Summary
Peroxynitrite is a reactive oxidant produced from nitric oxide (NO) and superoxide, which reacts with proteins, lipids and DNA and promotes cytotoxic and pro-inflammatory responses. Here we overview the role of peroxynitrite in various forms of circulatory shock. Immunohistochemical and biochemical evidence demonstrate the production of peroxynitrite in various experimental models of endotoxic and hemorrhagic shock, both in rodents and in large animals. In addition, biological markers of peroxynitrite have been identified in human tissues after circulatory shock. Peroxynitrite can initiate toxic oxidative reactions in vitro and in vivo. Initiation of lipid peroxidation, direct inhibition of mitochondrial respiratory chain enzymes, inactivation of glyceraldehyde-3-phosphate dehydrogenase, inhibition of membrane Na+/K+ ATP-ase activity, inactivation of membrane sodium channels, and other oxidative protein modifications contribute to the cytotoxic effect of peroxynitrite. In addition, peroxynitrite is a potent trigger of DNA strand breakage, with subsequent activation of the nuclear enzyme poly (ADP-ribose) polymerase (PARP), which promotes cellular energetic collapse and cellular necrosis. Additional actions of peroxynitrite that contribute to the pathogenesis of shock include inactivation of catecholamines and catecholamine receptors (leading to vascular failure), endothelial and epithelial injury (leading to endothelial and epithelial hyper-permeability and barrier dysfunction) as well as myocyte injury (contributing to loss of cardiac contractile function). Neutralization of peroxynitrite with potent peroxynitrite decomposition catalysts provides cytoprotective and beneficial effects in rodent and large animal models of circulatory shock.
Keywords: Nitric oxide, superoxide, endotoxin, inflammation, contraction, vascular dysfunction, poly (ADP-ribose) polymerase
Production and reactivity of peroxynitrite
Nitric oxide (•NO) and superoxide (O2·−) rapidly react to form the toxic reaction product, peroxynitrite anion (ONOO−) (1, 2). The oxidant reactivity of peroxynitrite is mediated by an intermediate with biological activity of hydroxyl radical, which is not hydroxyl radical per se, but, rather, peroxynitrous acid or its activated isomer. While •NO is a relatively stable and highly diffusible free radical, O2·− is much shorter lived and has restricted diffusion across biomembranes. Therefore, the sites of peroxynitrite formation are assumed to be spatially associated with the sources of O2·−, such as the plasma membrane NAD(P)H oxidases or the mitochondrial respiratory complexes. For additional information on the chemistry, decomposition and reactivity of peroxynitrite, peroxynitrous acid and its activated isomer, see: (2–6).
Peroxynitrite is highly reactive. One of the key reactions of ONOO− in biological systems is its fast reaction with carbon dioxide (in equilibrium with physiological levels of bicarbonate anion), which leads to the formation of carbonate (CO3•−) and nitrogen dioxide (•NO2) radicals (yield approximating 35 %), which are one-electron oxidants. Nitrogen dioxide can undergo diffusion-controlled radical-radical termination reactions with biomolecules resulting in nitrated species such as nitrotyrosine (which is commonly used as a marker or ‘footprint’ of peroxynitrite; see below). Alternatively, ONOOH can undergo homolytic fission to generate one-electron oxidants hydroxyl (•OH) and •NO2 radicals. The proton-catalyzed decomposition to form •OH and •NO2 radicals may become relevant in hydrophobic phases resulting in the initiation of lipid peroxidation processes (3, 4).
The activities of peroxynitrite include a rapid oxidation of sulfhydryl groups and thioethers, as well as nitration and hydroxylation of aromatic compounds, including tyrosine, tryptophan and guanine (7, 8). These reactions, when occurring during the reaction of peroxynitrite with enzymes, macromolecules and lipids, have been shown to influence numerous cellular functions (7–24). For instance, tyrosine nitration may lead to diminished function of the proteins, as has been shown or suggested in the case of superoxide dismutase (16) and neuronal tyrosine hydroxylase (15). Oxidation of critical sulfhydryl groups is responsible for the inhibition of mitochondrial and cytosolic aconitase (10, 13) and other critical enzymes in the mitochondrial respiratory chain (10, 13, 20, 21, 24) and disruption of the zinc-thiolate center at the active site of enzymes (11). There is also evidence that peroxynitrite can cause covalent modification of an active site thiol of glyceraldehyde-3-phosphate dehydrogenase (18). Peroxynitrite can also inhibit the activity of membrane Na+/K+ ATP-ase (9, 12, 14).
The reaction of peroxynitrite with lipids leads to peroxidation (malondialdehyde and conjugated diene formation) and formation of nitrito-, nitro-, nitrosoperoxo- and/or nitrated lipid oxidation adducts (25). Peroxynitrite also causes the oxidation of arachidonic acid, and the formation of F2-isoprostanes through the oxidation of low density lipoprotein (26). In addition, peroxynitrite has been shown to cause direct damage of pulmonary surfactant proteins (26–29).
Another important interaction of peroxynitrite occurs with nucleic acids, with the production of 8-hydroxydeoxyguanosine (30) or 8-nitroguanine (31). The mechanism of direct, peroxynitrite-induced the DNA strand breakage is probably related to abstraction of hydrogen atoms from the ribose of the DNA moiety, thereby opening the sugar ring (30, 32). However, in cells exposed to peroxynitrite, there is also an indirect mechanism of peroxynitrite-induced DNA damage which involves the secondary production of mitochondrially derived oxidants and free radicals (33, 34).
The reactivity and decomposition pathways of peroxynitrite are strongly influenced by the chemical environment. In the presence of plasma, proteins, glucose or glutathione, peroxynitrite can form intermediates, which act as NO donors (35, 36). In plasma, peroxynitrite oxidizes ascorbic acid, uric acid, tyrosine, and -SH groups of plasma proteins (37, 38). There is a delicate balance between peroxynitrite-mediated oxidant processes and endogenous antioxidant pathways, which limit the reactivity of peroxynitrite (39). This is illustrated by the example of the endogenous antioxidant glutathione: pharmacological depletion of glutathione renders cells and animals extremely sensitive to the cytotoxic effects of peroxynitrite (40, 41).
Pathophysiological actions of peroxynitrite in cultured cells
Pharmacological studies demonstrate that peroxynitrite is more cytotoxic than NO or superoxide in a variety of experimental systems (10, 13, 24, 42, 43) and can induce both necrosis and apoptosis (4, 6, 44). While NO itself only exerts a limited effect on aconitase activity, peroxynitrite is a potent inhibitor of this enzyme under the same experimental conditions (10, 13). Furthermore, peroxynitrite - and not NO - is a potent initiator of DNA single strand breakage (45–47).
Peroxynitrite can induce marked alterations in cellular energetics and DNA integrity (Table 1). For instance, in pulmonary type II cells, peroxynitrite inhibits membrane Na+/K+ ATP-ase activity and sodium uptake (14), and similar effects were seen in intestinal epithelial cells as well (9). Profound inhibition by peroxynitrite of mitochondrial respiration has been observed in a variety of cell types (24, 43, 45, 46). Peroxynitrite exposure or endogenous generation of peroxynitrite in immunostimulated cells can also lead to depletion of intracellular NAD+ and ATP levels in various cell types (46, 48–50).
Table 1.
Pathogenetic roles of peroxynitrite in circulatory shock
| Potential mechanisms | |
|---|---|
| Vascular dysfunction | Catecholamine oxidation; inhibition of catecholamine receptors and suppression of vascular smooth muscle function (via mitochondrial inhibition and via PARP activation) leading to suppression of contractile function. Direct damage to vascular endothelial cells via nitrosative damage and via PARP activation. Inhibition of endothelial function via activation of neutrophils. Inhibition of vascular prostacyclin synthetase. These alterations may culminate in tissue edema and in an inadequate perfusion of tissues. |
| Myocardial dysfunction | Inhibition of myocyte cellular respiration, nitration and inhibition of cardiac myofibrillar creatine kinase, inhibition of alpha-actinin and of myofibrillar proteins, activation of matrix metalloproteinases and activation of PARP, leading to suppression of myocardial contractile function. |
| Gut epithelial failure | Epithelial cell injury via mitochondrial dysfunction, DNA damage, PARP activation, cellular energetic failure, secondary intracellular oxidant generation, resulting in increased epithelial paracellular permeability, bacterial translocation and secondary positive feedback cycles of injury. |
| Renal failure | Epithelial cell injury via mitochondrial dysfunction, DNA damage, PARP activation, cellular energetic failure, secondary intracellular oxidant generation. |
| Hepatic failure | Hepatocyte injury via mitochondrial dysfunction, DNA damage, PARP activation, cellular energetic failure, secondary intracellular oxidant generation, resulting in hepatocyte death. |
| Pulmonary dysfunction | Epithelial cell injury; damage to pulmonary surfactants, leading to impaired pulmonary oxygen exchange. |
| Systemic inflammation | Upregulation of signal transduction mechanisms; enhanced production of pro-inflammatory cytokines and chemokines, upregulation of neutrophil adhesion molecules, resulting in further exacerbation of the inflammatory response and organ failure. |
Endogenous or exogenous peroxynitrite, is a potent trigger of DNA single strand breakage, which, in turn, activates the nuclear enzyme poly(ADP-ribose) polymerase (46). As overviewed elsewhere (6, 51–53), activation of PARP can rapidly deplete NAD+, slowing the rate of glycolysis, electron transport, and ATP formation, resulting in cell dysfunction and cell death via the necrotic route. In addition to these effects on mitochondrial function, PARP can also poly(ADP-ribosyl)ate GAPDH (54, 55), and this effect can lead to an inhibition of glycolysis, as demonstrated in ischemic kidneys (55).
Peroxynitrite is a cytotoxic molecule (Fig. 1). Exposure to high concentrations of peroxynitrite leads to rapid cell death, associated with rapid energetic derangements. On the other hand, lower concentrations of peroxynitrite, after several hours, can lead to apoptotic cell death (56–62). The cellular dysfunction can manifest itself in suppressed cellular functions (e.g. reduction in mitochondrial respiration), but can also lead to increases in paracellular permeability, as demonstrated in intestinal epithelial cells (63, 64): this effect may have significant implications for the pathogenesis of intestinal barrier dysfunction associated with circulatory shock (see below).
