β3-Adrenoceptors promote glucose uptake in brown adipose tissue via both cAMP-mediated increases in GLUT1 transcription and mTORC2-stimulated translocation of newly synthesized GLUT1 to the plasma membrane.
Abstract
Brown adipose tissue is the primary site for thermogenesis and can consume, in addition to free fatty acids, a very high amount of glucose from the blood, which can both acutely and chronically affect glucose homeostasis. Here, we show that mechanistic target of rapamycin (mTOR) complex 2 has a novel role in β3-adrenoceptor–stimulated glucose uptake in brown adipose tissue. We show that β3-adrenoceptors stimulate glucose uptake in brown adipose tissue via a signaling pathway that is comprised of two different parts: one part dependent on cAMP-mediated increases in GLUT1 transcription and de novo synthesis of GLUT1 and another part dependent on mTOR complex 2–stimulated translocation of newly synthesized GLUT1 to the plasma membrane, leading to increased glucose uptake. Both parts are essential for β3-adrenoceptor–stimulated glucose uptake. Importantly, the effect of β3-adrenoceptor on mTOR complex 2 is independent of the classical insulin–phosphoinositide 3-kinase–Akt pathway, highlighting a novel mechanism of mTOR complex 2 activation.
Introduction
The recent interest in brown adipose tissue (BAT) research stems from the insight that this tissue, when activated, expends energy in the form of heat production (thermogenesis) that could potentially affect whole body energy homeostasis in humans, with recent evidence demonstrating the presence and function of BAT in adult humans (Nedergaard et al., 2007). Besides its role in thermogenesis (Cannon and Nedergaard, 2004), another important function is that it can consume, in addition to free fatty acids, a very high amount of glucose per gram of tissue from the blood (Shibata et al., 1989; Liu et al., 1994). Studies in rodents have shown that the amount of glucose delivered to BAT is enough to both acutely and in the long term affect glucose homeostasis (Stanford et al., 2013). Because of these properties, BAT may prove to be a potential therapeutic target for several metabolic disorders that are dependent on glucose homeostasis, including type 2 diabetes.
Glucose uptake in BAT is stimulated in two metabolic states: sympathetically stimulated during active thermogenesis or by insulin during active anabolic processes. Although insulin-stimulated glucose uptake in tissues, including BAT, is well-characterized by the phosphoinositide 3-kinase-phosphoinositide–dependent kinase-1-Akt (PI3K–PDK1–Akt) pathway as resulting in the rapid translocation of glucose transporter 4 (GLUT4) from intracellular vesicles to the cell membrane (Huang and Czech, 2007; Zaid et al., 2008), the sympathetic pathway is poorly understood. Stimulation of the sympathetic nervous system via adrenoceptors, predominately the β3-adrenoceptor, increases non-shivering thermogenesis in mammals (Nedergaard et al., 2007), but also increases glucose uptake in BAT (Inokuma et al., 2005). β3-Adrenoceptor–stimulated glucose uptake is independent of the action of insulin in vivo and in vitro: glucose uptake in BAT in vivo is associated with decreases in plasma insulin levels (Shimizu and Saito, 1991), whereas in vitro β-adrenoceptor–mediated glucose uptake occurs in the absence of insulin (Marette and Bukowiecki, 1989; Chernogubova et al., 2004; Chernogubova et al., 2005) and via actions at GLUT1 and not GLUT4 (Shimizu and Saito, 1991; Dallner et al., 2006). Although other signaling pathways such as AMP-activated protein kinase can increase glucose uptake via an insulin-independent mechanism, we previously demonstrated that this mechanism is not likely to be involved in β3-adrenoceptor–mediated glucose uptake in BAT (Hutchinson et al., 2005). Hence, an alternative signaling pathway must be involved. One such candidate is mechanistic target of rapamycin (mTOR; Laplante and Sabatini, 2012).
mTOR is essential in the control of many aspects of cell growth, metabolism, and energy homeostasis (Polak and Hall, 2009; Laplante and Sabatini, 2012; Lamming and Sabatini, 2013). mTOR is the catalytic part of two functionally distinct multiprotein complexes: the well-studied mTOR complex 1 (mTORC1) and the less-studied mTOR complex 2 (mTORC2). They have different downstream targets, different biological functions, and, importantly, different sensitivity to the drug rapamycin. mTORC1 is pharmacologically inhibited by short-term rapamycin treatment, whereas mTORC2 is resistant to short-term rapamycin treatment, although long-term treatment can prevent mTORC2 complex assembly (Phung et al., 2006; Sarbassov et al., 2006). Recent studies of mTOR show that both complexes have important regulatory roles in white adipose tissue (Lamming and Sabatini, 2013). Most of the efforts have, however, been focused on studying white adipose tissue, leaving the role and the importance of both complexes of mTOR in BAT function relatively unexplored. Recent data indicate a role of mTORC2 in glucose homeostasis, with adipose-specific ablation of rictor, a component of the mTORC2 complex, depressing insulin-stimulated glucose uptake in adipose tissue and impairing glucose tolerance in vivo (Kumar et al., 2010). Adipose-specific deletion of raptor, a component of the mTORC1 complex, however, results in mice that are resistant to diet-induced obesity and are insulin sensitive (Polak and Hall, 2009), which indicates vastly different roles for mTORC1 and mTORC2 in adipose tissues.
In this study, we demonstrate that mTOR is necessary for β3-adrenoceptor–stimulated glucose uptake in mouse brown adipocytes and in human multipotent adipose-derived stem (hMADS) cells. Stimulation of β3-adrenoceptors increases glucose uptake via a pathway divided into two parts. The first part is dependent on increased GLUT1 transcription and de novo synthesis of GLUT1 via elevations in cAMP levels that are not dependent on either mTOR complexes. The second part involves translocation of GLUT1 to the plasma membrane by an mTORC2-mediated pathway. Both parts are necessary for β3-adrenoceptor–stimulated glucose uptake in BAT.
Results
β3-Adrenoceptor–stimulated glucose uptake in primary brown adipocytes is independent of insulin signaling
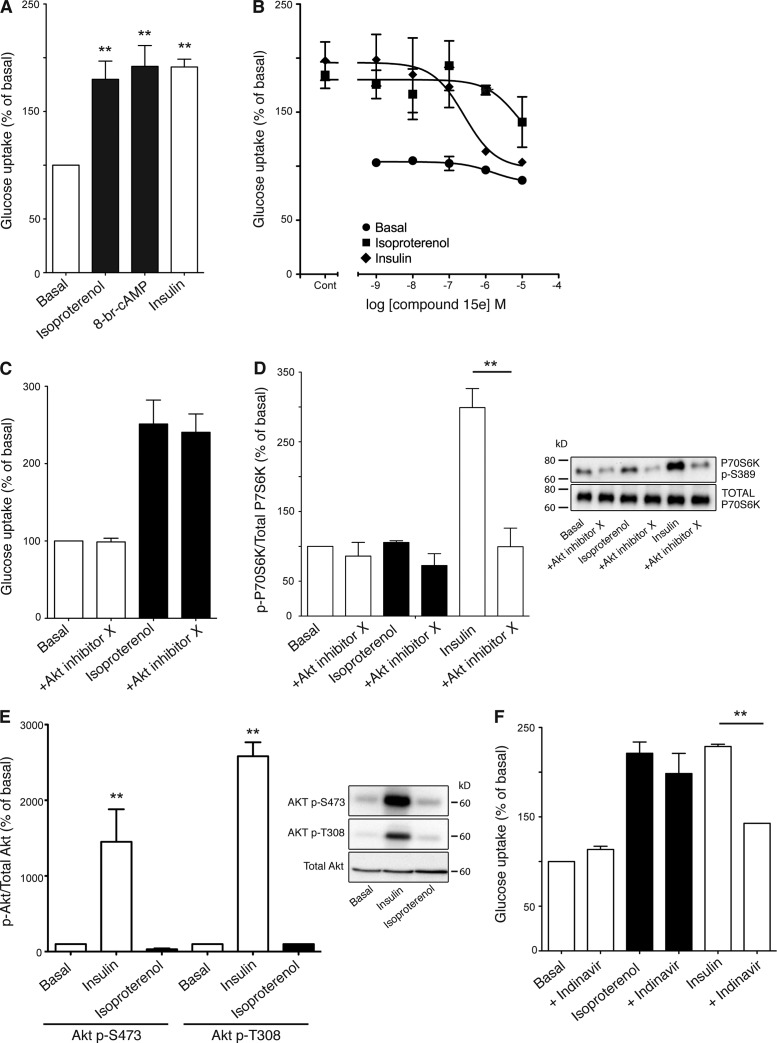
Isoproterenol (1 µM), a β-adrenoceptor agonist, and insulin (100 nM) increased glucose uptake approximately twofold in brown adipocytes (Fig. 1 A), which is consistent with our previous results (Chernogubova et al., 2004). The effect of isoproterenol was mimicked by the cell-permeable cAMP analogue 8-bromoadenosine-cAMP (8-br-cAMP; 1 mM; Fig. 1 A). Inhibition of PI3Kα by compound 15e (Fig. 1 B) inhibited insulin (IC50 256 nM) but not isoproterenol-mediated glucose uptake. Akt inhibitor X (100 µM) also failed to inhibit isoproterenol-stimulated glucose uptake (Fig. 1 C). However, it did block phosphorylation of p70S6K at Thr389 (a known substrate of Akt) upon insulin but not isoproterenol stimulation (Fig. 1 D). Similar results were obtained using Akt inhibitor VII (unpublished data). Isoproterenol also failed to phosphorylate Akt at either Thr308 or Ser473 (Fig. 1 E). Insulin-stimulated glucose uptake occurs primarily through GLUT4 translocation in both brown and white adipocytes (Dallner et al., 2006; Huang and Czech, 2007; Zaid et al., 2008). The GLUT4 inhibitor indinavir (Fig. 1 F) did not inhibit isoproterenol-stimulated glucose uptake while inhibiting insulin mediated glucose uptake, which highlights a contrast between the mechanisms of insulin and β3-adrenoceptor–mediated glucose uptake in brown adipocytes.
