Abstract
Fluid resuscitation is one of the most frequent and necessary practices in clinical medicine and is an integral part of the initial stabilization of critically ill, hypovolemic patients. Longstanding debate and conflicting evidence surround the use of both colloid and crystalloid fluid resuscitation in these patients. The basis of this debate is heavily rooted in the physiological understanding of Starling’s forces. In this review, we aim to highlight the ongoing debate of albumin versus crystalloid resuscitation both broadly and as it relates to lung function, and will discuss the current state-of-the-art, starting from an historic perspective and progressing through a review of both physiologic and clinical evidence. Despite the biologic and physiologic plausibility of therapeutic benefit, the current evidence base does not support the routine use of albumin administration to improve patient survival or prevent respiratory dysfunction.
Introduction
Respiratory dysfunction is common in critically ill patients, and is perhaps highest in patients with shock requiring large volume resuscitation. Since the time of World War II and the first use of albumin administration, debate has existed over whether albumin administration for hypovolemic patients is superior to crystalloid infusion. Further, it has been postulated that the risk of developing organ dysfunction, and specifically within the scope of this review, the clinical syndrome of acute respiratory distress syndrome (ARDS) potentiated by its pathophysiologic correlate, acute lung injury, might be mitigated by resuscitation with albumin. The basis of this debate is deeply rooted in the physiological understanding of Starling’s forces and the micro-physiologic environment of the alveolar-capillary barrier within the lung. To date, the collective body of evidence surrounding these questions does not support these claims, either for overall or respiratory related outcomes. This review will highlight the ongoing debate of albumin versus crystalloid resuscitation as it relates to respiratory dysfunction and will discuss the current state-of-the-art, starting from an historic perspective and progressing through a review of both physiologic and clinical evidence.
Methods
An online search was conducted via PubMed focusing on the most recent clinical studies, meta analyses, and systematic reviews in adult patient populations. Additional historical references and primary studies were reviewed and included as they pertain to the evolution of albumin use over time.
Historical Perspective
The first use of albumin occurred in the 1940s during World War II and was administered to injured soldiers in hemorrhagic shock who required aggressive volume expansion [1]. Since that time, it has been shown that this abundant plasma protein restores hemodynamic and tissue perfusion endpoints more rapidly and with smaller volumes than with crystalloid solutions. One of the earlier trials that addressed clinical outcomes was published by Shoemaker and colleagues in 1981[2]. In this prospective, randomized trial, hypotensive patients on a surgical inpatient service were randomized to one of several fluid resuscitation strategies, including albumin and crystalloid. Patients randomized to albumin resuscitation were found to have significantly faster restoration of mean arterial blood pressure, suggesting hemodynamic benefit in this patient population.
However, other studies performed in the same era produced conflicting results. In 1978, Weaver and colleagues showed that albumin resuscitation was detrimental to lung function in patients with hypovolemic shock [3]. The following year, Virgilio and colleagues showed no difference in rates of pulmonary edema in postsurgical patients resuscitated with albumin as compared to crystalloid [4]. Due to conflicting results, several meta-analyses were performed in the 1990s [5–8]. Collectively, they showed a trend toward higher mortality with albumin which caused a high level of concern about the safety of albumin resuscitation. However, early meta analyses were criticized for the heterogeneity of patient populations and exclusion of relevant trials. These arguments spurred the next wave of meta analyses and large randomized clinical trials [9].
Due to criticism of early meta analyses, Wilkes and colleagues performed one of the more recent analyses of the collective data set in 2001 that included 55 randomized clinical trials. Results showed no difference in mortality with the use of albumin versus crystalloid [10]. However, intensive care unit physicians worldwide continue to use colloids more frequently in resuscitation that crystalloids [11]. This may be due to the more potent hemodynamic improvements seen with colloids, or because potential differences in other clinically relevant endpoints such as lung and other organ dysfunction have not been extensively evaluated. However, the debate continues as a small pilot randomized, controlled clinical trial of morbidity endpoints has recently shown a beneficial dose-response relationship between albumin administration and reduced organ dysfunction [19].