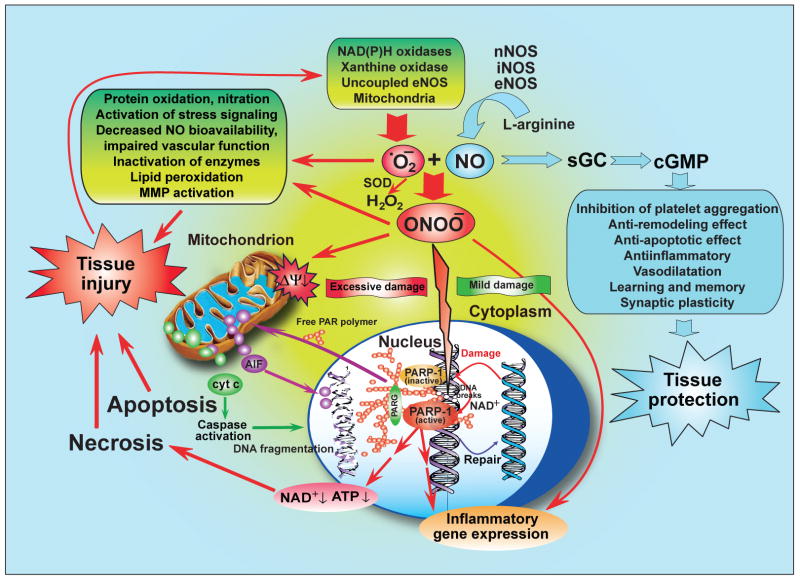
Figure 1. The nitric oxide – peroxynitrite - PARP pathway in circulatory shock.
Nitric oxide (NO) by activating soluble guanylate cyclase (sGC)-cyclic guanosine-3′,5′-monophosphate (cGMP) signal transduction pathway mediates various physiological/beneficial effects including vasodilation, inhibition of platelet aggregation, anti-inflammatory, anti-remodeling and anti-apoptotic effects. In circulatory shock nitric oxide and superoxide (·O2−) react to form peroxynitrite (ONOO−), which induces cell damage via lipid peroxidation, inactivation of enzymes and other proteins by oxidation and nitration and activation of stress signaling, matrix metalloproteinases (MMPs). Mitochondrial enzymes are particularly vulnerable to attacks by peroxynitrite, leading to reduced ATP formation and induction of mitochondrial permeability transition by opening of the permeability transition pore (PTP), which dissipates the mitochondrial membrane potential (ΔΨ). These events result in cessation of electron transport and ATP formation, mitochondrial swelling and permeabilization of the outer mitochondrial membrane, allowing the efflux of several pro-apoptotic molecules, including cytochrome c and apoptosis-inducing factor (AIF). In turn, cytochrome c and AIF activate a series of downstream effectors, which mediate caspase dependent and independent apoptotic death pathways. In addition to its damaging effects on mitochondria, peroxynitrite, in concert with other oxidants, causes oxidative injury to DNA, resulting in DNA strand breakage which in turn activate the nuclear enzyme poly(ADP-ribose) polymerase (PARP-1). Activated PARP-1 consumes NAD+ to build-up poly(ADP-ribose) polymers (PAR) which are then metabolized by the activity of poly(ADP-ribose) glycohydrolase (PARG). Peroxynitrite, at least in part via overactivated PARP-1, may also facilitate the expression of a variety of inflammatory genes leading to increased inflammation and associated tissue injury. (Reprinted from Am. J. Pathol 2008, 173:2–13 (5), with permission from the American Society for Investigative Pathology).
Peroxynitrite formation has been implicated as an important participant in positive feedback cycles of injury in various diseases. For instance, peroxynitrite can promote the oxidation of cofactors either by direct or free radical dependent mechanisms. Peroxynitrite-mediated oxidation of tetrahydrobiopterin (BH4) to 5,6-dihydrobiopterin (and subsequently to 7,8-dihydropterin) leads to the dysfunction (partial uncoupling) of NO synthase, since BH4 is an essential co-factor of NO synthase. It has been proposed that low levels of BH4 can, in turn, promote a cycle of its own destruction mediated by further peroxynitrite generation by the uncoupled NO synthase. This mechanism may contribute to vascular endothelial dysfunction induced by oxidative stress in various diseases (reviewed in (65). Reaction of NADH with peroxynitrite can result in the formation of NAD+ and superoxide and, subsequently, hydrogen peroxide (66, 67). This reaction can induce both an imbalance in cellular pyrimidine nucleotide levels, as well as a positive feedback cycle of intracellular oxidant generation. Inactivation of mitochondrial electron transport enzymes increases the amounts of superoxide and hydrogen peroxide generated by the mitochondria (20), which may further contribute to cellular injury, in an additive or synergistic fashion (68). Moreover, the inactivation of manganese superoxide dismutase (MnSOD) by peroxynitrite due to nitration of critical tyrosine- 34 may also amplify mitochondrial injury (69). Nitration of cytochrome c results in a marked increase in its peroxidase activity, which may exacerbate oxidative damage to mitochondrial proteins and membranes after peroxynitrite exposure (70). The peroxynitrite-induced DNA damage and PARP activation cycle represents another amplification mechanism, which contributes to peroxynitrite cytotoxicity (6, 51–53). Peroxynitrite can also trigger the release of mitochondrial pro-apoptotic factors and trigger cytochrome c-dependent apoptosis in the cytosol through peroxynitrite-dependent oxidation of permeability transition pore components and also possibly cardiolipin oxidation (71).
Peroxynitrite can play a role in promoting pro-inflammatory cellular responses. Potential biological targets of peroxynitrite include membrane as well as cytosolic and nuclear receptors. Putative targets for peroxynitrite include the EGF receptor, β1- and β2-adrenoceptor, platelet-endothelial cell adhesion molecule-1, IRS-1 and the peroxisome proliferator-activated receptor gamma (PPARγ) (overviewed in (72). Not only receptors, but also receptors ligands are targeted for modification. For instance, the reaction of peroxynitrite with fibroblast growth factor-1 induces extensive cysteine oxidation, tyrosine nitration, and irreversible inactivation of protein activity (73). Finally, peroxynitrite can react with catecholamines and lead to their inactivation, which may contribute to the development of vascular failure in shock (see below).
Peroxynitrite may participate in reactions that upregulate the inflammatory responses at multiple levels. For instance, peroxynitrite has been shown to play a role in the promotion of the expression of ICAM-1 and P-selectin in human endothelial cells (74). In human neutrophils, peroxynitrite triggers the down-regulation of L-selectin expression, and up-regulation of CD11b/CD18 expression (75). These effects are likely to be mediated, at least in part, by the ability of peroxynitrite to trigger and enhance NF-κB mediated pro-inflammatory signal transduction pathways by modifying proteins associated with the activation of this transcription factor. Peroxynitrite is also able to affect other signal transduction pathways, including protein kinase C (76), MAP kinase (77), and src tyrosine kinases (78, 79). Peroxynitrite can also contribute to the enhanced production of pro-inflammatory mediators by reduction of histone deacetylase HDAC2 activity through HDAC2 nitration (80). Elevated local levels of peroxynitrite during various forms of circulatory shock may upregulate localized inflammatory stress responses and possibly promote cellular and tissue injury (see below). Up-regulation of adhesion receptors by peroxynitrite may also result in an increased expression of endothelial adhesion molecules and such cells may represent a preferential site for adhesion and migration of neutrophils when simultaneously high concentrations of NO and neutrophil-derived superoxide are present.
Production of peroxynitrite in circulatory shock in animals and humans
The formation of peroxynitrite can be detected by its reactions (or ‘footprints’). The first evidence for peroxynitrite formation, by (a) increased nitrotyrosine immunoreactivity and (b) increased oxidation of the fluorescent probe dihydrorhodamine 123 to rhodamine 123 was obtained in rat models of endotoxin shock and hemorrhagic shock (7, 81). Subsequent studies have confirmed the formation of nitrotyrosine in various experimental models of shock (82–88). It must be noted, that peroxynitrite is not the only species that can yield nitrated tyrosine: myeloperoxidase-dependent nitrative reactions can also result in the formation of the same species (89, 90). Indeed, there is now experimental evidence from myeloperoxidase-deficient experimental models that supports the existence of peroxynitrite-independent (and myeloperoxidase-dependent or myeloperoxidase-independent) mechanisms of tyrosine nitration in animal models of circulatory shock (91, 92). Recently, by using the novel, potent porphyrinic antioxidants (‘peroxynitrite decomposition catalysts’), additional evidence has been obtained for the formation and pathophysiological significance of this species in circulatory shock (see below).
It is important to note that the markers of peroxynitrite generation have not only been documented in experimental models of shock, but also in human specimens obtained from patients suffering from circulatory shock. For instance, tyrosine nitration has been detected in the blood of septic patients (93, 94), in human samples after acute pulmonary injury (95), in chronic renal failure with patients with septic shock (96) and in myocardial and skeletal muscle samples after sepsis (97–101). There is a significant correlation between the degree of nitrotyrosine formation and the severity of the disease in human sepsis: in a preliminary study in a small set of patients with sepsis, Ohya and colleagues reported that plasma nitrotyrosine concentrations of the non-survivors and survivors were 0.7 nM versus 0.2 nM, respectively (93). In addition, Strand and colleagues have reported elevated levels of circulating nitrotyrosine in primary episodes of patients suffering from septic shock (94).
Role of peroxynitrite in the development of vascular changes in circulatory shock
One of the most important cardiovascular consequences of circulatory shock is the reduced responsiveness of arteries and veins to exogenous or endogenous vasoconstrictor agents (vascular hyporeactivity). This is usually coupled with a loss of endothelial function (reduced endothelium-dependent relaxations), as well as an increase of vascular permeability, leading to capillary extravasation and tissue edema. Many of the circulatory-shock-associated vascular alterations have been attributed to the formation of oxygen-derived oxidants and free radicals and the expression of a distinct inducible isoform of NOS (iNOS) in the vascular smooth muscle cells (49,102–105). As peroxynitrite is capable of mimicking many of the vascular alterations associated with shock (endothelial dysfunction, vascular hyporeactivity), this species may play a significant pathogenetic role in the vascular alterations associated with circulatory shock.
In 1994, Moncada and colleagues have reported the ability of peroxynitrite to impair the ability of endothelium-dependent relaxant agents to produce vascular relaxations (8). The degree of the endothelial dysfunction induced by peroxynitrite is dependent on the antioxidant milieu, as exemplified by glutathione: depletion of endogenous glutathione exacerbates (40) whereas supplementation of glutathione protects (106, 107) against peroxynitrite-induced endothelial dysfunction. The notion that endogenous peroxynitrite participates in the impairment of endothelium-dependent relaxant functions is supported by indirect evidence, i.e. data demonstrating that neutralization of superoxide protects against the development of endothelial dysfunction in a rodent model of shock (81), as well as by data with more selective neutralizers of peroxynitrite in rodent models of shock (peroxynitrite decomposition catalysts; see also below) (108).