Figure 1.
β-Adrenoceptor glucose uptake in mouse primary brown adipocyte cultures is independent of insulin. (A) Isoproterenol (1 µM), 8-br-cAMP (1 mM), and insulin (100 nM) significantly increased glucose uptake in mature brown adipocytes (isoproterenol P = 0.0086, 8-br-cAMP P = 0.0015, insulin P = 0.0012). Each value represents the mean ± SEM (error bars; n = 5). (B) The PI3K-p110α inhibitor compound 15 (10 µM) did not inhibit isoproterenol (1 µM)-stimulated glucose. There was a significant difference between insulin and isoproterenol (P = 0.0015). The results are expressed as a percentage of basal glucose uptake. Each value represents the mean ± SEM (error bars; n = 3–7). (C and D) The Akt inhibitor X (100 µM) fully blocked the phosphorylation on p70S6k, a known substrate of Akt (D; P = 0.0064), but did not significantly inhibit isoproterenol (1 µM)-stimulated glucose uptake (n = 3). (E) Western blot demonstrating Akt phosphorylation at Thr308 and Ser473 in response to 2 h stimulation of 100 nM insulin (P = 0.0052 and P = 0.0055, respectively) but not 1 µM isoproterenol. The blot is representative of three experiments performed. (F) Isoproterenol (1 µM)- and insulin (100 nM)-stimulated glucose uptake in response to the GLUT4 inhibitor Indinavir (1 mM). Indinavir did inhibit insulin-mediated glucose uptake (P = 0.0008) but did not inhibit isoproterenol-mediated glucose uptake. The results are expressed as a percentage of basal glucose uptake. Each value represents the mean ± SEM (error bars; n = 3). **, P < 0.01.
mTOR is a key factor in isoproterenol-stimulated glucose uptake
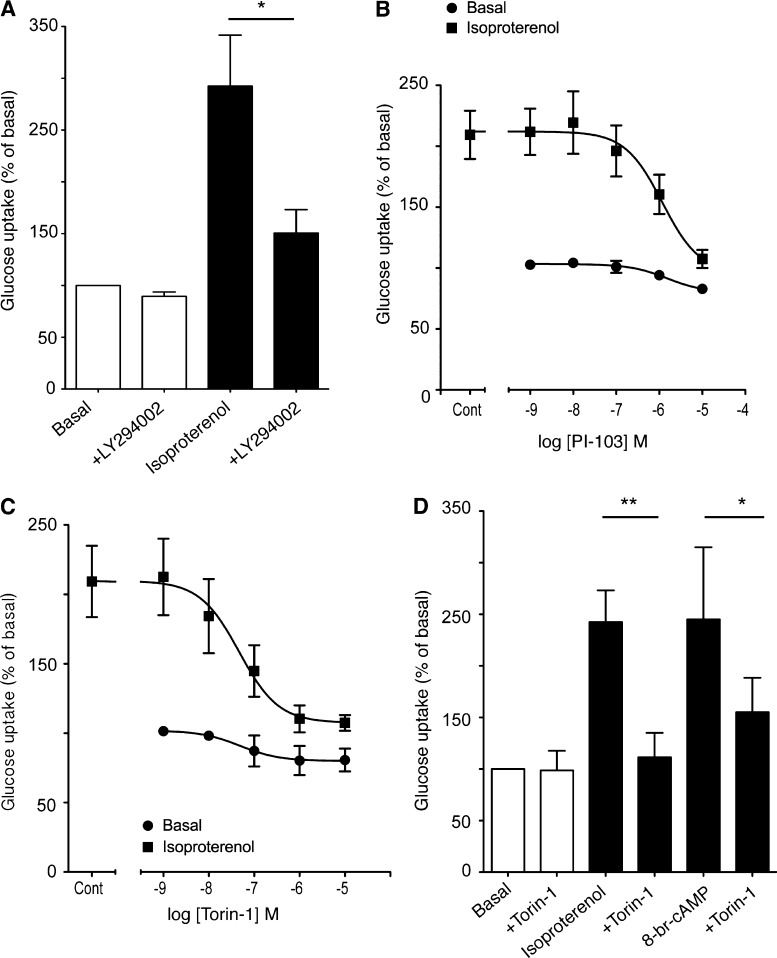
Pharmacological inhibition of PI3K by LY294002 inhibited isoproterenol-mediated glucose uptake in brown adipocytes (Fig. 2 A), which is consistent with our previous results (Chernogubova et al., 2004; Dallner et al., 2006), despite no concomitant phosphorylation of Akt (Fig. 1 C). This discrepancy may be caused by the nonspecific effects of these PI3K inhibitors (LY294002, wortmannin) on a wide range of other related PIKK family kinases (Knight and Shokat, 2007; Raynaud et al., 2007), including mTOR. The dual PI3K-mTOR inhibitor PI-103 (IC50 1.2 µM; Fig. 2 B) and the specific mTOR inhibitor Torin-1 (IC50 4.6 nM; Fig. 2 C) all significantly inhibited isoproterenol (1 µM)-stimulated glucose uptake. Furthermore, mimicking elevations in cAMP levels after isoproterenol treatment with 8-Br-cAMP (1 mM) increased glucose uptake to the same extent as isoproterenol, which was inhibited by Torin-1 (Fig. 2 D) and the commercially available mTOR inhibitor KU0063794 (unpublished data), which suggests that mTOR is downstream of cAMP.
Figure 2.
The effect of PI3K and PIKK inhibitors on β-adrenoceptor–mediated glucose uptake in mouse primary brown adipocytes. (A) Inhibition of isoproterenol (1 µM)-stimulated glucose uptake by the 10 µM PI3K and PIKK inhibitor LY294002 (P = 0.0306). Each value represents the mean ± SEM (error bars; n = 3). (B) Inhibition of isoproterenol (1 µM)-stimulated glucose uptake by the PIKK family inhibitor PI-103 (10 µM). Each value represents the mean ± SEM (error bars; n = 4). (C) Inhibition of isoproterenol (1 µM)-stimulated glucose uptake by the mTOR inhibitor Torin-1 (1 µM). Each value represents the mean ± SEM (error bars; n = 3). (D) The effect of Torin-1 (1 µM) on glucose uptake in response to 1 µM isoproterenol (P = 0.0042) and 1 mM of the cell-permeable cAMP analogue 8-br-cAMP (P = 0.0409). Each value represents the mean ± SEM (error bars; n = 4–6). *, P < 0.05; **, P < 0.01.