The most recent meta-analysis of albumin versus non-albumin resuscitation was published in 2011 by Delaney and others and reviewed trials of a specific patient population, those with sepsis, and found a lower mortality rate with the use of albumin (OR 0.82) as compared with non-albumin containing fluids. Unlike the extensive patient heterogeneity inherent in prior meta-analyses, these results suggest that albumin may provide therapeutic benefit as a niche therapy for selected patient populations rather than hypovolemic patients at large.
Biochemical Properties
Human albumin is a small, negatively-charged, globular protein that accounts for roughly 50% of human plasma protein and over 75% of plasma oncotic pressure [12–15]. Albumin is continuously synthesized in the liver up to 10–15 grams per day and serves many functions. Although only 30–40% of albumin is retained in the vascular space, it remains the primary determinant of colloid osmotic pressure (COP). Continuous leak of albumin from the vascular space into the interstitial space is balanced by lymphatic drainage and recirculation into the vascular space at roughly the same rate. The half-life of albumin ranges from 2–3 weeks in healthy individuals [16]. Degradation occurs primarily in the muscle, liver and kidney, and the balance of synthesis and degradation is tightly regulated by multiple variables including colloid osmotic pressure [12–14].
Albumin is comprised of three domains which fold into a heart-shaped tertiary structure that contains a hydrophobic interior and hydrophilic exterior. Binding sites allow for interaction with a range of ligands including cations, metal ions, bilirubin, hormones, fatty acids and pharamacologic agents, making albumin a vital factor in many enzymatic and endocrine functions, detoxification, and drug bioavailability. In addition to contributing to colloid osmotic pressure and having extensive binding capacity, albumin has also been associated with other important functions, some of which have only recently been elucidated. It appears that albumin administration may affect vascular permeability, cell signaling, neutrophil adhesion, redox homeostasis [12, 17, 18]. It also has anticoagulant and antithrombotic properties [12, 17, 18]. In total, the extra-circulatory effects of albumin make a biologically plausible case that infusion of this protein may have therapeutic benefit based on factors separate from the hemodynamic and physiologic functions of the molecule.
Clinical use, however, is not without risk. Potential adverse effects of prescribing human serum albumin include acute, allergic reactions ranging from itching and urticarial to anaphylaxis [19, 20]. Rapid infusion into elderly individuals has been associated with abrupt fall in systemic arterial blood pressure. Caution is also advised in patients in or at risk of hypervolemic states including congestive heart failure, chronic renal disease and chronic liver disease [19, 20].
Physiology and Pathophysiology
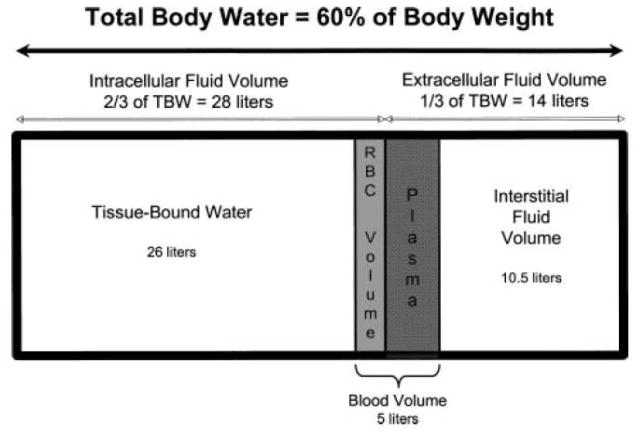
The human body is functionally divided into compartments that permit volume buffering during times of physiological stress [21]. (Figure 1) Water is an important component of all tissues and constitutes 50 to 70% of total body weight, present in inverse proportion to age and body fat. The distribution of total body water (TBW) is two thirds (40%) to the intracellular compartment and one third (20%) to the extracellular compartments. The extracellular fluid (ECF) is then subdivided into an interstitial component, constituting 75% of the ECF, and an intravascular component, constituting 25% of the ECF and representing the effective plasma volume.
Figure 1.
Depiction of body fluid compartments and distribution of body water for an average 70 kg male. TBW, total body water; RBCvolume, red blood cell volume, normally 2 L. Plasma volume normally occupies 3.5 L, or 8% of TBW. [Reproduced with permission from reference [22]].