The impairment of endothelial function by peroxynitrite may contribute to the pathogenesis of organ failure in circulatory shock in many different ways: (a) it may exacerbate local vasospasm, may increase local neutrophil adhesion and migration into inflamed tissues; (b) it may exacerbate platelet activation and aggregation and (c) it may lead to hypo-perfusion of certain parts of various organs. Endothelial dysfunction induced by peroxynitrite may also be associated with increased endothelial permeability, and may lead to extravasation and local tissue edema. With respect to endothelial barrier dysfunction and peroxynitrite, a variety of cellular mechanisms have been described that may contribute to the deleterious effects of peroxynitrite, including PARP activation (109), disorganization of junctional proteins and dephosphorylation of phosphorylated focal adhesion kinase (FAK) at tyrosine 397 (109, 110) and an increase in protein phosphatase type 2A (111).
The mechanisms by which peroxynitrite may contribute to the impairment of vascular contractile function in circulatory shock are also multiple. Some of these mechanisms may be related to a direct impairment of vascular smooth muscle energy generation, via inhibition of mitochondrial function and/or activation of PARP (5, 81, 103) Other mechanisms may be related to nitration of F-actin in vascular smooth muscle leading to depolymerization and the subsequent loss of myogenic tone (112), and direct activation of potassium channels on the vascular smooth muscle (113, 114). An additional mechanism of peroxynitrite-mediated impairment of vascular function may involve inactivation of the sarcoplasmic reticulum Ca2+ pump function (115, 116). Other mechanisms that may contribute to the inhibition of contractile responses by peroxynitrite may be related to direct oxidative inactivation of vasoconstrictor catecholamines norepinephrine and dopamine, as well as inactivation of receptors for the vasoconstrictor hormones noradrenaline: such as peroxynitrite-mediated inhibition of α-adrenoceptor function and peroxynitrite-mediated inhibition of vasopressin receptors (117–121). Importantly, inhibition of superoxide production in rodent models of endotoxin shock increased the plasma levels of noradrenaline, and decreased plasma levels of the inactive noradrenaline metabolite adrenochrome (122), indicating that a reactive oxidant-mediated (possibly peroxynitrite-mediated) mechanism is operative in vivo and consumes the endogenous catecholamines during circulatory shock.
Part of the vascular dysfunction elicited by peroxynitrite may be related to modulation of local mechanisms of vascular mediator production and coagulation. The selective nitration and inactivation of prostacyclin synthase by peroxynitrite may result in the accumulation of the intermediate PGH2, which is capable to activate the thromboxane A2 receptor on the surface of smooth muscle cells to promote vasoconstriction (123, 124). The nitration of prostacyclin-synthase thus functions as endogenous posttranslational switch that shuts off the prostacyclin-mediated vasodilatory, anti-aggregatory, and anti-adhesive conditions and may promote a pro-aggregatory and vasoconstrictive type vascular response in circulatory shock. Degradation of extracellular matrix proteins by peroxynitrite (125) may also contribute to pathophysiological vascular alterations, even though the potential role of this process in models of circulatory shock has not yet been explored.
Potential role of peroxynitrite in mediating myocardial hypocontractility in circulatory shock
Suppression of myocardial contractility is a common feature in patients with circulatory shock. Peroxynitrite is recognized as an endogenous myocardial depressant factor, with a potential role in the pathogenesis of myocardial hypocontractility in shock. The direct cytotoxic effects of peroxynitrite on cardiac myocytes have been demonstrated in multiple studies (126, 127). Infusion of peroxynitrite causes a reduction in myocardial contractility in isolated perfused hearts (128–131) and aggravates myocardial ischemic and reperfusion injury (132). The mechanism of peroxynitrite-mediated myocyte injury involves multiple pathways including nitration and inhibition of cardiac myofibrillar creatine kinase (133), alpha-actinin (134) and of myofibrillar proteins (135), activation of matrix metalloproteinases (127, 136, 137) and activation of PARP in the cardiac myocytes (138–140). Simultaneous generation of NO and superoxide, yielding peroxynitrite, has been demonstrated in hearts exhibiting myocardial dysfunction after endotoxemia (141). Neutralization of peroxynitrite mercaptoethylguanidine and 5,10,15,20-tetrakis(4-sulfonatophenyl)-porphyrinato iron (III) (FeTPPS), restored myocardial contractility in various models of shock and endotoxemia (142). There are no published data implicating the pathogenetic role of peroxynitrite in the myocardial dysfunction associated with human circulatory shock, but indirect evidence supports a potential relationship: tyrosine nitration has been demonstrated in cardiac specimens from patients with sepsis (97–101). PARP activation has also been demonstrated in patients who have died from septic shock: the extent of PARP activation shows a significant positive correlation with the release of cardiac enzymes in sepsis as well as with the extent of myocardial contractile dysfunction (143). Finally, studies conducted in human myocardial preparations exposed to endotoxin in vitro have indirectly implicated the potential role of peroxynitrite in the development of myocardial dysfunction during human sepsis (144). Taken together, the above data strongly support the view that peroxynitrite acts as a myocardial depressant factor both in animals and humans suffering from circulatory shock.
Potential role of peroxynitrite in the development of hepatic dysfunction in circulatory shock
Hepatic dysfunction is another common pathophysiological event in patients with various forms of circulatory shock. Similar to other organs, the liver is both a source and a target of peroxynitrite. Multiple cell types of the liver, including hepatocytes, Kupffer cells, stellate cells, endothelial cells as well as infiltrating leukocytes have the capacity to generate nitric oxide, superoxide and peroxynitrite (145, 146). The respiratory burst oxidase of neutrophils, eosinophils, monocytes, and macrophages is an important source of superoxide and other reactive oxygen species. The major source of reactive nitrogen-derived radicals in circulatory shock is iNOS, an enzyme expressed in leukocytes, hepatocytes and the vascular smooth muscle cells. It has been demonstrated in several models of liver damage that NO and peroxynitrite contribute to the functional and morphological alterations. D’Ambrosio and colleagues demonstrated that S-nitroso-N-acetylpenicillamine-amine (SNAP), which generates NO, and 3-morpholinosydnonimine (SIN-1), which produces equal molar concentrations of superoxide and NO (resulting in peroxynitrite production), exhibit different levels of cytotoxicity in cultured human hepatocytes, with SIN-1 being markedly more cytotoxic than SNAP. Nitrotyrosine, a marker of peroxynitrite formation, was detected in hepatocytes treated with SIN-1 or SNAP. From these data it appears that hepatocytes generate significant amounts of intracellular superoxide, which reacts with the exogenous NO derived from SNAP to produce intracellular peroxynitrite, resulting in cytotoxicity. SIN-1 (and to a lesser degree SNAP) induced dose- DNA damage, as well as cell-cycle arrest in the S-phase, growth inhibition, and hepatocyte apoptosis. These data support the view that the functional and morphological changes observed in liver following chronic exposure to reactive nitrogen species are, in part, the result of mitochondrial and nuclear DNA damage (147). In another in vitro study Watanabe and colleagues observed the endogenously released NO and oxidative DNA alterations in hepatocytes co-cultured with splenic macrophages isolated from Wistar rats and incubated with either lipopolysaccharide (LPS) or interferon-gamma (148). Increased NO release, nitrotyrosine production and ratio of 8-hydroxy-deoxyguanosine (8-OH-dG) to deoxyguanosine (dG) were also noted in the hepatocytes. Part of the peroxynitrite-induced metabolic cellular dysfunction in hepatocytes resulted from an inhibition of mitochondrial respiration, in part via a direct mitochondrial action, and in part via activation of PARP (149).
Septic patients frequently suffer from acidosis. The stability and reactivity of many reactive nitrogen and oxygen species are dependent on the pH, which affects the degree of the resulting hepatocellular damage. Shu and colleagues demonstrated that acidification (pH 7.0) of the medium in normal and C. parvum-primed hepatocytes exposed to a mixture of pro-inflammatory cytokines and LPS produces a significant increase of peroxynitrite and hydroxyl radicals (150). Importantly, an enhanced degree of hepatocellular damage was noted in acidotic conditions, as compared to the responses at physiological (pH 7.4) or alkaline (pH 7.8) conditions. These results suggest that hepatocellular damage is partly regulated by the surrounding pH: acidosis and reactive oxidant production are likely to act in concert to produce hepatocellular damage in circulatory shock.
In accordance with the above outlined in vitro studies, several independent in vivo studies suggest that peroxynitrite may also contribute to the hepatocellular damage in animal models of circulatory shock. Cimen and colleagues investigated the in vivo effect of bacterial LPS on Na+,K+-ATPase activity of guinea pig liver and investigated the possible contribution of various reactive nitrogen species (151, 152) and found a good correlation between the inhibition of Na+,K+-ATPase activity and the increase 3-nitrotyrosine levels in the livers of LPS-treated animals, suggesting that peroxynitrite may contribute to the inhibition of cell-membrane Na+,K+-ATPase.
Several series of studies, using various pharmacological interventions aimed at indirectly reducing peroxynitrite formation have resulted in improvements in hepatic function in various models of circulatory shock. These approaches included oxygen and nitrogen-derived radicals scavengers and neutralizers such as (−)-epitechin 3-O-gallate (153, 154), tempol (155–157) and Hypericum perforatum extract (158) or iNOS enzyme inhibitors such as N6-(iminoethyl)-L-lysine (159), aminoguanidine (160), and 1400W (108). The putative peroxynitrite scavenger uric acid has also improved hepatic function, as demonstrated in a rat model of hemorrhagic shock (159). The most definitive proof for the specific role of peroxynitrite in the pathogenesis of hepatic dysfunction in circulatory shock comes from studies by Cuzzocrea and colleagues who have examined the contribution of peroxynitrite formation in the pathophysiology of endotoxin-induced shock in the rat (108) using the peroxynitrite decomposition catalyst, 5,10,15,20-tetrakis(4-sulfonatophenyl) porphyrinato iron III chloride (FeTTPs). In this model, FeTTPs markedly attenuated the degree of hepatic injury, and significantly improved mortality rate.
‘Cytopathic hypoxia’ (a common feature of circulatory shock, where tissues (that are nominally adequately perfused) lose their ability to extract and utilize oxygen due to the inhibition of cellular metabolism (161, 162). The evidence demonstrating that peroxynitrite has the ability to suppress hepatocyte metabolism in circulatory shock may be consistent with the hypothesis that peroxynitrite contributes to the pathogenesis of cytopathic hypoxia in circulatory shock. However, to date, no studies have been published to directly test the effect of specific peroxynitrite decomposition catalysts on tissue oxygen utilization or arterio-venous oxygen differences in animal models of circulatory shock.