The effect of long-term stimulation of rapamycin on β3-adrenoceptor–mediated glucose uptake and mTOR phosphorylation suggests mTORC2 involvement
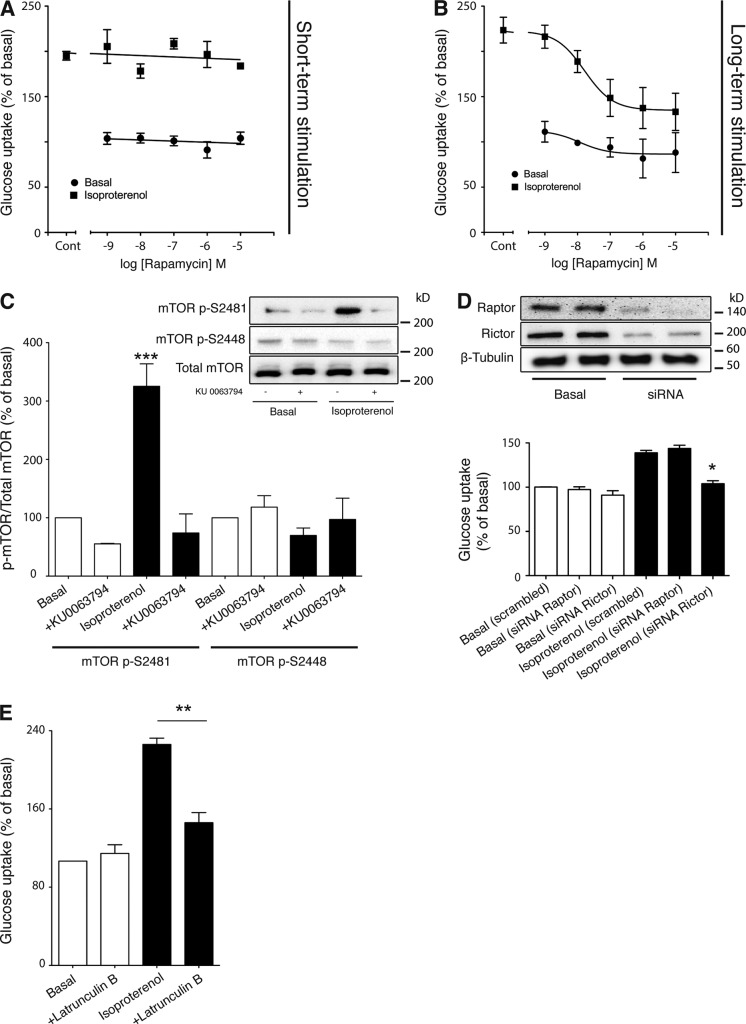
To further investigate the role of mTOR in isoproterenol-stimulated glucose uptake, we examined which mTOR complex was involved by using rapamycin. Short-term treatment (30 min) of rapamycin that inhibits mTORC1 (Brown et al., 1994; Sabatini et al., 1994) did not affect isoproterenol-mediated glucose uptake (Fig. 3 A), but did completely block insulin-stimulated phosphorylation of p70S6K, a downstream substrate of mTORC1 (not depicted). However, long-term rapamycin treatment that inhibits the assembly of the mTORC2 complex (Phung et al., 2006; Sarbassov et al., 2006) significantly inhibited isoproterenol-mediated glucose uptake (IC50 16 nM; Fig. 3 B).
Figure 3.
mTORC2 is a key factor in isoproterenol-mediated glucose uptake in mouse primary brown adipocytes. (A) The effect of rapamycin on glucose uptake in response to isoproterenol (1 µM). Brown adipocyte primary cultures were pretreated with rapamycin for 30 min followed by stimulation with isoproterenol (1 µM for 2 h). The results are expressed as a percentage of basal glucose uptake. Each point represents the mean ± SEM (error bars; n = 3–7). (B) The effect of rapamycin on glucose uptake in response to isoproterenol (1 µM). Brown adipocyte primary cultures were pretreated with rapamycin for 46 h followed by stimulation with isoproterenol (1 µM for 2 h). The results are expressed as a percentage of basal glucose uptake. Each point represents the mean ± SEM (error bars; n = 3–7). (C) mTOR phosphorylation at Ser2481 (P < 0.001) but not at Ser2448 in response to 2 h of stimulation of 1 µM isoprenaline in the presence or absence of the mTOR inhibitor KU 0063794 (1 µM). The immunoblot is representative of three experiments performed. (D) Knockdown of raptor and rictor with the K2 transfection system resulted in a large reduction of both proteins. However, only knockdown of rictor led to inhibition of isoproterenol-stimulated glucose uptake (P = 0.0105). Each value represents the mean ± SEM (error bars; n = 3). (E) Isoproterenol (1 µM)-stimulated glucose uptake in the presence and absence of 20 µM Latrunculin B (P = 0.0027). Each value represents the mean ± SEM (error bars; n = 4). *, P < 0.5; **, P < 0.01; ***, P < 0.001.
mTOR is phosphorylated at several sites including Ser2481 and Ser2448. These phosphorylation sites are located predominantly on different complexes: Ser2448 on complex 1 and Ser2481 on complex 2 (Copp et al., 2009). Isoproterenol phosphorylated mTOR at Ser2481 but not Ser2448 (Fig. 3 C). Phosphorylation at Ser2481 by isoproterenol was abolished in the presence of the commercially available mTOR inhibitor KU 0063794. S2448 phosphorylation, although predominately associated with mTORC1, is not completely specific for mTORC1, with some S2448-phosphorylated mTOR associated with rictor in HEK293 cells and some other cancer cell lines (Copp et al., 2009). Hence, we have knocked down either rictor or raptor to fully elucidate the mTOR complex involved. Knockdown of raptor or rictor, the regulatory subunit of mTORC1 and mTORC2, respectively, resulted in ∼85% knockdown of raptor protein levels or 75% of rictor protein levels (Fig. 3 D). Isoproterenol-stimulated glucose uptake was abolished by knockdown of rictor and not raptor, confirming the involvement of mTORC2.
mTORC2 is believed to be involved in the regulation of the cytoskeleton by reorganization of actin filaments (Sarbassov et al., 2004). Isoproterenol-mediated glucose uptake was significantly inhibited by Latrunculin B, which disrupts the actin cytoskeleton (Wakatsuki et al., 2001; Fig. 3 E), indicating the involvement of actin filaments and giving further evidence of mTORC2 involvement.
β-Adrenoceptor–mediated glucose uptake is dependent on mTOR in both human MADS cells and in vivo
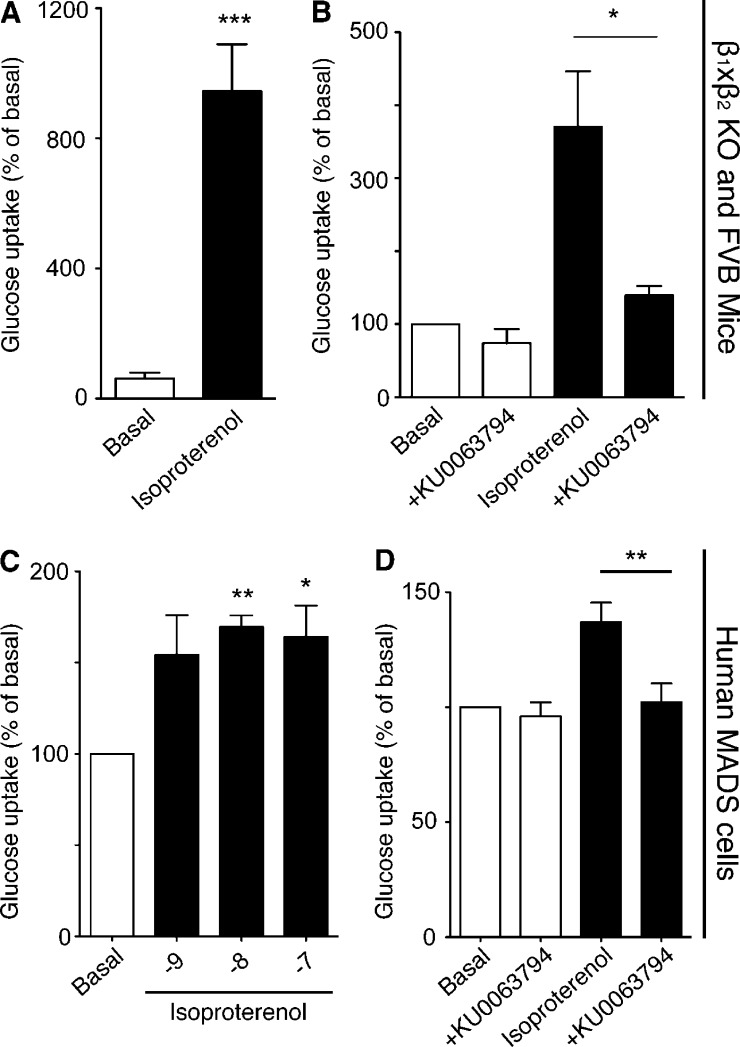
To further confirm our findings in primary brown adipocyte cultures, glucose uptake in BAT was measured in vivo in β1/β2 knockout (to exclude any effects on β1- or β2-adrenoceptors) and wild-type FVB mice that express all three β-adrenoceptor subtypes (Fig. 4, A and B). Isoproterenol increased glucose uptake in BAT in vivo in FVB and β1/β2 knockout mice, which indicates that isoproterenol acts via β3-adrenoceptors. This effect in FVB mice in vivo was abolished by treatment of mice with the mTOR inhibitor KU 0063794.