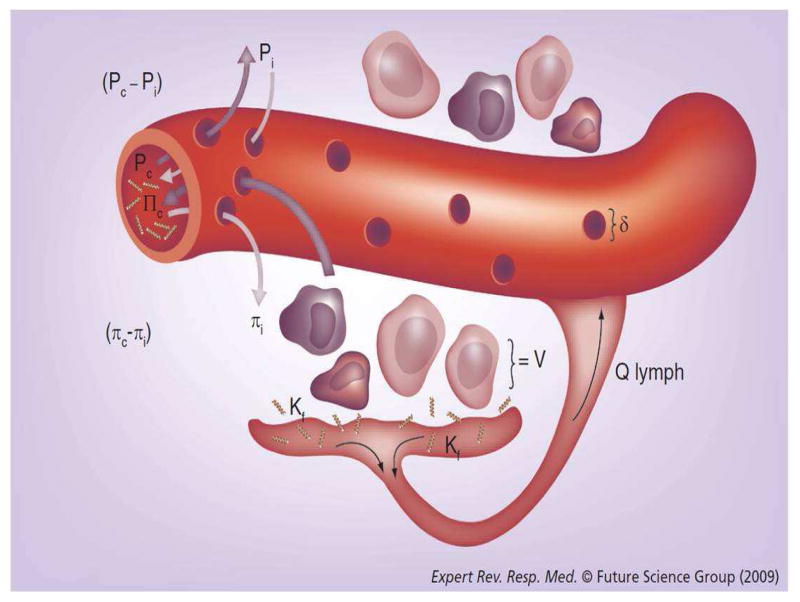
The development of albumin as a resuscitation fluid was initially based on the physiological principles of Starling. (See Figure 2). The rate of filtration across a capillary membrane may be quantified by the balance of forces described from the Starling equation, including hydrostatic and oncotic pressures in the capillaries and in the interstitial spaces. Iso-oncotic albumin (i.e. 4% or 5%) maintains colloid osmotic pressure (COP) at 20–30mm Hg and results in maximum plasma volume expansion of up to 100% [22]. In cardiac surgery patients, 5% albumin administration increases cardiac output and is five times more efficient for plasma volume expansion than isotonic saline (52% vs. 9%, p<0.05) [23]. In critically ill patients with sepsis, 5% albumin administration maintains COP and increases the plasma volume in an amount equal to the volume infused [24].
Figure 2. Visual illustration of Starling’s law as applied to the alveolar-capillary barrier.
The Starling equation is written as: Qf = Kf [(Pc−PIF) − σ (πc−πIF)], where Qf is flow or fluid flux across a membrane, Kf is the capillary filtration coefficient, Pc is the capillary hydrostatic pressure, PIF is the interstitial hydrostatic pressure, σ is the oncotic reflection coefficient (usually 0.8 for non-damaged lung endothelium), πc is the capillary colloid osmotic pressure, and πIF is the interstitial colloid osmotic pressure. V represents the cellular component of filtered fluid; QLYMPH is the lymphatic flow. The driving forces that favor fluid filtration are Pc and πIF, while PIF and πc oppose it. [Reproduced with permission from reference [35]
On the other hand, crystalloids have the potential to decrease COP resulting in fluid extravasation and maximum plasma volume expansion of only 25%. In critically ill patients at risk for the acute respiratory distress syndrome, the alveolar capillary membrane is a unique microenvironment where leaky capillaries and diffuse alveolar damage may result in extravasation of not only crystalloid resuscitation fluids, but also albumin. In this situation, interstitial oncotic pressure may be increased and result in worsening of edema associated with capillary leak syndromes. With respect to pulmonary edema, it has been shown that administration of albumin to patients with ARDS does not worsen pulmonary edema as long as hydrostatic pressures do not increase and contribute to tissue-directed fluid flux [25].