Potential role of peroxynitrite in the development of renal dysfunction in circulatory shock
Renal dysfunction is another common feature of circulatory shock. Peroxynitrite can be directly toxic to renal epithelial cells in culture (153, 163). Paller and colleagues have implicated the potential pathogenetic role of peroxynitrite in primary cultures of rat proximal tubular epithelial cells exposed to hypoxia and reoxygenation (164). Hypoxia and reoxygenation produced a marked increase in cellular generation of reactive oxidant species and triggered a significant degree of LDH release. Similar to the studies demonstrating the generation of peroxynitrite in vivo (see above), intracellular peroxynitrite generation was assessed by measuring the conversion of dihydrorhodamine 123 to rhodamine 123. PARP inhibitors of various structural classes have also been demonstrated to exert protective effects in various models of cultured kidney epithelial cells exposed to pro-oxidant conditions or hypoxia-reoxygenation (165–167).
Several lines of in vivo studies also point to the potential pathogenetic role of peroxynitrite in the development of renal injury associated with circulatory shock. The antioxidant tempol proved to be also effective in reducing the renal dysfunction and injury associated with ischemia/reperfusion of the kidney (157), in a model of multiple organ injury (including renal dysfunction) associated with hemorrhagic shock (155) as well as in another rodent model of multiple organ injury induced by cell wall components of S. aureus (lipoteichoic acid and peptidoglycan) (156). Finally, the peroxynitrite decomposition catalyst FeTTPs was shown to attenuate endotoxin-induced renal injury in a rat model of endotoxic shock (108).
Potential role of peroxynitrite in the development of pulmonary dysfunction in circulatory shock
Similarly to cultured hepatocytes and kidney epithelial cells, peroxynitrite has the capacity to induce injury to pulmonary epithelial cells: an effect which occurs via a combination of mechanisms including direct metabolic inhibition, activation of PARP, activation of caspases and other cell death effector pathways (168–170). In addition (as mentioned earlier), peroxynitrite can induce damage to pulmonary surfactant (26–29), which may lead to pro-inflammatory changes and self-amplifying cycles of pulmonary injury in shock. In various animal models of circulatory shock, formation of nitrotyrosine has been demonstrated in pulmonary tissue sections (171–173). Furthermore, pharmacological neutralization of this species has been shown to reduce pulmonary histological damage and improve pulmonary oxygen function, as demonstrated by the effects of the metalloporphyrinic compound FP-15 in a rat model of pulmonary reperfusion injury (173) or the metalloporphyrinic compound WW-85 in a large animal model of systemic inflammation and pulmonary dysfunction induced by IL-2 (174).
As patients with septic shock are generally subjected to mechanical ventilation, part of the pulmonary injury in critical illness is not the result of the primary disease, but a iatrogenic effect. Even though there are attempts to minimize the development of VILI (ventilator-induced lung injury), it is a fact of life that VILI develops a significant number of patients with circulatory shock, and it is, therefore, connected to the pathogenesis of circulatory shock itself. Hence, we briefly mention in our review that several studies have investigated the molecular pathogenesis of VILI and have implicated the potential role of peroxynitrite and related reactive species (175).
Conclusions
Multiple lines of evidence indicates that peroxynitrite, a labile, cytotoxic species, (a) is produced in various forms of circulatory shock; (b) has the capacity to induce cell and organ damage including cellular metabolic suppression and cell death (apoptosis and necrosis); and (c) its pharmacological neutralization exerts beneficial effects in various models of circulatory shock, as evidenced by peroxynitrite decomposition catalysts (Table 2), as well as compounds that act as combined inhibitors of iNOS and scavengers of peroxynitrite (176–180). The pathogenetic roles of peroxynitrite not only include the promotion of vascular and myocardial dysfunction and hepatic, renal and pulmonary dysfunction (key components of organ failure), but also intestinal dysfunction (86, 181), pancreatic injury (108, 182), as well as skeletal muscle dysfunction (183, 184). In addition, peroxynitrite may possibly also contribute to the pathogenesis of cellular metabolic failure (‘cytopathic hypoxia’). Several studies also demonstrate a correlation between its formation and the severity of the disease in human circulatory shock. Based on these findings, the conclusion can be formed that peroxynitrite is a pathogenetic factor and a potential drug development target in circulatory shock. We must keep in mind, however, that peroxynitrite is a cytotoxic byproduct of nitric oxide, and nitric oxide exerts multiple vital physiological roles (Fig. 1). Therefore, selective neutralization of peroxynitrite formation (e.g. using catalytic inhibitors of superoxide formation or by compounds that promote the catalytic decomposition of peroxynitrite) appears to represent a preferred approach over non-selective pharmacological inhibition of NO generation.
Table 2.
Effects of peroxynitrite neutralizing agents in animal models of shock
| Decompositio n catalyst | Shock model | Main effects | References |
|---|---|---|---|
| FeTMPS, FeTMPyP | Endotoxin- induced duodenal and intestinal damage in rats. | Reduction of microvascular leakage, lipid peroxidation, and epithelial cell injury. | (185, 186) |
| FeTMPS | Splanchnic artery occlusion shock in rats. | Reduction of bowel injury and improvement of survival rate. Reduction of the intensity P-selectin and ICAM-1 expression. | (187) |
| FeTPPS | Endotoxin shock models in rats. | Improvement in myocardial contractile function. Protection against LPS-induced vascular failure, hypotension, tissue injury, and mortality. | (142) (108) |
| FP15 | Rat and mouse models of endotoxic shock and polymicrobial sepsis. | Reduction in hepatic injury, improvement in survival rate. | Soriano and Szabo, unpublished observations. |
| WW85 | IL-2-induced pulmonary injury and systemic inflammation in sheep. | Improvement in lung transvascular fluid flux, decreased lipid peroxidation, prevention of tachycardia, and reduction in fever. | (174) |
| Mouse model of cecal ligation induced sepsis. | Improvement in survival rate. | Radermacher and Szabo, unpublished observations. | |
| MnTCPP | Rodent models of endotoxic and zymosan induced shock. | Prevention of vascular dysfunction and cellular energetic alterations. Reduction in peritoneal exudation, polymorphonuclear migration and peroxynitrite formation. Improvement in cellular energetics ex vivo. | (81) (188) |
| MnTE-2-PyP | Cecal ligation and puncture in rats. | Preservation of mitochondrial function and diaphragmatic contractility. | (189) |
Acknowledgments
This work was supported by the US National Institutes of Health: R01 GM66189 and by a grant from the Oszkar Asboth project grant of the National Office for Research and Technology (Budapest, Hungary).
Abbreviations used
- AIF
apoptosis-inducing factor
- BH4
tetrahydrobiopterin
- cGMP
soluble guanylate cyclase (sGC)-cyclic guanosine-3′,5′-monophosphate
- CO3•−
carbonate
- dG
deoxyguanosine
- eNOS
endothelial nitric oxide synthase
- FAK
phosphorylated focal adhesion kinase
- FeTPPS
5,10,15,20-tetrakis(4-sulfonatophenyl)-porphyrinato iron (III)
- FeTTPs
5,10,15,20-tetrakis(4-sulfonatophenyl) porphyrinato iron III chloride
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HDAC2
histone deacetylase
- iNOS
inducible nitric oxide synthase
- LNIL
N6-(iminoethyl)-L-lysine
- LPS
bacterial lipopolysaccharide
- MMPs
matrix metalloproteinases
- MnSOD
manganese superoxide dismutase
- NAD+
Nicotinamide adenine dinucleotide
- NO
nitric oxide
- •NO2
nitrogen dioxide radical
- O2·−
superoxide
- 8-OH-dG
8-hydroxy-deoxyguanosine
- •OH
hydroxyl radical
- ONOO−
peroxynitrite anion
- PAR
poly(ADP-ribose) polymer
- PARG
poly(ADP-ribose) glycohydrolase
- PARP
poly (ADP-ribose) polymerase
- PPARγ
peroxisome proliferator-activated receptor gamma
- PTP
permeability transition pore
- SIN-1
3-morpholinosydnonimine
- SNAP
S-nitroso-N-acetylpenicillamine-amine
- VILI
ventilator-induced lung injury
References
- 1.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 3.Rubbo H, Radi R, Trujillo M, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 4.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 5.Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140–141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- 7.Szabo C, Salzman AL, Ischiropoulos H. Peroxynitrite-mediated oxidation of dihydrorhodamine 123 occurs in early stages of endotoxic and hemorrhagic shock and ischemia-reperfusion injury. FEBS Lett. 1995;372:229–232. doi: 10.1016/0014-5793(95)00984-h. [DOI] [PubMed] [Google Scholar]
- 8.Villa LM, Salas E, Darley-Usmar M, Radomski MW, Moncada S. Peroxynitrite induces both vasodilatation and impaired vascular relaxation in the isolated perfused rat heart. Proc Natl Acad Sci USA. 1994;91:12383–12387. doi: 10.1073/pnas.91.26.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer ML, Beckman JS, Bridges RJ, Fuller CM, Matalon S. Peroxynitrite inhibits sodium uptake in rat colonic membrane vesicles. Biochim Biophys Acta. 1992;1104:87–94. doi: 10.1016/0005-2736(92)90135-9. [DOI] [PubMed] [Google Scholar]