Figure 4.
Adrenoceptor-mediated glucose uptake in human MADS cells and in mice occurs through mTOR. (A) Isoproterenol stimulated glucose uptake in vivo in β1×β2 knockout mice. Mice were stimulated with 1 mg/kg i.p. isoproterenol (P = 0.0002). Each value represents the mean ± SEM (error bars; n = 5). (B) Isoproterenol-stimulated glucose uptake in vivo in FVB mice. Mice were stimulated with 1 mg/kg i.p. isoproterenol. Isoproterenol-stimulated glucose uptake was significantly reduced (P = 0.0175) by the mTOR inhibitor KU 0063794 (1 µM). Each value represents the mean ± SEM (error bars; n = 5). (C) Isoproterenol (10 and 100 nM) significantly increased (P = 0.0023 and P = 0.0357, respectively) glucose uptake in human MADS cells. Each value represents the mean ± SEM (error bars; n = 3). (D) 1 µM KU 0063794 significantly blocked (P = 0.0025) isoproterenol-stimulated glucose uptake in human MADS cells. Each value represents the mean ± SEM (error bars; n = 4). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Human multipotent adipose-derived stem hMADS cells are a mesenchymal stem cell population from human adipose tissue. Differentiated hMADS cells respond to both insulin and β-adrenoceptor agonists, have up-regulated uncoupling protein 1 expression after stimulation of a β3-adrenoceptor agonist, and have increased uncoupling protein 1–dependent respiratory capacity after activation of PPARγ (Elabd et al., 2009). Isoproterenol significantly increased glucose uptake in hMADS cells (Fig. 4 C), which was significantly inhibited by the mTOR inhibitor KU 0063794 (Fig. 4 D).
mTORC2 mediates translocation of GLUT1 in BAT
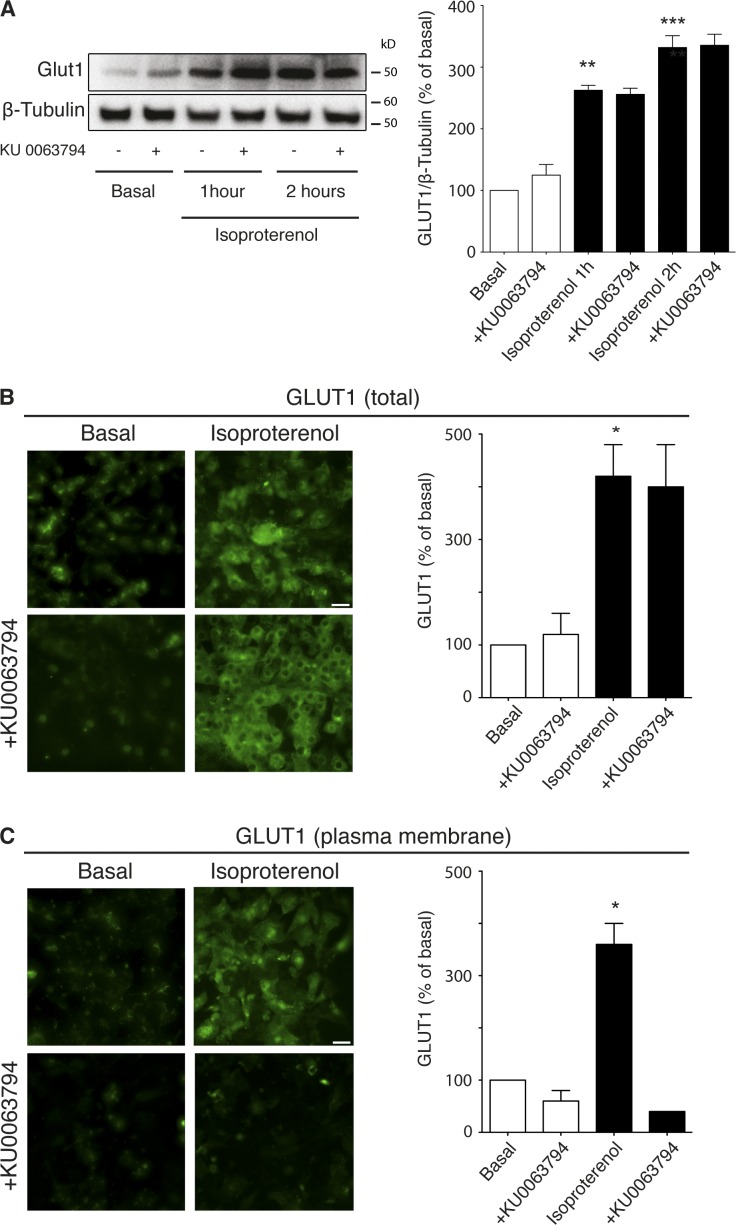
β3-Adrenoceptor–stimulated glucose uptake in brown adipocytes is dependent on de novo synthesis and translocation of GLUT1 but not GLUT4 (Dallner et al., 2006). Isoproterenol increased total GLUT1 protein content in a time-dependent manner, with total GLUT1 protein levels not affected by the mTOR inhibitor KU 0063794 when measuring total GLUT1 protein content by immunoblotting (Fig. 5 A) or using confocal microscopy in permeabilized cells (Fig. 5 B). However, visualization of only cell surface GLUT1 in nonpermeabilized cells showed that isoproterenol increased expression of cell surface GLUT1 in a KU 0063794–sensitive manner (Fig. 5 C). These results indicate that mTORC2 may have an important role in transporting the newly synthesized glucose transporters to the plasma membrane.
Figure 5.
KU 0063794 prevents the translocation of GLUT1 to the plasma membrane after stimulation of isoproterenol in mature brown adipocytes. (A) Western blot showing GLUT1 protein content in mature brown adipocytes after isoproterenol stimulation (1 µM, 0–2 h) in the presence or absence of 1 µM KU 0063794. Isoproterenol significantly increase the amount of GLUT1 in an mTOR-independent manner (P = 0.0015 and P = 0.0003, respectively; n = 3). (B) Permeabilized mature brown adipocytes treated for 2 h with 1 µM isoproterenol (P = 0.0334) showing total cellular GLUT1 in the presence or absence of 1 µM KU 0063794 (n = 3). (C) Nonpermeabilized mature brown adipocytes after 2 h of 1 µM isoproterenol treatment in the presence or absence of 1 µM KU 0063794 (P = 0.0153). The histogram shows significant inhibition of GLUT1 transport to the plasma membrane (n = 3). *, P < 0.05; **, P < 0.01; ***, P < 0.001. Bars, 100 µm.