A reduction in plasma COP is a strong predictor of the development of respiratory dysfunction and ARDS [26, 27]. Although albumin is the major component of serum total protein and the greatest contributor to plasma COP, reductions in serum total protein are predictive of ARDS, with an area under the receiver operating characteristic curve of 0.95 using a cutoff of 59 g/L [27]. COP is a potent mediator of fluid flux, such that reductions in COP will increase lung lymphatic flow (QL) twice as much as an equivalent rise in hydrostatic pressure. Furthermore, experimental reductions in COP impair and prolong pulmonary edema clearance [28–30]. Comparatively, increases in COP normalize QL and reduce tissue-directed fluid flux in the lung [31, 32].
The clinical application of these physiological principles has ignited much debate regarding the utility of restoring colloid osmotic pressure as a strategy to prevent edema, both pulmonary and peripheral. Ernest et al attempted to answer this question in critically ill patients by studying the volume of distribution of infused normal saline and 5% albumin in septic patients [23]. In this prospective trial of 18 patients, plasma volume (PV) and extracellular fluid volume (ECFV) were measured by dilution of 131I-albumin and 35S Sodium Sulfate, respectively. Interstitial fluid volume (ISFV) was calculated by subtracting PV from the ECFV. In the saline group, the increase in ECFV approximated the volume of saline infused, while only 21% of the volume remained as plasma volume. In the albumin group, the increase in ECFV was more than double the volume of albumin infused, and the total volume was distributed evenly between the intravascular and extravascular spaces. However, there was no significant difference between the absolute amounts of extravascular fluid volume in either group, refuting the hypothesis that colloid therapy reduces pulmonary edema.
It is well established that reducing hydrostatic pressure in patients with acute lung injury (ALI), this term now being used as a pathophysiologic concept, results in improved lung function [33]. In the Fluid and Catheter Treatment Trial (FACTT), patients with acute lung injury were randomized to a fluid liberal or fluid conservative strategy [33]. Patients in the fluid conservative arm had improved measures of lung function and an additional 2.5 ventilator free days as compared with the fluid liberal arm. The question that naturally flows from these finding is whether augmenting both hydrostatic pressure, by means of a fluid conservative strategy and diuretics, and colloid osmotic pressure, by means of albumin infusion, improves respiratory endpoints more than either strategy alone.
Our group has conducted a series of studies examining the role of colloids in altering measures of pulmonary edema in patients with acute lung injury [34, 35]. In a prospective, randomized, placebo-controlled clinical trial, 37 mechanically ventilated patients with acute lung injury were enrolled and treated with either the combination of intermittent doses of 25% albumin and continuous infusion furosemide, or two matching placebos [35]. Patients in the experimental group had significantly better oxygenation as well as higher urine output and mean arterial pressure and lower heart rate within the first four days as compared to the control group. There was a non-significant trend toward shorter duration of mechanical ventilation in the experimental group and mortality rates were not different between the two groups. This study was followed by a second randomized, placebo-controlled clinical trial of 40 patients with acute lung injury, to study the specific effects of albumin [36]. In this study, similar patients with acute lung injury were randomly allocated to treatment either with an identical combination of albumin and furosemide, or with placebo and furosemide. Patients treated with albumin had greater oxygenation within 24 hours of therapy, and greater hemodynamic stability despite greater net fluid loss. There was no difference in clinical outcomes, but again, a similar numerical trend was observed for a shorter duration of mechanical ventilation.
Review of the Literature
Until recently, most of the research related to the albumin versus crystalloid controversy has focused on outcomes related to overall mortality and measures of hemodynamic perfusion. The body of evidence that specifically focuses on the use of albumin as a means of reducing lung dysfunction is small and conflicting. More than ten years ago, several meta-analyses were performed in an attempt to identify a collective signal of benefit or harm associated with colloids [5–8]. This body of evidence refuted the hypothesis that colloid therapy results in improved outcomes and inconsistently included outcome measures of lung function.