- 10.Castro L, Rodriguez M, RR Aconitase is readily inactivated by peroxynitrite, but not by its precursor, nitric oxide. J Biol Chem. 1994;269:29409–29415. [PubMed] [Google Scholar]
- 11.Crow JPBJ, McCord JM. Sensitivity of the essential zinc-thiolate moiety of yeast alcohol dehydrogenase to hypochlorite and peroxynitrite. Biochemistry. 1995;34:3544–3552. doi: 10.1021/bi00011a008. [DOI] [PubMed] [Google Scholar]
- 12.Guzman NJ, Fang MZ, Tang SS, Ingelfinger JR, Garg LC. Autocrine inhibition of Na+/K(+)-ATPase by nitric oxide in mouse proximal tubule epithelial cells. J Clin Invest. 1995;95:2083–2088. doi: 10.1172/JCI117895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hausladen A, Fridovich I. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J Biol Chem. 1994;269:29405–29408. [PubMed] [Google Scholar]
- 14.Hu P, Ischiropoulos H, Beckman JS, Matalon S. Peroxynitrite inhibition of oxygen consumption and sodium transport in alveolar type II cells. Am J Physiol. 1994;266:L628–L634. doi: 10.1152/ajplung.1994.266.6.L628. [DOI] [PubMed] [Google Scholar]
- 15.Ischiropoulos H, Duran D, Horwitz J. Peroxynitrite-mediated inhibition of DOPA synthesis in PC12 cells. J Neurochem. 1995;65:2366–2372. doi: 10.1046/j.1471-4159.1995.65052366.x. [DOI] [PubMed] [Google Scholar]
- 16.Ischiropoulos H, Zhu L, Chen J, et al. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 17.Miles AM, Bohle DS, Glassbrenner PA, Hansert B, Wink DA, Grisham MB. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J Biol Chem. 1996;271:40–47. doi: 10.1074/jbc.271.1.40. [DOI] [PubMed] [Google Scholar]
- 18.Mohr S, Stamler JS, Brune B. Mechanism of covalent modification of glyceraldehyde-3-phosphate dehydrogenase at its active site thiol by nitric oxide, peroxynitrite and related nitrosating agents. FEBS Lett. 1994;348:223–227. doi: 10.1016/0014-5793(94)00596-6. [DOI] [PubMed] [Google Scholar]
- 19.Pou S, Nguyen SY, Gladwell T, Rosen GM. Does peroxynitrite generate hydroxyl radical? Biochim Biophys Acta. 1995;1244:62–68. doi: 10.1016/0304-4165(94)00197-6. [DOI] [PubMed] [Google Scholar]
- 20.Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 21.Rubbo H, Denicola A, Radi R. Peroxynitrite inactivates thiol-containing enzymes of Trypanosoma cruzi energetic metabolism and inhibits cell respiration. Arch Biochem Biophys. 1994;308:96–102. doi: 10.1006/abbi.1994.1014. [DOI] [PubMed] [Google Scholar]
- 22.Salman-Tabcheh S, Guerin MC, Torreilles J. Nitration of tyrosyl-residues from extra- and intracellular proteins in human whole blood. Free Radic Biol Med. 1995;19:695–698. doi: 10.1016/0891-5849(95)00075-9. [DOI] [PubMed] [Google Scholar]
- 23.Selden LA, Gersham LC, Estes JE, Ferro TJ, AJ Peroxynitrite-induced tyrosine nitration mediates decreased polymerization of actin. Mol Biol Cell. 1995;6 [Google Scholar]
- 24.Bolanos JP, Heales SJ, Land JM, Clark JB. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J Neurochem. 1995;64:1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- 25.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation:the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 26.Moore KP, Darley-Usmar V, Morrow J, Roberts LJ., 2nd Formation of F2-isoprostanes during oxidation of human low-density lipoprotein and plasma by peroxynitrite. Circ Res. 1995;77:335–341. doi: 10.1161/01.res.77.2.335. [DOI] [PubMed] [Google Scholar]
- 27.Cifuentes J, Ruiz-Oronoz J, Myles C, Nieves B, Carlo WA, Matalon S. Interaction of surfactant mixtures with reactive oxygen and nitrogen species. J Appl Physiol. 1995;78:1800–1805. doi: 10.1152/jappl.1995.78.5.1800. [DOI] [PubMed] [Google Scholar]
- 28.Haddad IY, Crow JP, Hu P, Ye Y, Beckman J, Matalon S. Concurrent generation of nitric oxide and superoxide damages surfactant protein A. Am J Physiol. 1994;267:L242–L249. doi: 10.1152/ajplung.1994.267.3.L242. [DOI] [PubMed] [Google Scholar]
- 29.Haddad IY, Ischiropoulos H, Holm BA, Beckman JS, Baker JR, Matalon S. Mechanisms of peroxynitrite-induced injury to pulmonary surfactants. Am J Physiol. 1993;265:L555–L564. doi: 10.1152/ajplung.1993.265.6.L555. [DOI] [PubMed] [Google Scholar]
- 30.Inoue S, Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- 31.Yermilov V, Rubio J, Becchi M, Friesen MD, Pignatelli B, Ohshima H. Formation of 8-nitroguanine by the reaction of guanine with peroxynitrite in vitro. Carcinogenesis. 1995;16:2045–2050. doi: 10.1093/carcin/16.9.2045. [DOI] [PubMed] [Google Scholar]
- 32.King PA, Anderson VE, Edwards JO, Gustav G, Plumb RC, Suggs JW. A stable solid that generates hydroxyl radical dissolutions in aqueous solutions: reaction with proteins and nucleic acid. J Am Chem Soc. 1992;114:5430–5432. [Google Scholar]
- 33.Salgo MG, Bermudez E, Squadrito GL, Pryor WA. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes. Arch Biochem Biophys. 1995;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- 34.Salgo MG, Stone K, Squadrito GL, Battista JR, Pryor WA. Peroxynitrite causes DNA nicks in plasmid pBR322. Biochem Biophys Res Commun. 1995;210:1025–1030. doi: 10.1006/bbrc.1995.1759. [DOI] [PubMed] [Google Scholar]
- 35.Moro MA, Darley-Usmar VM, Goodwin DA, et al. Paradoxical fate and biological action of peroxynitrite on human platelets. Proc Natl Acad Sci USA. 1994;91:6702–6706. doi: 10.1073/pnas.91.14.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moro MA, Darley-Usmar VM, Lizasoain I, et al. The formation of nitric oxide donors from peroxynitrite. Br J Pharmacol. 1995;116:1999–2004. doi: 10.1111/j.1476-5381.1995.tb16404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lymar SV, JKH Rapid reaction between peroxynitrite ion and carbon dioxide: implications for biological activity. J Am Chem Soc. 1995;117:8867–8868. [Google Scholar]
- 38.Van der Vliet A, Smith D, O’Neill CA, et al. Interactions of peroxynitrite with human plasma and its constituents: oxidative damage and antioxidant depletion. Biochem J. 1994;303:295–301. doi: 10.1042/bj3030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darley-Usmar V, Wiseman H, Halliwell B. Nitric oxide and oxygen radicals: a question of balance. FEBS Lett. 1995;369:131–135. doi: 10.1016/0014-5793(95)00764-z. [DOI] [PubMed] [Google Scholar]
- 40.Mayer B, Schrammel A, Klatt P, Koesling D, Schmidt K. Peroxynitrite-induced accumulation of cyclic GMP in endothelial cells and stimulation of purified soluble guanylyl cyclase. Dependence on glutathione and possible role of S-nitrosation. J Biol Chem. 1995;270:17355–17360. doi: 10.1074/jbc.270.29.17355. [DOI] [PubMed] [Google Scholar]
- 41.Tarpey MM, Beckman JS, Ischiropoulos H, Gore JZ, Brock TA. Peroxynitrite stimulates vascular smooth muscle cell cyclic GMP synthesis. FEBS Lett. 1995;364:314–318. doi: 10.1016/0014-5793(95)00413-4. [DOI] [PubMed] [Google Scholar]
- 42.Brunelli L, Crow JP, Beckman JS. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch Biochem Biophys. 1995;316:327–334. doi: 10.1006/abbi.1995.1044. [DOI] [PubMed] [Google Scholar]
- 43.Szabó C, Salzman AL. Endogenous peroxynitrite is involved in the inhibition of cellular respiration in immuno-stimulated J774.2 macrophages. Biochem Biophys Res Commun. 1995;209:739–743. doi: 10.1006/bbrc.1995.1561. [DOI] [PubMed] [Google Scholar]
- 44.Szabo C. Mechanisms of cell necrosis. Crit Care Med. 2005;33:S530–S534. doi: 10.1097/01.ccm.0000187002.88999.cf. [DOI] [PubMed] [Google Scholar]
- 45.Szabó C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages, using a novel mesoporphyrin superoxide dismutase analog and peroxynitrite scavenger. FEBS Lett. 1996;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- 46.Szabó C, Zingarelli B, O’Connor M, Salzman AL. DNA strand breakage, activation of poly-ADP ribosyl synthetase, and cellular energy depletion are involved in the cytotoxicity in macrophages and smooth muscle cells exposed to peroxynitrite. Proc Natl Acad Sci USA. 1996;93:1753–1758. doi: 10.1073/pnas.93.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu M, Pritchard KA, Jr, Kaminski PM, Fayngersh RP, Hintze TH, Wolin MS. Involvement of nitric oxide and nitrosothiols in relaxation of pulmonary arteries to peroxynitrite. Am J Physiol. 1994;266:H2108–H2113. doi: 10.1152/ajpheart.1994.266.5.H2108. [DOI] [PubMed] [Google Scholar]
- 48.deRojas-Walker T, Tamir S, Ji H, Wishnok JS, Tannenbaum SR. Nitric oxide induces oxidative damage in addition to deamination in macrophage DNA. Chem Res Toxicol. 1995;8:473–477. doi: 10.1021/tx00045a020. [DOI] [PubMed] [Google Scholar]
- 49.Szabó C, Zingarelli B, Salzman AL. Role of poly-ADP ribosyltransferase activation in the nitric oxide- and peroxynitrite-induced vascular failure. Circ Res. 1996;78:1051–1063. doi: 10.1161/01.res.78.6.1051. [DOI] [PubMed] [Google Scholar]
- 50.Zingarelli B, O’Connor M, Wong H, Salzman AL, Szabo C. Peroxynitrite-mediated DNA strand breakage activates poly-ADP ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol. 1996;156:350–358. [PubMed] [Google Scholar]
- 51.Szabo C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett. 2003;140–141:105–112. doi: 10.1016/s0378-4274(02)00507-6. [DOI] [PubMed] [Google Scholar]
- 52.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 53.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 54.Du X, Matsumura T, Edelstein D, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Devalaraja-Narashimha K, Padanilam BJ. PARP-1 inhibits glycolysis in ischemic kidneys. J Am Soc Nephrol. 2009;20:95–103. doi: 10.1681/ASN.2008030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, SL Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with NMDA or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estevez AGRR, Barbeito L, Shin JT, Thompson JA, Beckman JS. Peroxynitrite-induced cytotoxicity in PC12 cells: evidence for an apoptotic mechanism differentially modulated by neurotropic factors. J Neurochem. 1995;65:1543–1550. doi: 10.1046/j.1471-4159.1995.65041543.x. [DOI] [PubMed] [Google Scholar]