Discussion
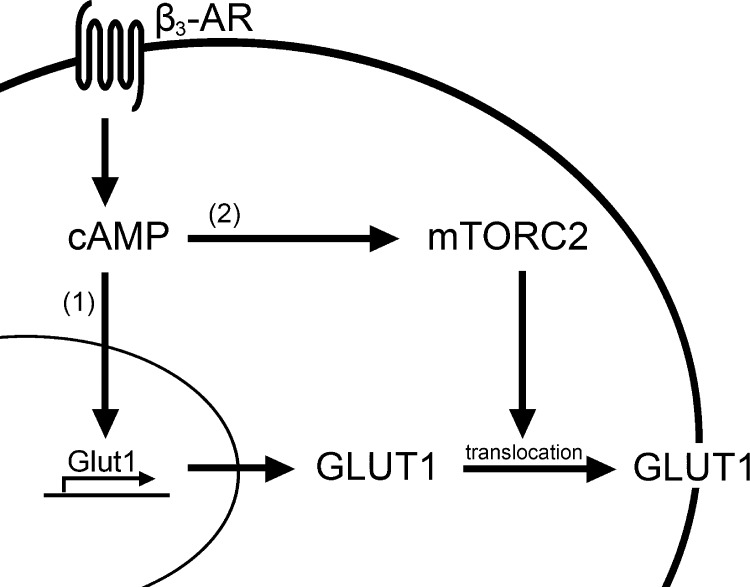
Stimulation of glucose uptake has been used as an important parameter for understanding BAT existence, localization, and function in animals (Cannon and Nedergaard, 2004; Nedergaard et al., 2007). Together with the potential importance that BAT has in regulating total energy homeostasis in the body (Cannon and Nedergaard, 2004), it is important to understand the mechanisms that regulate glucose uptake in this tissue. Curiously however, the signaling pathway and mechanism used to increase glucose uptake in brown adipocytes and BAT are not well characterized. In this article, we show that β3-adrenoceptor–stimulated glucose uptake in brown adipocytes occurs through a pathway divided into two parts that both involve cAMP elevation. The first part is rapid de novo synthesis of GLUT1, which is mTOR independent. The second part of translocation of the newly synthesized GLUT1 to the plasma membrane requires mTORC2 (Fig. 6).
Figure 6.
Schematic summary of the mechanism of β3-adrenceptor–stimulated glucose uptake in BAT. Activation of β3-adrenoceptors result in increased levels of intracellular cAMP that lead to increased de novo synthesis of GLUT1 (1) and mTORC2 phosphorylation (2). The newly produced GLUT1 is then translocated to the plasma membrane with help of actin filaments.
In this paper, we have used the β-adrenoceptor agonist isoproterenol for all the in vivo and in vitro studies. We have extensively shown (Chernogubova et al., 2004, 2005; Hutchinson and Bengtsson, 2006; Dallner et al., 2006) that the β3-adrenoceptor is the only β-adrenoceptor subtype that increases glucose uptake via increased de novo synthesis of GLUT1 in brown adipocytes from wild-type mice in vitro through the use of specific agonists and antagonists (Chernogubova et al., 2004, 2005; Dallner et al., 2006). The exception occurs in β3-adrenoceptor knockout mice, where there is compensation by both the α1- and β1-adrenoceptors that is not evident in cultures derived from wild-type mice (Chernogubova et al., 2005). Hence we strongly believe that the effects of isoproterenol in vitro are due solely to activation of β3-adrenoceptors. With respect to glucose uptake in vivo, isoproterenol is still effective in promoting glucose uptake into BAT in β1β2-adrenoceptor knockout mice (Fig. 4 A), highlighting the role of β3-adrenoceptors in vivo. CL316243, a highly specific β3-adrenoceptor agonist, also increases in vivo glucose uptake in BAT in FVB mice (unpublished data).
mTOR is a key central regulator of metabolism. It is involved in multiple physiological processes such as lipogenesis, lipolysis, and adipogenesis, and has recently been implicated in insulin signaling and sensitivity in white adipose tissue (Polak et al., 2008; Kumar et al., 2010), with a large focus on the role of mTOR in white adipose tissue. Little is known about mTOR function in brown adipocytes, with recent reviews highlighting the lack of knowledge in the field (Lamming and Sabatini, 2013). Our results clearly show that β3-adrenoceptor–mediated glucose uptake in brown adipocytes in vitro, BAT in vivo, and, importantly, in human MADS cells in vitro, is through mTOR.
mTOR exists in two distinct complexes: mTORC1 and mTORC2. In this paper, we first tried to characterize which of these complexes was required for β3-adrenoceptor–mediated glucose uptake in brown adipocytes. The specific mTORC1 inhibitor rapamycin has been instrumental in giving mechanistic insight into mTORC1 action. mTORC2 is less characterized due to the lack of specific inhibitors, but long-term treatment with rapamycin can inhibit mTORC2 formation (Phung et al., 2006; Sarbassov et al., 2006). We show that long-term, but not short-term, treatment with rapamycin in mouse brown adipocytes inhibits β3-adrenoceptor–mediated glucose uptake, which indicates the involvement of mTORC2. This was confirmed by knockdown of the regulatory subunit rictor that abolished β3-adrenoceptor–mediated glucose uptake, and that β3-adrenoceptor stimulation phosphorylated mTOR at Ser2481, a phosphorylation site predominately associated with mTORC2 activation. Collectively, this is the first time it has been shown that β3-adrenoceptors can specifically regulate mTORC2 in adipocytes.
mTORC2 responds to growth factors such as insulin, and it has been suggested that activation of mTORC2 is dependent on PI3K activation. However, the PI3K inhibitor Compound15e failed to inhibit β3-adrenoceptor–mediated glucose uptake in brown adipocytes. In conjunction with no inhibition of isoproterenol-stimulated glucose uptake in response to Akt inhibitor X, and no Akt phosphorylation at either Thr308 or Ser473 after β3-adrenoceptor activation, this indicates a lack of PI3K–Akt pathway in β3-adrenoceptor signaling in brown adipocytes (Feng et al., 2004; Surucu et al., 2008), and that β3-adrenoceptors stimulate mTORC2 differently from insulin. After insulin-mediated increases in PI3K activity, phosphatidylinositol (3,4,5)-trisphosphate (PIP3) recruits inactive Akt and phosphoinositide-dependent kinase-1 (PDK1) to the plasma membrane via their N-terminal PH domain, allowing Akt phosphorylation at Thr308 by PDK1. In parallel, PI3K phosphorylates mTORC2 at Ser2481. The subsequent conformational change in Akt allows mTORC2 to phosphorylate Akt at Ser473, thereby fully activating Akt, resulting in mTORC1 phosphorylation at Ser2448 and subsequent AS160 and GLUT4 translocation. In contrast, β3-adrenoceptors activate mTORC2 in a PI3K-Akt–independent manner (as indicated by no Akt phosphorylation, glucose uptake responses that are insensitive to Akt inhibition [using Akt inhibitor X], or PI3K inhibition [using compound 15e]). These results are consistent with our results in skeletal muscle that show activation of mTORC2 independently of PI3K and Akt after β2-adrenoceptor stimulation (Sato et al., 2014). The precise mechanism whereby β3-adrenoceptors activate mTORC2 needs further investigation, but cAMP is implied, with 8-Br-cAMP mimicking the effect of isoproterenol. It should be noted that the exact mechanism of activation of mTORC2 is still unknown even in the insulin field, most likely due to the absence of specific inhibitors for this complex. However, our novel finding that G protein–coupled receptors (GPCRs), via cAMP, can activate mTORC2 could have wider implications on how GPCRs stimulate mTOR. Most studies investigating links between GPCRs and mTOR have focused on mTORC1, where GPCR activation of PI3K subsequently leads to mTORC1 activation (Shimobayashi and Hall, 2014). Almost nothing is known about GPCR stimulation of mTORC2 without PI3K involvement, except in a study in Dictyostelium discoideum (Lee et al., 2005).