One of the largest meta-analyses was published by Choi and others and included randomized trials on colloid versus crystalloid resuscitation [8]. Not only did they analyze overall mortality risk, but they also identified six studies that assessed outcomes related to lung function, albeit in a non-uniform fashion. Although they found an increase in mortality of trauma patients treated with colloids, there was no difference in overall respiratory outcomes in any subgroup of the study. Importantly, however, the studies that evaluated lung function were not powered to detect a significant difference in respiratory outcomes, and the combined sample size was small (n=180)
These results were confirmed in a more recent study by van der Heijden and coworkers [34] in a prospective randomized trial of septic and non-septic mechanically ventilated patients who required fluid resuscitation. Patients were randomized to 90 minutes of fluid loading with either 0.9% NaCl, gelatin 4%, hydroxyethyl starch 6%, or albumin 5% titrated to predetermined cardiac filling pressures. Outcome parameters included pulmonary leak index of Gallium-labeled transferrin, extravascular lung water (EVLW) and the lung injury score. As would be expected, colloid osmotic pressure (COP) increased in the colloid groups and decreased in the crystalloid group. However, pulmonary edema and lung injury scores were not affected by the type of resuscitation fluid, adding to the argument that pulmonary edema is more dependent on hydrostatic forces than COP in the setting of increased capillary permeability, and likewise, dependent also on overall extracellular fluid volume and fluid balance.
Large randomized controlled trials powered to detect a difference in pulmonary function are lacking. One notable exception is the SAFE (Saline versus Albumin Fluid Evaluation) trial that randomized 7,000 acutely ill patients to receive iso-oncotic albumin or isotonic crystalloid for early fluid resuscitation [35]. The study population was heterogeneous and showed no difference in organ function (e.g. respiratory failure) or 28-day mortality between the two groups. Among the pre-defined subgroups, the SAFE study reported a higher relative risk of death (RR=1.36) for traumatic brain injury patients treated with albumin as compared to crystalloid, and a lower risk of death for sepsis patients treated with albumin versus saline (adjusted RR=0.71) [37]. Although there was no significant difference in mortality in the subgroup of patients with ARDS, this group only included patients with ARDS at the time of hospital arrival and thus represented only ~2% of the SAFE study population, making firm conclusions impossible.
Finally, it has been shown that hypoproteinemic states, as an indicator of reduced oncotic pressure, correlate with the development of acute lung injury and ARDS. In a study published by Mangialardi and colleagues in 2000 [24], a regression modeling approach was used to compare outcomes in 455 critically ill patients with severe sepsis and low serum protein levels to compare sepsis patients with normal serum protein levels. Patients with low serum protein levels were found to have a significantly higher risk of developing ARDS, to require longer durations of mechanical ventilation, and to have a higher rate of mortality. These findings have led to further research aimed at determining whether treating the hypoproteinemic state reduces the risk of respiratory distress syndrome by augmenting Starling’s forces. This hypothesis has been tested by our group and has shown that treatment with a combination of albumin and furosemide results in a non-significant trend toward shorter duration of mechanical ventilation in hypoproteinemic patients with acute lung injury.
The American Thoracic Society published a consensus statement on the use of colloid therapy in the critically ill in 2004 [12]. The study group concluded that “colloids restore intravascular volume and tissue perfusion more rapidly than crystalloids in all shock states” and “although hydrostatic pressure is more important than COP [colloid osmotic pressure] for accumulation of pulmonary edema, colloid administration reduces tissue edema and may ameliorate pulmonary edema as a consequence of shock resuscitation”. Both of these conclusions were based on evidence obtained from well-designed controlled trials without randomization or randomized trials without blinding [12]. Together, these data suggest that further study of specific patient populations is needed, including hypoproteinemic patients with sepsis.
Summary Opinion
Fluid resuscitation is one of the most frequent and necessary practices in intensive care, being applied to critically ill, hypovolemic patients as an integral part of initial stabilization. The collective body of evidence to date, including multiple meta-analyses and the SAFE trial, suggest there is no difference in overall mortality between iso-oncotic albumin and common isotonic crystalloid solutions. Likewise, the evidence supporting the use of colloid resuscitation to reduce the risk of respiratory dysfunction and pulmonary edema is conflicting. To date, there is not clear and convincing evidence that albumin resuscitation prevents the development of respiratory dysfunction as compared to crystalloid resuscitation. However, albumin may have a role as a niche drug in specified patient populations such as ARDS and sepsis. Despite the biological and physiological plausibility of efficacy, we do not recommend the routine use of colloid administration for preventing pulmonary edema or the development of ARDS. Further investigation with large randomized clinical trials in specific patient populations is needed.