- 58.Lin KT, Xue JY, Nomen M, Spur B, Wong PY. Peroxynitrite-induced apoptosis in HL-60 cells. J Biol Chem. 1995;270:16487–16490. doi: 10.1074/jbc.270.28.16487. [DOI] [PubMed] [Google Scholar]
- 59.Salgo MG, Squadrito GL, Pryor WA. Peroxynitrite causes apoptosis in rat thymocytes. Biochem Biophys Res Commun. 1995;215:1111–1118. doi: 10.1006/bbrc.1995.2578. [DOI] [PubMed] [Google Scholar]
- 60.Shacka JJ, Sahawneh MA, Gonzalez JD, Ye YZ, D’Alessandro TL, Estevez AG. Two distinct signaling pathways regulate peroxynitrite-induced apoptosis in PC12 cells. Cell Death Differ. 2006;13:1506–1514. doi: 10.1038/sj.cdd.4401831. [DOI] [PubMed] [Google Scholar]
- 61.Virag L, Marmer DJ, Szabo C. Crucial role of apopain in the peroxynitrite-induced apoptotic DNA fragmentation. Free Radic Biol Med. 1998;25:1075–1082. doi: 10.1016/s0891-5849(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 62.Zhuang S, Simon G. Peroxynitrite-induced apoptosis involves activation of multiple caspases in HL-60 cells. Am J Physiol Cell Physiol. 2000;279:C341–C351. doi: 10.1152/ajpcell.2000.279.2.C341. [DOI] [PubMed] [Google Scholar]
- 63.Kennedy M, Denenberg AG, Szabo C, Salzman AL. Poly(ADP-ribose) synthetase activation mediates increased permeability induced by peroxynitrite in Caco-2BBe cells. Gastroenterology. 1998;114:510–518. doi: 10.1016/s0016-5085(98)70534-7. [DOI] [PubMed] [Google Scholar]
- 64.Khan AU, Delude RL, Han YY, et al. Liposomal NAD(+) prevents diminished O(2) consumption by immunostimulated Caco-2 cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1082–L1091. doi: 10.1152/ajplung.00358.2001. [DOI] [PubMed] [Google Scholar]
- 65.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease:from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 66.Goldstein S, Czapski G. Reactivity of peroxynitrite versus simultaneous generation of (*)NO and O(2)(*)(−) toward NADH. Chem Res Toxicol. 2000;13:736–741. doi: 10.1021/tx000099n. [DOI] [PubMed] [Google Scholar]
- 67.Kirsch M, de Groot H. Reaction of peroxynitrite with reduced nicotinamide nucleotides, the formation of hydrogen peroxide. J Biol Chem. 1999;274:24664–24670. doi: 10.1074/jbc.274.35.24664. [DOI] [PubMed] [Google Scholar]
- 68.Boczkowski J, Lisdero CL, Lanone S, Carreras MC, Aubier M, Poderoso JJ. Peroxynitrite-mediated mitochondrial dysfunction. Biol Signals Recept. 2001;10:66–80. doi: 10.1159/000046876. [DOI] [PubMed] [Google Scholar]
- 69.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cassina AM, Hodara R, Souza JM, et al. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 71.Quijano C, Cassina A, Castro L, Rodríguez M, Radi R. In: In Nitric Oxide, Cell Signaling and Gene Expression. Lamas S, Cadenas E, editors. CRC; Boca Raton: 2005. p. 2005. [Google Scholar]
- 72.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bagnasco P, MacMillan-Crow LA, Greendorfer JS, Young CJ, Andrews L, Thompson JA. Peroxynitrite modulates acidic fibroblast growth factor (FGF-1) activity. Arch Biochem Biophys. 2003;419:178–189. doi: 10.1016/j.abb.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 74.Zingarelli B, Salzman AL, Szabo C. Genetic disruption of poly (ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res. 1998;83:85–94. doi: 10.1161/01.res.83.1.85. [DOI] [PubMed] [Google Scholar]
- 75.Zouki C, Zhang SL, Chan JS, Filep JG. Peroxynitrite induces integrin-dependent adhesion of human neutrophils to endothelial cells via activation of the Raf-1/MEK/Erk pathway. FASEB J. 2001;15:25–27. doi: 10.1096/fj.00-0521fje. [DOI] [PubMed] [Google Scholar]
- 76.Knapp LT, Kanterewicz BI, Hayes EL, Klann E. Peroxynitrite-induced tyrosine nitration and inhibition of protein kinase C. Biochem Biophys Res Commun. 2001;286:764–770. doi: 10.1006/bbrc.2001.5448. [DOI] [PubMed] [Google Scholar]
- 77.Bapat S, Verkleij A, Post JA. Peroxynitrite activates mitogen-activated protein kinase (MAPK) via a MEK-independent pathway: a role for protein kinase C. FEBS Lett. 2001;499:21–26. doi: 10.1016/s0014-5793(01)02511-x. [DOI] [PubMed] [Google Scholar]
- 78.Mallozzi C, Di Stasi AM, Minetti M. Nitrotyrosine mimics phosphotyrosine binding to the SH2 domain of the src family tyrosine kinase lyn. FEBS Lett. 2001;503:189–195. doi: 10.1016/s0014-5793(01)02726-0. [DOI] [PubMed] [Google Scholar]
- 79.Mallozzi C, Di Stasi MA, Minetti M. Peroxynitrite-dependent activation of src tyrosine kinases lyn and hck in erythrocytes is under mechanistically different pathways of redox control. Free Radic Biol Med. 2001;30:1108–1117. doi: 10.1016/s0891-5849(01)00509-3. [DOI] [PubMed] [Google Scholar]
- 80.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315:240–245. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 81.Zingarelli B, Day BJ, Crapo JD, Salzman AL, Szabo C. The potential role of peroxynitrite in the vascular contractile and cellular energetic failure in endotoxic shock. Br J Pharmacol. 1997;120:259–267. doi: 10.1038/sj.bjp.0700872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cuzzocrea S, Zingarelli B, Costantino G, et al. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. Br J Pharmacol. 1997;121:1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Doursout MF, Oguchi T, Fischer UM, et al. Distribution of NOS isoforms in a porcine endotoxin shock model. Shock. 2008;29:692–702. doi: 10.1097/shk.0b013e3181598b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Enkhbaatar P, Joncam C, Traber L, et al. Novel ovine model of methicillin-resistant Staphylococcus aureus-induced pneumonia and sepsis. Shock. 2008;29:642–649. doi: 10.1097/shk.0b013e318158125b. [DOI] [PubMed] [Google Scholar]
- 85.Lehnert M, Arteel GE, Smutney OM, et al. Dependence of liver injury after hemorrhage/resuscitation in mice on NADPH oxidase-derived superoxide. Shock. 2003;19:345–351. doi: 10.1097/00024382-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 86.Liaudet L, Soriano FG, Szabo E, et al. Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose)polymerase. Proc Natl Acad Sci USA. 2000;97:10203–10208. doi: 10.1073/pnas.170226797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murakami K, Enkhbaatar P, Shimoda K, et al. Inhibition of poly (ADP-ribose) polymerase attenuates acute lung injury in an ovine model of sepsis. Shock. 2004;21:126–133. doi: 10.1097/01.shk.0000108397.56565.4a. [DOI] [PubMed] [Google Scholar]
- 88.Wu L, Mayeux PR. Effects of the inducible nitric-oxide synthase inhibitor L-N(6)-(1-iminoethyl)-lysine on microcirculation and reactive nitrogen species generation in the kidney following lipopolysaccharide administration in mice. J Pharmacol Exp Ther. 2007;320:1061–1067. doi: 10.1124/jpet.106.117184. [DOI] [PubMed] [Google Scholar]
- 89.Eiserich JP, Hristova M, Cross CE, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 90.Pfeiffer S, Lass A, Schmidt K, Mayer B. Protein tyrosine nitration in cytokine-activated murine macrophages. Involvement of a peroxidase/nitrite pathway rather than peroxynitrite. J Biol Chem. 2001;276:34051–34058. doi: 10.1074/jbc.M100585200. [DOI] [PubMed] [Google Scholar]
- 91.Brovkovych V, Gao XP, Ong E, et al. Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L96–L103. doi: 10.1152/ajplung.00450.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang R, Brennan ML, Shen Z, et al. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J Biol Chem. 2002;277:46116–46122. doi: 10.1074/jbc.M209124200. [DOI] [PubMed] [Google Scholar]
- 93.Ohya M, Marukawa S, Inoue T, et al. Plasma nitrotyrosine concentration relates to prognosis in human septic shock. Shock. 2002;18:116–118. doi: 10.1097/00024382-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 94.Strand OA, Leone A, Giercksky KE, Kirkeboen KA. Nitric oxide indices in human septic shock. Crit Care Med. 2000;28:2779–2785. doi: 10.1097/00003246-200008000-00017. [DOI] [PubMed] [Google Scholar]
- 95.Kooy NW, Royall JA, Ye YZ, Kelly DR, Beckman JS. Evidence for in vivo peroxynitrite production in human acute lung injury. Am J Respir Crit Care Med. 1995;151:1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- 96.Fukuyama N, Takebayashi Y, Hida M, Ishida H, Ichimori K, Nakazawa H. Clinical evidence of peroxynitrite formation in chronic renal failure patients with septic shock. Free Radic Biol Med. 1997;22:771–774. doi: 10.1016/s0891-5849(96)00401-7. [DOI] [PubMed] [Google Scholar]
- 97.Capasso M, Di Muzio A, Pandolfi A, et al. Possible role for nitric oxide dysregulation in critical illness myopathy. Muscle Nerve. 2008;37:196–202. doi: 10.1002/mus.20907. [DOI] [PubMed] [Google Scholar]
- 98.Kooy NW, Lewis SJ, Royall JA, Ye YZ, Kelly DR, Beckman JS. Extensive tyrosine nitration in human myocardial inflammation:evidence for the presence of peroxynitrite. Crit Care Med. 1997;25:812–819. doi: 10.1097/00003246-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 99.Lanone S, Manivet P, Callebert J, et al. Inducible nitric oxide synthase (NOS2) expressed in septic patients is nitrated on selected tyrosine residues: implications for enzymic activity. Biochem J. 2002;366:399–404. doi: 10.1042/BJ20020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lanone S, Mebazaa A, Heymes C, et al. Muscular contractile failure in septic patients: role of the inducible nitric oxide synthase pathway. Am J Respir Crit Care Med. 2000;162:2308–2315. doi: 10.1164/ajrccm.162.6.2001097. [DOI] [PubMed] [Google Scholar]
