. 2005;49:1302–1310. doi: 10.1111/j.1399-6576.2005.00831.x. [DOI] [PubMed] [Google Scholar]
- 21.Barnes P.J. Theophylline. Am J Respir Crit Care Med. 2013;188:901–906. doi: 10.1164/rccm.201302-0388PP. [DOI] [PubMed] [Google Scholar]
- 22.Adkins W.K., Barnard J.W., May S., Seibert A.F., Haynes J., Taylor A.E. Compounds that increase cAMP prevent ischemia-reperfusion pulmonary capillary injury. J Appl Physiol. 1992;72:492–497. doi: 10.1152/jappl.1992.72.2.492. [DOI] [PubMed] [Google Scholar]
- 23.Uhlig S., Wollin L. An improved setup for the isolated perfused rat lung. J Pharmacol Toxicol Methods. 1994;31:85–94. doi: 10.1016/1056-8719(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 24.Gum R.J., McLaughlin M.M., Kumar S., Wang Z., Bower M.J., Lee J.C. Acquisition of sensitivity of stress-activated protein kinases to the p38 inhibitor, SB 203580, by alteration of one or more amino acids within the ATP binding pocket. J Biol Chem. 1998;273:15605–15610. doi: 10.1074/jbc.273.25.15605. [DOI] [PubMed] [Google Scholar]
- 25.Araujo E.G., Bianchi C., Faro R., Sellke F.W. Oscillation in the activities of MEK/ERK1/2 during cardiopulmonary bypass in pigs. Surgery. 2001;130:182–191. doi: 10.1067/msy.2001.115826. [DOI] [PubMed] [Google Scholar]
- 26.Araujo E.G., Bianchi C., Sato K., Faro R., Li X.A., Sellke F.W. Inactivation of the MEK/ERK pathway in the myocardium during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2001;121:773–781. doi: 10.1067/mtc.2001.112933. [DOI] [PubMed] [Google Scholar]
- 27.Schuh K., Pahl A. Inhibition of the MAP kinase ERK protects from lipopolysaccharide-induced lung injury. Biochem Pharmacol. 2009;77:1827–1834. doi: 10.1016/j.bcp.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Uhlig U., Haitsma J.J., Goldmann T., Poelma D.L., Lachmann B., Uhlig S. Ventilation-induced activation of the mitogen-activated protein kinase pathway. Eur Respir J. 2002;20:946–956. doi: 10.1183/09031936.02.01612001. [DOI] [PubMed] [Google Scholar]
- 29.Chen C., Fang X., Wang Y., Li Y., Wang D., Zhao X. Preventive and therapeutic effects of phosphoinositide 3-kinase inhibitors on acute lung injury. Chest. 2011;140:391–400. doi: 10.1378/chest.10-3060. [DOI] [PubMed] [Google Scholar]
- 30.Miyoshi K., Yanagi S., Kawahara K., Nishio M., Tsubouchi H., Imazu Y. Epithelial Pten controls acute lung injury and fibrosis by regulating alveolar epithelial cell integrity. Am J Respir Crit Care Med. 2013;187:262–275. doi: 10.1164/rccm.201205-0851OC. [DOI] [PubMed] [Google Scholar]
- 31.Lionetti V., Lisi A., Patrucco E., De Giuli P., Milazzo M.G., Ceci S. Lack of phosphoinositide 3-kinase-gamma attenuates ventilator-induced lung injury. Crit Care Med. 2006;34:134–141. doi: 10.1097/01.ccm.0000190909.70601.2c. [DOI] [PubMed] [Google Scholar]
- 32.Kolliputi N., Waxman A.B. IL-6 cytoprotection in hyperoxic acute lung injury occurs via PI3K/Akt-mediated Bax phosphorylation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L6–L16. doi: 10.1152/ajplung.90381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Featherstone R.L., Chambers D.J. Long-term hypothermic lung preservation: does adenosine A1 receptor antagonism have a role in ischemic preconditioning protection? Interact Cardiovasc Thorac Surg. 2004;3:182–187. doi: 10.1016/S1569-9293(03)00274-3. [DOI] [PubMed] [Google Scholar]
- 34.To Y., Ito K., Kizawa Y., Failla M., Ito M., Kusama T. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:897–904. doi: 10.1164/rccm.200906-0937OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howell R.E., Muehsam W.T., Kinnier W.J. Mechanism for the emetic side effect of xanthine bronchodilators. Life Sci. 1990;46:563–568. doi: 10.1016/0024-3205(90)90123-9. [DOI] [PubMed] [Google Scholar]