Key Messages.
Fluid resuscitation is a core intervention for hypovolemic and many critically ill patients.
Albumin infusion restores hemodynamic endpoints more quickly than crystalloid options.
Albumin therapy does not appear to affect mortality as compared to crystalloid options.
Respiratory endpoints are not improved with albumin therapy as compared to crystalloid in a general patient population; however, certain populations including patients with sepsis and ARDS may benefit from albumin therapy. Further study is needed in this area.
Bibliography
- 1.Moss GS, et al. Colloid or crystalloid in the resuscitation of hemorrhagic shock: a controlled clinical trial. Surgery. 1981;89(4):434–8. [PubMed] [Google Scholar]
- 2.Shoemaker WC, SM, Hopkins JA, et al. Comparison of the Relative Effectiveness of Colloids and Crystalloids in Emergency Resuscitation. Am J Surg. 1981;142:73–81. doi: 10.1016/s0002-9610(81)80015-3. [DOI] [PubMed] [Google Scholar]
- 3.Weaver DW, et al. Pulmonary effects of albumin resuscitation for severe hypovolemic shock. Arch Surg. 1978;113(4):387–92. doi: 10.1001/archsurg.1978.01370160045006. [DOI] [PubMed] [Google Scholar]
- 4.Virgilio RW, et al. Crystalloid vs. colloid resuscitation: is one better? A randomized clinical study. Surgery. 1979;85(2):129–39. [PubMed] [Google Scholar]
- 5.Velanovich V. Crystalloid versus colloid fluid resuscitation: a meta-analysis of mortality. Surgery. 1989;105(1):65–71. [PubMed] [Google Scholar]
- 6.Schierhout G, Roberts I. Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ. 1998;316(7136):961–4. doi: 10.1136/bmj.316.7136.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Human albumin administration in critically ill patients: systematic review of randomised controlled trials. Cochrane Injuries Group Albumin Reviewers. BMJ. 1998;317(7153):235–40. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi PT, et al. Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med. 1999;27(1):200–10. doi: 10.1097/00003246-199901000-00053. [DOI] [PubMed] [Google Scholar]
- 9.Webb AR. The appropriate role of colloids in managing fluid imbalance: a critical review of recent meta-analytic findings. Crit Care. 2000;4(Suppl 2):S26–32. doi: 10.1186/cc967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkes MM, Navickis RJ. Patient survival after human albumin administration. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;135(3):149–64. doi: 10.7326/0003-4819-135-3-200108070-00007. [DOI] [PubMed] [Google Scholar]
- 11.Finfer S, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14(5):R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin GS, MM Evidence-based Colloid Use in the Critically Ill: American Thoracic Society Consensus Statement. Am J Respir Crit Care Med. 2004;170:1247–1259. doi: 10.1164/rccm.200208-909ST. [DOI] [PubMed] [Google Scholar]
- 13.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41(6):1211–9. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 14.Peters T. All about albumin: biochemistry, genetics, and medical applications. San Diego: Academic Press; 1996. p. xx.p. 432. [Google Scholar]
- 15.Farrugia A. Albumin usage in clinical medicine: tradition or therapeutic? Transfus Med Rev. 2010;24(1):53–63. doi: 10.1016/j.tmrv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Beeken WL, et al. Studies of I-131-albumin catabolism and distribution in normal young male adults. J Clin Invest. 1962;41:1312–33. doi: 10.1172/JCI104594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radi R, et al. Reaction of xanthine oxidase-derived oxidants with lipid and protein of human plasma. Arch Biochem Biophys. 1991;286(1):117–25. doi: 10.1016/0003-9861(91)90016-c. [DOI] [PubMed] [Google Scholar]
- 18.Powers KA, et al. Twenty-five percent albumin prevents lung injury following shock/resuscitation. Crit Care Med. 2003;31(9):2355–63. doi: 10.1097/01.CCM.0000084846.45830.AA. [DOI] [PubMed] [Google Scholar]