- 101.Rossi MA, Celes MR, Prado CM, Saggioro FP. Myocardial structural changes in long-term human severe sepsis/septic shock may be responsible for cardiac dysfunction. Shock. 2007;27:10–18. doi: 10.1097/01.shk.0000235141.05528.47. [DOI] [PubMed] [Google Scholar]
- 102.Hecker M, Cattaruzza M, Wagner AH. Regulation of inducible nitric oxide synthase gene expression in vascular smooth muscle cells. Gen Pharmacol. 1999;32:9–16. doi: 10.1016/s0306-3623(98)00082-2. [DOI] [PubMed] [Google Scholar]
- 103.Szabo C. Alterations in nitric oxide production in various forms of circulatory shock. New Horizons. 1995;3:2–32. [PubMed] [Google Scholar]
- 104.Kilbourn RG, Traber DL, Szabo C. Nitric oxide and shock. Dis Mon. 1997;43:277–348. doi: 10.1016/s0011-5029(97)90028-6. [DOI] [PubMed] [Google Scholar]
- 105.Howes LG, Brillante DG. Expert opinion on tilarginine in the treatment of shock. Expert Opin Investig Drugs. 2008;17:1573–1580. doi: 10.1517/13543784.17.10.1573. [DOI] [PubMed] [Google Scholar]
- 106.Cuzzocrea S, Zingarelli B, O’Connor M, Salzman AL, Szabo C. Effect of L-buthionine-(S,R)-sulphoximine, an inhibitor of gamma-glutamylcysteine synthetase on peroxynitrite- and endotoxic shock-induced vascular failure. Br J Pharmacol. 1998;123:525–537. doi: 10.1038/sj.bjp.0701612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakamura M, Thourani VH, Ronson RS, et al. Glutathione reverses endothelial damage from peroxynitrite, the byproduct of nitric oxide degradation, in crystalloid cardioplegia. Circulation. 2000;102:III-332–338. doi: 10.1161/01.cir.102.suppl_3.iii-332. [DOI] [PubMed] [Google Scholar]
- 108.Cuzzocrea S, Mazzon E, Di Paola R, et al. A role for nitric oxide-mediated peroxynitrite formation in a model of endotoxin-induced shock. J Pharmacol Exp Ther. 2006;319:73–81. doi: 10.1124/jpet.106.108100. [DOI] [PubMed] [Google Scholar]
- 109.Mazzon E, De Sarro A, Caputi AP, Cuzzocrea S. Role of tight junction derangement in the endothelial dysfunction elicited by exogenous and endogenous peroxynitrite and poly(ADP-ribose) synthetase. Shock. 2002;18:434–439. doi: 10.1097/00024382-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y, Zhao S, Gu Y, Lewis DF, Alexander JS, Wang Y. Effects of peroxynitrite and superoxide radicals on endothelial monolayer permeability: potential role of peroxynitrite in preeclampsia. J Soc Gynecol Investig. 2005;12:586–592. doi: 10.1016/j.jsgi.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 111.Wu F, Wilson JX. Peroxynitrite-dependent activation of protein phosphatase type 2A mediates microvascular endothelial barrier dysfunction. Cardiovasc Res. 2009;81:38–45. doi: 10.1093/cvr/cvn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maneen MJ, Cipolla MJ. Peroxynitrite diminishes myogenic tone in cerebral arteries:role of nitrotyrosine and F-actin. Am J Physiol Heart Circ Physiol. 2007;292:H1042–H1050. doi: 10.1152/ajpheart.00800.2006. [DOI] [PubMed] [Google Scholar]
- 113.Pan BX, Zhao GL, Huang XL, Jin JQ, Zhao KS. Peroxynitrite induces arteriolar smooth muscle cells membrane hyperpolarization with arteriolar hyporeactivity in rats. Life Sci. 2004;74:1199–1210. doi: 10.1016/j.lfs.2003.07.039. [DOI] [PubMed] [Google Scholar]
- 114.Zhao KS, Liu J, Yang GY, Jin C, Huang Q, Huang X. Peroxynitrite leads to arteriolar smooth muscle cell membrane hyperpolarization and low vasoreactivity in severe shock. Clin Hemorheol Microcirc. 2000;23:259–267. [PubMed] [Google Scholar]
- 115.Grover AK, Samson SE, Robinson S, Kwan CY. Effects of peroxynitrite on sarcoplasmic reticulum Ca2+ pump in pig coronary artery smooth muscle. Am J Physiol Cell Physiol. 2003;284:C294–C301. doi: 10.1152/ajpcell.00297.2002. [DOI] [PubMed] [Google Scholar]
- 116.Walia M, Samson SE, Schmidt T, Best K, Kwan CY, Grover AK. Effects of peroxynitrite on pig coronary artery smooth muscle. Cell Calcium. 2003;34:69–74. doi: 10.1016/s0143-4160(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 117.Benkusky NA, Lewis SJ, Kooy NW. Peroxynitrite-mediated attenuation of alpha- and beta-adrenoceptor agonist-induced vascular responses in vivo. Eur J Pharmacol. 1999;364:151–158. doi: 10.1016/s0014-2999(98)00791-2. [DOI] [PubMed] [Google Scholar]
- 118.Lewis SJ, Hoque A, Sandock K, Robertson TP, Bates JN, Kooy NW. Differential effects of peroxynitrite on the function of arginine vasopressin V(1a) receptors and alpha(1)-adrenoceptors in vivo. Vascul Pharmacol. 2007;46:24–34. doi: 10.1016/j.vph.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 119.Shelkovnikov S, Merlic CA, Gonick HC. Influence of nitric oxide donors and peroxynitrite on the contractile effect and concentration of norepinephrine. Life Sci. 2004;74:2919–2928. doi: 10.1016/j.lfs.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 120.Takakura K, Xiaohong W, Takeuchi K, Fukuda S. Peroxynitrite decreases dopamine’s vasoconstrictive activity. Anesth Analg. 2003;97:1492–1496. doi: 10.1213/01.ANE.0000082248.30437.0B. [DOI] [PubMed] [Google Scholar]
- 121.Takakura K, Xiaohong W, Takeuchi K, Yasuda Y, Fukuda S. Deactivation of norepinephrine by peroxynitrite as a new pathogenesis in the hypotension of septic shock. Anesthesiology. 2003;98:928–934. doi: 10.1097/00000542-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 122.Macarthur H, Westfall TC, Riley DP, Misko TP, Salvemini D. Inactivation of catecholamines by superoxide gives new insights on the pathogenesis of septic shock. Proc Natl Acad Sci USA. 2000;97:9753–9758. doi: 10.1073/pnas.97.17.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schildknecht S, Ullrich V. Peroxynitrite as regulator of vascular prostanoid synthesis. Arch Biochem Biophys. 2009;484:183–189. doi: 10.1016/j.abb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 124.Zou MH. Peroxynitrite and protein tyrosine nitration of prostacyclin synthase. Prostaglandins Other Lipid Mediat. 2007;82:119–127. doi: 10.1016/j.prostaglandins.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 125.Kennett EC, Davies MJ. Degradation of extracellular matrix by peroxynitrite/peroxynitrous acid. Free Radic Biol Med. 2008;45:716–725. doi: 10.1016/j.freeradbiomed.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 126.Ishida H, Ichimori K, Hirota Y, Fukahori M, Nakazawa H. Peroxynitrite-induced cardiac myocyte injury. Free Radic Biol Med. 1996;20:343–350. doi: 10.1016/0891-5849(96)02060-6. [DOI] [PubMed] [Google Scholar]
- 127.Leon H, Baczko I, Sawicki G, Light PE, Schulz R. Inhibition of matrix metalloproteinases prevents peroxynitrite-induced contractile dysfunction in the isolated cardiac myocyte. Br J Pharmacol. 2008;153:676–683. doi: 10.1038/sj.bjp.0707621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Digerness SB, Harris KD, Kirklin JW, et al. Peroxynitrite irreversibly decreases diastolic and systolic function in cardiac muscle. Free Radic Biol Med. 1999;27:1386–1392. doi: 10.1016/s0891-5849(99)00184-7. [DOI] [PubMed] [Google Scholar]
- 129.Panas D, Khadour FH, Szabo C, Schulz R. Proinflammatory cytokines depress cardiac efficiency by a nitric oxide-dependent mechanism. Am J Physiol. 1998;275:H1016–H1023. doi: 10.1152/ajpheart.1998.275.3.H1016. [DOI] [PubMed] [Google Scholar]
- 130.Schulz R, Dodge KL, Lopaschuk GD, Clanachan AS. Peroxynitrite impairs cardiac contractile function by decreasing cardiac efficiency. Am J Physiol. 1997;272:H1212–H1219. doi: 10.1152/ajpheart.1997.272.3.H1212. [DOI] [PubMed] [Google Scholar]
- 131.Xie YW, Kaminski PM, Wolin MS. Inhibition of rat cardiac muscle contraction and mitochondrial respiration by endogenous peroxynitrite formation during posthypoxic reoxygenation. Circ Res. 1998;82:891–897. doi: 10.1161/01.res.82.8.891. [DOI] [PubMed] [Google Scholar]
- 132.Ma XL, Lopez BL, Liu GL, Christopher TA, Ischiropoulos H. Peroxynitrite aggravates myocardial reperfusion injury in the isolated perfused rat heart. Cardiovasc Res. 1997;36:195–204. doi: 10.1016/s0008-6363(97)00179-x. [DOI] [PubMed] [Google Scholar]
- 133.Mihm MJ, Bauer JA. Peroxynitrite-induced inhibition and nitration of cardiac myofibrillar creatine kinase. Biochimie. 2002;84:1013–1019. doi: 10.1016/s0300-9084(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 134.Borbely A, Toth A, Edes I, et al. Peroxynitrite-induced alpha-actinin nitration and contractile alterations in isolated human myocardial cells. Cardiovasc Res. 2005;67:225–233. doi: 10.1016/j.cardiores.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 135.Snook JH, Li J, Helmke BP, Guilford WH. Peroxynitrite inhibits myofibrillar protein function in an in vitro assay of motility. Free Radic Biol Med. 2008;44:14–23. doi: 10.1016/j.freeradbiomed.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gao CQ, Sawicki G, Suarez-Pinzon WL, et al. Matrix metalloproteinase-2 mediates cytokine-induced myocardial contractile dysfunction. Cardiovasc Res. 2003;57:426–433. doi: 10.1016/s0008-6363(02)00719-8. [DOI] [PubMed] [Google Scholar]
- 137.Wang W, Sawicki G, Schulz R. Peroxynitrite-induced myocardial injury is mediated through matrix metalloproteinase-2. Cardiovasc Res. 2002;53:165–174. doi: 10.1016/s0008-6363(01)00445-x. [DOI] [PubMed] [Google Scholar]
- 138.Pacher P, Cziraki A, Mabley JG, Liaudet L, Papp L, Szabo C. Role of poly(ADP-ribose) polymerase activation in endotoxin-induced cardiac collapse in rodents. Biochem Pharmacol. 2002;64:1785–1791. doi: 10.1016/s0006-2952(02)01421-1. [DOI] [PubMed] [Google Scholar]
- 139.Pacher P, Liaudet L, Mabley JG, Cziraki A, Hasko G, Szabo C. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int J Mol Med. 2006;17:369–375. [PMC free article] [PubMed] [Google Scholar]
- 140.Gero D, Modis K, Nagy N, et al. Oxidant-induced cardiomyocyte injury: identification of the cytoprotective effect of a dopamine 1 receptor agonist using a cell-based high-throughput assay. Int J Mol Med. 2007;20:749–761. [PubMed] [Google Scholar]