As very little is known specifically about mTORC2 and its effectors in the insulin signaling pathway, not much is known about its downstream targets that can regulate glucose uptake. However, mTORC2 is involved in actin cytoskeleton reorganization (Sarbassov et al., 2004), and it is believed that this reorganization is the mechanism responsible for insulin-mediated translocation of GLUT4 to the plasma membrane (Tsakiridis et al., 1994; Huang and Czech, 2007; Zaid et al., 2008). This is in agreement with studies showing that knockdown of mTORC2-specific components in cells resulted in alteration of the actin cytoskeleton (Sarbassov et al., 2004). Consistent with actin as a downstream target, latrunculin B, which disrupts actin polymerization, impairs β3-adrenoceptor–mediated glucose uptake. However, one major difference between insulin- and β3-adrenoceptor–mediated glucose uptake revolves around the required GLUT isoform. Whereas insulin translocates GLUT4 from intracellular vesicles to the cell surface in brown adipocytes (Pessin et al., 1999; Konrad et al., 2002), β3-adrenoceptor–mediated glucose uptake involves de novo synthesis of GLUT1. Surprisingly, mTOR does not appear to be required for the de novo synthesis of GLUT1, but is specifically required for its subsequent translocation to the cell surface. The difference between the GLUT isoforms required for insulin and β3-adrenoceptor stimulation of glucose uptake further implies the difference between the two signaling pathways. The significance of mTORC2 and GLUT1 translocation in brown adipocyte glucose uptake can be used in future experiments aimed specifically at inhibiting or stimulating glucose uptake in BAT. Furthermore, the difference in both signaling and GLUT isoforms between insulin- and β3-adrenoceptor–stimulated glucose uptake should be considered in the search for compounds that are aimed at activating BAT function. It could also aid in experiments aimed at elucidating the localization, the amount, and the potential therapeutic value of BAT in humans.
Sympathetic-mediated glucose uptake is not limited to BAT. We have previously shown that stimulation of adrenoceptors can increase glucose uptake in skeletal muscle and other cells (Nevzorova et al., 2002; Nevzorova et al., 2006; Hutchinson and Bengtsson, 2006; Catus et al., 2011), which indicates the broad significance of this finding and the importance of understanding this mechanism. Our recent findings in skeletal muscle show involvement of mTORC2 in β2-adrenoceptor activation of glucose uptake via GLUT4 translocation (Sato et al., 2014).
In summary, our results show that β3-adrenoceptor activation increases glucose uptake in brown adipocytes and BAT both in vitro and in vivo. This results in rapid de novo synthesis of GLUT1 by cAMP, and subsequent translocation of GLUT1 to the plasma membrane via mTORC2. This new knowledge and the physiological relevance of these results may contribute to new exciting possibilities in understanding BAT function and provide therapeutic value in the field of metabolic diseases.
Materials and methods
Chemicals
Isoproterenol, insulin, collagenase type II, 8-Br-cAMP, latrunculin B, and DMEM (4.5 g d-glucose/liter) were all obtained from Sigma-Aldrich. Compound 15e (3-[4-(4-morpholinyl)thieno[3,2-d]pyrimidin-2-yl]-phenol), PI-103 (3-(4-(4-morpholinyl), pyrido[3′,2’:4,5] furo [3,2-d]pyrimidin-2-yl)phenol), LY294002, and rapamycin were from Alexis Biochemical. KU 0063794 was obtained from Axon Medchem. Akt inhibitor C was obtained from EMD Millipore, 2-deoxy-d-[1-3H]-glucose ([3H]2DG; specific activity 7.5 Ci/mmol) from PerkinElmer. Torin1 was provided by D.M. Sabatini (Whitehead Institute, Cambridge, MA).
Brown fat precursor cell isolation
3–4-wk-old NMRI mice of either sex were purchased from Nova-SCB AB. NMRI mice are known to produce BAT with high quality, and have been used extensively for more than 25 years in characterizing brown adipocyte physiology (Cannon and Nedergaard, 2001). Animals were euthanized by CO2, and brown fat precursor cells were isolated from the intrascapular, axillary, and cervical brown adipocyte depots (Néchad et al., 1987; Rehnmark et al., 1990). The tissue was minced and transferred to a Hepes-buffered solution, pH 7.4, containing 0.2% (wt/vol) crude collagenase type II. Routinely, tissue from six mice was digested in 10 ml of the Hepes-buffered solution. The tissue was digested for 30 min at 37°C, with constant vortexing. The digest was filtered through a 250-µm filter and the solution incubated on ice for 15 min to allow the mature adipocytes and fat droplets to float. The infranatant was filtered through a 25-µm filter and centrifuged (10 min, 700 g). The pellet was then resuspended in DMEM (4.5 g d-glucose/liter) and recentrifuged. The pellet was finally resuspended in 0.5 ml cell culture medium per mouse dissected. All experiments were conducted with ethical permission (N388/12) from the North Stockholm Animal Ethics Committee.
Primary brown adipocyte cell culture
The cell culture medium consisted of DMEM (4.5 g d-glucose/liter) supplemented with 10% newborn calf serum, 2.4 nM insulin, 10 nM Hepes, 50 IU/ml penicillin, 50 µg/ml streptomycin, and 25 µg/ml sodium ascorbate. Aliquots of 0.1-ml cell suspension were cultured in 12-well culture dishes with 0.9 ml of cell culture medium. Cultures were incubated in a 37°C humidified atmosphere of 8% CO2 in air. On days 1, 3, and 5, the cell culture medium was renewed. Cells were used on day 7.
hMADS cell culture
Human multipotent adipose-derived stem hMADS cells that have previously been established as a model system for human brown fat (Elabd et al., 2009) were seeded in DMEM supplemented with 10% fetal calf serum, 2.5 ng/ml hFGF2, 60 µg/ml penicillin, and 50 µg/ml streptomycin. The medium was changed every other day and hFGF2 removed when the cells reached confluence and were triggered for differentiation on day 2 after confluence. Cells were then maintained in DMEM-Ham’s F-12 medium supplemented with 10 µg/ml transferrin, 0.85 µM insulin, 0.2 nM triiodothyronine, 1 µM dexamethasone, and 500 µM isobutylmethylxanthine. 3 d later, the medium was changed (dexamethasone and isobutylmethylxanthine were omitted) and 100 nM rosiglitazone was added. Medium was changed every other day until day 7, when they were used.
[3H]2DG uptake in primary brown adipocytes, hMADS cells, and in vivo
Brown adipocytes were grown and differentiated in 12-well plates, and serum and insulin starved the night before the experiment. On day 7, the cells were treated with inhibitors for 30 min before addition of insulin, isoproterenol, or 8-br-cAMP for 2 h, unless otherwise indicated. 10 min before [3H]2DG uptake measurement, the medium was discarded, and cells washed with prewarmed PBS (10 mM phosphate buffer, 2.7 mM KCl, and 137 mM NaCl, pH 7.4). Glucose-free DMEM (containing 0.5% BSA and 0.25 mM sodium ascorbate) was added and drugs re-added with trace amounts of [3H]2DG (50 nM; specific activity 7.5 Ci/mmol) for 10 min. Reactions were terminated by washing in ice-cold PBS, cells were lysed (400 µl of 0.2 M NaOH, 1 h at 60°C), and the incorporated radioactivity was determined by liquid scintillation counting.
For in vivo glucose uptake, groups of β1×β2 knockout (Devic et al., 2001) and FVB mice were fasted for 5 h before study and anesthetized with pentobarbital (60 mg/kg of body weight, i.p.). If stated, mice were then injected with KU 0063794 (10 mg/kg i.p.) or DMSO. Insulin (1 mg/kg i.p.), isoproterenol (1 mg/kg i.p.), or saline were injected after 10 min and [3H]2DG (130 µCi/kg body weight, i.p.) 20 min before the indicated end time. BAT was dissected 1 h after [3H]2DG injection, and tissues were digested with 0.5 M NaOH overnight. Glucose uptake was measured by liquid scintillation counting. All experiments were conducted with ethical permission (N388/12) from the North Stockholm Animal Ethics Committee.