- 19.Liumbruno GM, et al. Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009;7(3):216–34. doi: 10.2450/2009.0094-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent JL, Wilkes MM, Navickis RJ. Safety of human albumin--serious adverse events reported worldwide in 1998–2000. Br J Anaesth. 2003;91(5):625–30. doi: 10.1093/bja/aeg233. [DOI] [PubMed] [Google Scholar]
- 21.Dubois MJ, et al. Albumin administration improves organ function in critically ill hypoalbuminemic patients: A prospective, randomized, controlled, pilot study. Crit Care Med. 2006;34(10):2536–40. doi: 10.1097/01.CCM.0000239119.57544.0C. [DOI] [PubMed] [Google Scholar]
- 22.Martin GS, Lewis CA. Fluid management in shock. Semin Respir Crit Care Med. 2004;25(6):683–93. doi: 10.1055/s-2004-860982. [DOI] [PubMed] [Google Scholar]
- 23.Ernest D, Belzberg AS, Dodek PM. Distribution of normal saline and 5% albumin infusions in cardiac surgical patients. Crit Care Med. 2001;29(12):2299–302. doi: 10.1097/00003246-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Ernest D, Belzberg AS, Dodek PM. Distribution of normal saline and 5% albumin infusions in septic patients. Crit Care Med. 1999;27(1):46–50. doi: 10.1097/00003246-199901000-00025. [DOI] [PubMed] [Google Scholar]
- 25.Sibbald WJ, et al. The short-term effects of increasing plasma colloid osmotic pressure in patients with noncardiac pulmonary edema. Surgery. 1983;93(5):620–33. [PubMed] [Google Scholar]
- 26.Mangialardi RJ, et al. Hypoproteinemia predicts acute respiratory distress syndrome development, weight gain, and death in patients with sepsis. Ibuprofen in Sepsis Study Group. Crit Care Med. 2000;28(9):3137–45. doi: 10.1097/00003246-200009000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Arif SK, et al. Hypoproteinemia as a marker of acute respiratory distress syndrome in critically ill patients with pulmonary edema. Intensive Care Med. 2002;28(3):310–7. doi: 10.1007/s00134-002-1220-y. [DOI] [PubMed] [Google Scholar]
- 28.Kramer GC, et al. Effects of hypoproteinemia and increased vascular pressure on lung fluid balance in sheep. J Appl Physiol. 1983;55(5):1514–22. doi: 10.1152/jappl.1983.55.5.1514. [DOI] [PubMed] [Google Scholar]
- 29.Guyton AC, Lindsey AW. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ Res. 1959;7(4):649–57. doi: 10.1161/01.res.7.4.649. [DOI] [PubMed] [Google Scholar]
- 30.Cummings JJ, et al. Hypoproteinemia slows lung liquid clearance in young lambs. J Appl Physiol. 1993;74(1):153–60. doi: 10.1152/jappl.1993.74.1.153. [DOI] [PubMed] [Google Scholar]
- 31.Demling RH, Kramer G, Harms B. Role of thermal injury-induced hypoproteinemia on fluid flux and protein permeability in burned and nonburned tissue. Surgery. 1984;95(2):136–44. [PubMed] [Google Scholar]
- 32.Wareing TH, et al. Increased plasma oncotic pressure inhibits pulmonary fluid transport when pulmonary pressures are elevated. J Surg Res. 1989;46(1):29–34. doi: 10.1016/0022-4804(89)90178-9. [DOI] [PubMed] [Google Scholar]
- 33.National Heart L et al., . Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 34.Lewis CA, Martin GS. Understanding and managing fluid balance in patients with acute lung injury. Curr Opin Crit Care. 2004;10(1):13–7. doi: 10.1097/00075198-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Martin GS, et al. Albumin and furosemide therapy in hypoproteinemic patients with acute lung injury. Crit Care Med. 2002;30(10):2175–82. doi: 10.1097/00003246-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 36.van der Heijden M, et al. Crystalloid or colloid fluid loading and pulmonary permeability, edema, and injury in septic and nonseptic critically ill patients with hypovolemia. Crit Care Med. 2009;37(4):1275–81. doi: 10.1097/CCM.0b013e31819cedfd. [DOI] [PubMed] [Google Scholar]
- 37.Finfer S, et al. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]