Immunoblotting
Brown adipocytes were grown and differentiated in 12-well plates, and serum and insulin starved the night before the experiment. On day 7, the cells were challenged with inhibitors for 30 min before being stimulated with drugs as indicated. Lysates were prepared in prewarmed (65°C) sample buffer (62.5 mM Tris, pH 6.8, 2% SDS, 10% glycerol, 50 mM dithiothreitol, and 0.1% bromophenol blue) and boiled for 5 min. Samples were loaded on a 8 or 12% acrylamide gel and separated for 2 h at 100 V. Proteins were transferred to Hybond-P polyvinylidene difluoride membranes (pore size 0.45 µm; GE Healthcare). The primary antibodies used were: rabbit anti-AKT (1:1,000), rabbit anti–p-AKT Thr308 (1:1,000), rabbit anti–p-AKT Ser473 (1:1,000), rabbit anti-mTOR (1:1,000), rabbit anti–p-mTOR Ser2448 (1:1,000), rabbit anti–p-mTOR Ser2481 (1:1,000), rabbit anti-P70S6K (1:1,000), rabbit anti–p-P70S6K S389 (1:1,000), rabbit anti-rictor (1:1,000), rabbit anti-raptor (1:1,000), and rabbit anti–β-tubulin (diluted 1:1,000) were from Cell Signaling Technology. Rabbit anti-GLUT1 (diluted 1:500) was from Abcam. All primary antibodies were detected using a secondary antibody (horseradish peroxidase–linked anti–rabbit IgG; Cell Signaling Technology) diluted 1:2,000 and enhanced chemiluminescence (ECL; GE Healthcare). Images were quantified using ImageJ 1.46r.
Immunocytochemistry
Brown adipocytes were isolated as described in the “Primary brown adipocyte cell culture” section and seeded onto BD Falcon culture chamber slides (BD). Cells were serum starved the night before the experiment. On day 7, the cells were challenged with inhibitors for 30 min before being stimulated for 1–2 h with drugs as indicated. Cells were washed with warm PBS and fixed for 15 min (4% formaldehyde in PBS). Cells were washed with PBS and formaldehyde quenched with 50 mM glycine in PBS, and washed three times for 5 min each with PBS. Cells were blocked for 1 h at room temperature with 8% BSA in PBS, and washed three times for 5 min each with PBS. For permeabilizing the cells, the cells were treated with 10% Triton X-100 in PBS (dilution 1:40) before blocking. Primary antibody (2 µg/ml GLUT1 antibody [Abcam], 1.5% BSA in PBS) was added and slides were incubated overnight at 4°C. The next day the cells were washed three times for 5 min each with PBS. Slides were then incubated with secondary antibody (3 µg/ml Alexa Fluor 488–conjugated goat anti–rabbit IgG [Invitrogen], 3% BSA in PBS) and washed three times for 5 min each with PBS. Slides were mounted with mounting media (8% 1,4-diazabicyclooctane, 75% glycerol in PBS) and sealed. Validation of the specificity of the antibody was performed using immunoblotting, with a strong specific band at the molecular mass of 50 kD.
Images were acquired at room temperature on an inverted laser-scanning microscope (Axiovert 200M; Carl Zeiss). The objective lens used was EC Plan Neofluar 10× dry/0.3 NA (Carl Zeiss) and a Cascade 1K camera (Photometrics). All images were acquired and processed using Slidebook (Version 6) software. The mean fluorescents from a set of pictures for all the different stimulations (2–4 pictures/stimulation) from three different experiments were quantified and analyzed with ImageJ (1.48v). The basal level was set to 100%.
Transient transfection of siRNA
siRNA constructs directed against mouse raptor (sequence not disclosed; Santa Cruz Biotechnology, Inc.) and mouse rictor were used (5′-CAGAAAGCAATCGCAACTCACCACA-3′; Sigma-Aldrich). Primary brown adipocytes were used on day 3 and transfected with K2 Transfection System (Biontex Laboratories GmbH) according to the manufacturer’s protocol using 2.5 µg/ml of siRNA. 24 h after transfection, glucose uptake or Western blotting was performed as described in the “[3H]2DG uptake in primary brown adipocytes, hMADS cells, and in vivo” and “Immunoblotting” sections.
Statistics
All experiments were performed in duplicate, and the results were expressed as mean ± SEM. The responses to agonists were calculated as a percentage compared to control, which was set to 100%. The statistical significance of differences was analyzed by a Student’s unpaired two-tailed t test unless stated otherwise.
Acknowledgments
The authors thank IFSU and Anna-Stina Höglund for technical help. Torin1 was kindly provided by D.M. Sabatini.
Dr. Masaaki Sato is supported by the Wenner-Gren Foundations, an Australian Research Council Linkage International fellowship (LX0989791), and a National Health and Medical Research Council (NHMRC) CJ Martin Overseas Biomedical Fellowship (606763). Dr. Dana Hutchinson is supported by NHMRC Career Development Fellowship 545952. Prof. Tore Bengtsson is supported by the Vetenskapsrådet Medicin (VR-M) from the Swedish Research Council, Novonordiskfonden, Stiftelsen Svenska Diabetesförbundets Forskningsfond, the Magnus Bergvall foundation, and the Carl Tryggers foundation.
The authors declare no competing financial interests.
Author contributions: J.M. Olsen and M. Sato wrote the manuscript and researched data. O.S. Dallner, A.L. Sandström, D.F. Pisani, and J.-C. Chambard researched data. E.-Z. Amri, D.S. Hutchinson, and T. Bengtsson reviewed and edited the manuscript. T. Bengtsson is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Abbreviations used in this paper:
- [3H]2DG
- 2-deoxy-d-[1-3H]-glucose
- 8-Br-cAMP
- 8-bromoadenosine-cAMP
- BAT
- brown adipose tissue
- GLUT4
- glucose transporter 4
- hMADS
- human multipotent adipose-derived stem
- mTOR
- mechanistic target of rapamycin
- mTORC1
- mTOR complex 1
- mTORC2
- mTOR complex 2
References
- Brown E.J., Albers M.W., Shin T.B., Ichikawa K., Keith C.T., Lane W.S., and Schreiber S.L.. 1994. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 369:756–758 10.1038/369756a0 [DOI] [PubMed] [Google Scholar]
- Cannon B., and Nedergaard J.. 2001. Cultures of adipose precursor cells from brown adipose tissue and of clonal brown-adipocyte-like cell lines. Methods Mol. Biol. 155:213–224. [DOI] [PubMed] [Google Scholar]
- Cannon B., and Nedergaard J.. 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84:277–359 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- Catus S.L., Gibbs M.E., Sato M., Summers R.J., and Hutchinson D.S.. 2011. Role of β-adrenoceptors in glucose uptake in astrocytes using β-adrenoceptor knockout mice. Br. J. Pharmacol. 162:1700–1715 10.1111/j.1476-5381.2010.01153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernogubova E., Cannon B., and Bengtsson T.. 2004. Norepinephrine increases glucose transport in brown adipocytes via β3-adrenoceptors through a cAMP, PKA, and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology. 145:269–280 10.1210/en.2003-0857 [DOI] [PubMed] [Google Scholar]
- Chernogubova E., Hutchinson D.S., Nedergaard J., and Bengtsson T.. 2005. α1- and β1-adrenoceptor signaling fully compensates for β3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology. 146:2271–2284 10.1210/en.2004-1104 [DOI] [PubMed] [Google Scholar]
- Copp J., Manning G., and Hunter T.. 2009. TORC-specific phosphorylation of mammalian target of rapamycin (mTOR): phospho-Ser2481 is a marker for intact mTOR signaling complex 2. Cancer Res. 69:1821–1827 10.1158/0008-5472.CAN-08-3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallner O.S., Chernogubova E., Brolinson K.A., and Bengtsson T.. 2006. β3-adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology. 147:5730–5739 10.1210/en.2006-0242 [DOI] [PubMed] [Google Scholar]
- Devic E., Xiang Y., Gould D., and Kobilka B.. 2001. β-Adrenergic receptor subtype-specific signaling in cardiac myocytes from β(1) and β(2) adrenoceptor knockout mice. Mol. Pharmacol. 60:577–583. [PubMed] [Google Scholar]
- Elabd C., Chiellini C., Carmona M., Galitzky J., Cochet O., Petersen R., Pénicaud L., Kristiansen K., Bouloumié A., Casteilla L., et al. 2009. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells. 27:2753–2760 10.1002/stem.200 [DOI] [PubMed] [Google Scholar]
- Feng J., Park J., Cron P., Hess D., and Hemmings B.A.. 2004. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279:41189–41196 10.1074/jbc.M406731200 [DOI] [PubMed] [Google Scholar]
- Huang S., and Czech M.P.. 2007. The GLUT4 glucose transporter. Cell Metab. 5:237–252 10.1016/j.cmet.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Hutchinson D.S., and Bengtsson T.. 2006. AMP-activated protein kinase activation by adrenoceptors in L6 skeletal muscle cells: mediation by alpha1-adrenoceptors causing glucose uptake. Diabetes. 55:682–690 10.2337/diabetes.55.03.06.db05-0901 [DOI] [PubMed] [Google Scholar]
- Hutchinson D.S., Chernogubova E., Dallner O.S., Cannon B., and Bengtsson T.. 2005. β-Adrenoceptors, but not α-adrenoceptors, stimulate AMP-activated protein kinase in brown adipocytes independently of uncoupling protein-1. Diabetologia. 48:2386–2395 10.1007/s00125-005-1936-7 [DOI] [PubMed] [Google Scholar]
- Inokuma K., Ogura-Okamatsu Y., Toda C., Kimura K., Yamashita H., and Saito M.. 2005. Uncoupling protein 1 is necessary for norepinephrine-induced glucose utilization in brown adipose tissue. Diabetes. 54:1385–1391 10.2337/diabetes.54.5.1385 [DOI] [PubMed] [Google Scholar]
- Knight Z.A., and Shokat K.M.. 2007. Chemically targeting the PI3K family. Biochem. Soc. Trans. 35:245–249 10.1042/BST0350245 [DOI] [PubMed] [Google Scholar]
- Konrad D., Bilan P.J., Nawaz Z., Sweeney G., Niu W., Liu Z., Antonescu C.N., Rudich A., and Klip A.. 2002. Need for GLUT4 activation to reach maximum effect of insulin-mediated glucose uptake in brown adipocytes isolated from GLUT4myc-expressing mice. Diabetes. 51:2719–2726 10.2337/diabetes.51.9.2719 [DOI] [PubMed] [Google Scholar]
- Kumar A., Lawrence J.C. Jr, Jung D.Y., Ko H.J., Keller S.R., Kim J.K., Magnuson M.A., and Harris T.E.. 2010. Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes. 59:1397–1406 10.2337/db09-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamming D.W., and Sabatini D.M.. 2013. A central role for mTOR in lipid homeostasis. Cell Metab. 18:465–469 10.1016/j.cmet.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M., and Sabatini D.M.. 2012. mTOR signaling in growth control and disease. Cell. 149:274–293 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Comer F.I., Sasaki A., McLeod I.X., Duong Y., Okumura K., Yates J.R. III, Parent C.A., and Firtel R.A.. 2005. TOR complex 2 integrates cell movement during chemotaxis and signal relay in Dictyostelium. Mol. Biol. Cell. 16:4572–4583 10.1091/mbc.E05-04-0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Pérusse F., and Bukowiecki L.J.. 1994. Chronic norepinephrine infusion stimulates glucose uptake in white and brown adipose tissues. Am. J. Physiol. 266:R914–R920. [DOI] [PubMed] [Google Scholar]
- Marette A., and Bukowiecki L.J.. 1989. Stimulation of glucose transport by insulin and norepinephrine in isolated rat brown adipocytes. Am. J. Physiol. 257:C714–C721. [DOI] [PubMed] [Google Scholar]
- Néchad M., Nedergaard J., and Cannon B.. 1987. Noradrenergic stimulation of mitochondriogenesis in brown adipocytes differentiating in culture. Am. J. Physiol. 253:C889–C894. [DOI] [PubMed] [Google Scholar]
- Nedergaard J., Bengtsson T., and Cannon B.. 2007. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293:E444–E452 10.1152/ajpendo.00691.2006 [DOI] [PubMed] [Google Scholar]
- Nevzorova J., Bengtsson T., Evans B.A., and Summers R.J.. 2002. Characterization of the β-adrenoceptor subtype involved in mediation of glucose transport in L6 cells. Br. J. Pharmacol. 137:9–18 10.1038/sj.bjp.0704845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevzorova J., Evans B.A., Bengtsson T., and Summers R.J.. 2006. Multiple signalling pathways involved in β2-adrenoceptor-mediated glucose uptake in rat skeletal muscle cells. Br. J. Pharmacol. 147:446–454 10.1038/sj.bjp.0706626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessin J.E., Thurmond D.C., Elmendorf J.S., Coker K.J., and Okada S.. 1999. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J. Biol. Chem. 274:2593–2596 10.1074/jbc.274.5.2593 [DOI] [PubMed] [Google Scholar]
- Phung T.L., Ziv K., Dabydeen D., Eyiah-Mensah G., Riveros M., Perruzzi C., Sun J., Monahan-Earley R.A., Shiojima I., Nagy J.A., et al. 2006. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell. 10:159–170 10.1016/j.ccr.2006.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak P., and Hall M.N.. 2009. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 21:209–218 10.1016/j.ceb.2009.01.024 [DOI] [PubMed] [Google Scholar]
- Polak P., Cybulski N., Feige J.N., Auwerx J., Rüegg M.A., and Hall M.N.. 2008. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 8:399–410 10.1016/j.cmet.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Raynaud F.I., Eccles S., Clarke P.A., Hayes A., Nutley B., Alix S., Henley A., Di-Stefano F., Ahmad Z., Guillard S., et al. 2007. Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 67:5840–5850 10.1158/0008-5472.CAN-06-4615 [DOI] [PubMed] [Google Scholar]
- Rehnmark S., Néchad M., Herron D., Cannon B., and Nedergaard J.. 1990. α- and β-adrenergic induction of the expression of the uncoupling protein thermogenin in brown adipocytes differentiated in culture. J. Biol. Chem. 265:16464–16471. [PubMed] [Google Scholar]
- Sabatini D.M., Erdjument-Bromage H., Lui M., Tempst P., and Snyder S.H.. 1994. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 78:35–43 10.1016/0092-8674(94)90570-3 [DOI] [PubMed] [Google Scholar]
- Sarbassov D.D., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., and Sabatini D.M.. 2004. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14:1296–1302 10.1016/j.cub.2004.06.054 [DOI] [PubMed] [Google Scholar]
- Sarbassov D.D., Ali S.M., Sengupta S., Sheen J.H., Hsu P.P., Bagley A.F., Markhard A.L., and Sabatini D.M.. 2006. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 22:159–168 10.1016/j.molcel.2006.03.029 [DOI] [PubMed] [Google Scholar]
- Sato M., Dehvari N., Öberg A.I., Dallner O.S., Sandström A.L., Olsen J.M., Csikasz R.I., Summers R.J., Hutchinson D.S., and Bengtsson T.. 2014. Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes. 10.2337/db13-1860 [DOI] [PubMed] [Google Scholar]
- Shibata H., Pérusse F., Vallerand A., and Bukowiecki L.J.. 1989. Cold exposure reverses inhibitory effects of fasting on peripheral glucose uptake in rats. Am. J. Physiol. 257:R96–R101. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., and Saito M.. 1991. Activation of brown adipose tissue thermogenesis in recovery from anesthetic hypothermia in rats. Am. J. Physiol. 261:R301–R304. [DOI] [PubMed] [Google Scholar]
- Shimobayashi M., and Hall M.N.. 2014. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 15:155–162 10.1038/nrm3757 [DOI] [PubMed] [Google Scholar]
- Stanford K.I., Middelbeek R.J., Townsend K.L., An D., Nygaard E.B., Hitchcox K.M., Markan K.R., Nakano K., Hirshman M.F., Tseng Y.H., and Goodyear L.J.. 2013. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123:215–223 10.1172/JCI62308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surucu B., Bozulic L., Hynx D., Parcellier A., and Hemmings B.A.. 2008. In vivo analysis of protein kinase B (PKB)/Akt regulation in DNA-PKcs-null mice reveals a role for PKB/Akt in DNA damage response and tumorigenesis. J. Biol. Chem. 283:30025–30033 10.1074/jbc.M803053200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiridis T., Vranic M., and Klip A.. 1994. Disassembly of the actin network inhibits insulin-dependent stimulation of glucose transport and prevents recruitment of glucose transporters to the plasma membrane. J. Biol. Chem. 269:29934–29942. [PubMed] [Google Scholar]
- Wakatsuki T., Schwab B., Thompson N.C., and Elson E.L.. 2001. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J. Cell Sci. 114:1025–1036. [DOI] [PubMed] [Google Scholar]
- Zaid H., Antonescu C.N., Randhawa V.K., and Klip A.. 2008. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem. J. 413:201–215 10.1042/BJ20080723 [DOI] [PubMed] [Google Scholar]