- 141.Khadour FH, Panas D, Ferdinandy P, et al. Enhanced NO and superoxide generation in dysfunctional hearts from endotoxemic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1108–H1115. doi: 10.1152/ajpheart.00549.2001. [DOI] [PubMed] [Google Scholar]
- 142.Lancel S, Tissier S, Mordon S, et al. Peroxynitrite decomposition catalysts prevent myocardial dysfunction and inflammation in endotoxemic rats. J Am Coll Cardiol. 2004;43:2348–2358. doi: 10.1016/j.jacc.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 143.Soriano FG, Nogueira AC, Caldini EG, et al. Potential role of poly(adenosine 5′-diphosphate-ribose) polymerase activation in the pathogenesis of myocardial contractile dysfunction associated with human septic shock. Crit Care Med. 2006;34:1073–1079. doi: 10.1097/01.CCM.0000206470.47721.8D. [DOI] [PubMed] [Google Scholar]
- 144.Flesch M, Kilter H, Cremers B, et al. Effects of endotoxin on human myocardial contractility involvement of nitric oxide and peroxynitrite. J Am Coll Cardiol. 1999;33:1062–1070. doi: 10.1016/s0735-1097(98)00660-3. [DOI] [PubMed] [Google Scholar]
- 145.Alexander B. The role of nitric oxide in hepatic metabolism. Nutrition. 1998;14:376–390. doi: 10.1016/s0899-9007(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 146.Spapen H. Liver perfusion in sepsis, septic shock, and multiorgan failure. Anat Rec (Hoboken) 2008;291:714–720. doi: 10.1002/ar.20646. [DOI] [PubMed] [Google Scholar]
- 147.D’Ambrosio SM, Gibson-D’Ambrosio RE, Brady T, Oberyszyn AS, Robertson FM. Mechanisms of nitric oxide-induced cytotoxicity in normal human hepatocytes. Environ Mol Mutagen. 2001;37:46–54. doi: 10.1002/1098-2280(2001)37:1<46::aid-em1005>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 148.Watanabe N, Miura S, Zeki S, Ishii H. Hepatocellular oxidative DNA injury induced by macrophage-derived nitric oxide. Free Radic Biol Med. 2001;30:1019–1028. doi: 10.1016/s0891-5849(01)00498-1. [DOI] [PubMed] [Google Scholar]
- 149.Gero D, Szabo C. Role of the peroxynitrite-poly (ADP-ribose) polymerase pathway in the pathogenesis of liver injury. Curr Pharm Des. 2006;12:2903–2910. doi: 10.2174/138161206777947579. [DOI] [PubMed] [Google Scholar]
- 150.Shu Z, Jung M, Beger HG, et al. pH-dependent changes of nitric oxide, peroxynitrite, and reactive oxygen species in hepatocellular damage. Am J Physiol. 1997;273:G1118–G1126. doi: 10.1152/ajpgi.1997.273.5.G1118. [DOI] [PubMed] [Google Scholar]
- 151.Seven I, Türközkan N, Cimen B. The effects of nitric oxide synthesis on the Na+, K(+)-ATPase activity in guinea pig kidney exposed to lipopolysaccharides. Mol Cell Biochem. 2005;271:107–112. doi: 10.1007/s11010-005-5616-1. [DOI] [PubMed] [Google Scholar]
- 152.Cimen B, Türközkan N, Seven I, Unlü A, CK Impaired Na+,K+-ATPase activity as a mechanism of reactive nitrogen species-induced cytotoxicity in guinea pig liver exposed to lipopolysaccharides. Mol Cell Biochem. 2004;259:53–57. doi: 10.1023/b:mcbi.0000021344.64317.a2. [DOI] [PubMed] [Google Scholar]
- 153.Yokozawa T, Rhyu DY, Cho EJ, Aoyagi K. Protective activity of (−)-epicatechin 3-O-gallate against peroxynitrite-mediated renal damage. Free Radic Res. 2003;37:561–571. doi: 10.1080/1071576031000083134. [DOI] [PubMed] [Google Scholar]
- 154.Yokozawa T, Rhyu DY, Cho EJ. (−)-Epicatechin 3-O-gallate ameliorates the damages related to peroxynitrite production by mechanisms distinct from those of other free radical inhibitors. J Pharm Pharmacol. 2004;56:231–239. doi: 10.1211/0022357022601. [DOI] [PubMed] [Google Scholar]
- 155.Mota-Filipe H, McDonald MC, Cuzzocrea S, Thiemermann C. A membrane-permeable radical scavenger reduces the organ injury in hemorrhagic shock. Shock. 1999;12:255–261. doi: 10.1097/00024382-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 156.Zacharowski K, Olbrich A, Cuzzocrea S, Foster SJ, Thiemermann C. Membrane-permeable radical scavenger, tempol, reduces multiple organ injury in a rodent model of gram-positive shock. Crit Care Med. 2000;28:1953–1961. doi: 10.1097/00003246-200006000-00044. [DOI] [PubMed] [Google Scholar]
- 157.Chatterjee PK, Cuzzocrea S, Brown PA, et al. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58:658–673. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 158.Di Paola R, Mazzon E, Muia C, et al. Protective effect of Hypericum perforatum in zymosan-induced multiple organ dysfunction syndrome: relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. Nitric Oxide. 2007;16:118–130. doi: 10.1016/j.niox.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 159.Tsukada K, Hasegawa T, Tsutsumi S, et al. Effect of uric acid on liver injury during hemorrhagic shock. Surgery. 2000;127:439–446. doi: 10.1067/msy.2000.104486. [DOI] [PubMed] [Google Scholar]
- 160.Ogetman Z, Dirlik M, Caglikulekci M, et al. The effect of aminoguanidine on blood and tissue lipid peroxidation in jaundiced rats with endotoxemia induced with LPS. J Invest Surg. 2006;19:19–30. doi: 10.1080/08941930500444396. [DOI] [PubMed] [Google Scholar]
- 161.Harrois A, Huet O, Duranteau J. Alterations of mitochondrial function in sepsis and critical illness. Curr Opin Anaesthesiol. 2009;22:143–149. doi: 10.1097/ACO.0b013e328328d1cc. [DOI] [PubMed] [Google Scholar]
- 162.Fink MP. Bench-to-bedside review: Cytopathic hypoxia. Crit Care. 2002;6:491–499. doi: 10.1186/cc1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rhyu DY, Kang KS, Sekiya M, Tanaka T, Park JC, Yokozawa T. Active compounds isolated from traditional Chinese prescription Wen-Pi-Tang protecting against peroxynitrite-induced LLC-PK(1) cell damage. Am J Chin Med. 2008;36:761–770. doi: 10.1142/S0192415X08006211. [DOI] [PubMed] [Google Scholar]
- 164.Paller MS, Weber K, Patten M. Nitric oxide-mediated renal epithelial cell injury during hypoxia and reoxygenation. Ren Fail. 1998;20:459–469. doi: 10.3109/08860229809045135. [DOI] [PubMed] [Google Scholar]
- 165.Jung JS, Park MY, Lee RH, Jun JS, Kim YK. Protection against hydrogen peroxide induced injury in renal proximal tubule cell lines by inhibition of poly(ADP-ribose) synthase. Kidney Blood Press Res. 2000;23:14–19. doi: 10.1159/000025949. [DOI] [PubMed] [Google Scholar]
- 166.Filipovic DM, Meng X, Reeves WB. Inhibition of PARP prevents oxidant-induced necrosis but not apoptosis in LLC-PK1 cells. Am J Physiol. 1999;277:F428–F436. doi: 10.1152/ajprenal.1999.277.3.F428. [DOI] [PubMed] [Google Scholar]
- 167.Tikoo K, Lau SS, Monks TJ. Histone H3 phosphorylation is coupled to poly-(ADP-ribosylation) during reactive oxygen species-induced cell death in renal proximal tubular epithelial cells. Mol Pharmacol. 2001;60:394–402. doi: 10.1124/mol.60.2.394. [DOI] [PubMed] [Google Scholar]
- 168.Szabo C, Saunders C, O’Connor M, Salzman AL. Peroxynitrite causes energy depletion and increases permeability via activation of poly (ADP-ribose) synthetase in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 1997;16:105–109. doi: 10.1165/ajrcmb.16.2.9032115. [DOI] [PubMed] [Google Scholar]
- 169.Janssen-Heininger YM, Persinger RL, Korn SH, et al. Reactive nitrogen species and cell signaling: implications for death or survival of lung epithelium. Am J Respir Crit Care Med. 2002;166:S9–S16. doi: 10.1164/rccm.2206008. [DOI] [PubMed] [Google Scholar]
- 170.Ho YS, Liou HB, Lin JK, et al. Lipid peroxidation and cell death mechanisms in pulmonary epithelial cells induced by peroxynitrite and nitric oxide. Arch Toxicol. 2002;76:484–493. doi: 10.1007/s00204-002-0373-3. [DOI] [PubMed] [Google Scholar]
- 171.Lobo SM, Orrico SR, Queiroz MM, et al. Pneumonia-induced sepsis and gut injury: effects of a poly-(ADP-ribose) polymerase inhibitor. J Surg Res. 2005;129:292–297. doi: 10.1016/j.jss.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 172.Javeshghani D, Magder S. Presence of nitrotyrosine with minimal inducible nitric oxide synthase induction in lipopolysaccharide-treated pigs. Shock. 2001;16:304–311. doi: 10.1097/00024382-200116040-00013. [DOI] [PubMed] [Google Scholar]
- 173.Naidu BV, Fraga C, Salzman AL, Szabo C, Verrier ED, Mulligan MS. Critical role of reactive nitrogen species in lung ischemia-reperfusion injury. J Heart Lung Transplant. 2003;22:784–793. doi: 10.1016/s1053-2498(02)00556-9. [DOI] [PubMed] [Google Scholar]
- 174.Maybauer DM, Maybauer MO, Szabo C, et al. Lung-protective effects of the metalloporphyrinic peroxynitrite decomposition catalyst WW-85 in interleukin-2 induced toxicity. Biochem Biophys Res Commun. 2008;377:786–791. doi: 10.1016/j.bbrc.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Martinez-Caro L, Lorente JA, Marin-Corral J, et al. Role of free radicals in vascular dysfunction induced by high tidal volume ventilation. Intensive Care Med. 2009;35:1110–1119. doi: 10.1007/s00134-009-1469-5. [DOI] [PubMed] [Google Scholar]
- 176.Szabo C, Ferrer-Sueta G, Zingarelli B, Southan GJ, Salzman AL, Radi R. Mercaptoethylguanidine and guanidine inhibitors of nitric-oxide synthase react with peroxynitrite and protect against peroxynitrite-induced oxidative damage. J Biol Chem. 1997;272:9030–9036. doi: 10.1074/jbc.272.14.9030. [DOI] [PubMed] [Google Scholar]
- 177.Zingarelli B, Ischiropoulos H, Salzman AL, Szabo C. Amelioration by mercaptoethylguanidine of the vascular and energetic failure in haemorrhagic shock in the anesthetised rat. Eur J Pharmacol. 1997;338:55–65. doi: 10.1016/s0014-2999(97)01325-3. [DOI] [PubMed] [Google Scholar]
- 178.Cuzzocrea S, Zingarelli B, Hake P, Salzman AL, Szabo C. Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic Biol Med. 1998;24:450–459. doi: 10.1016/s0891-5849(97)00280-3. [DOI] [PubMed] [Google Scholar]
- 179.Szabo A, Hake P, Salzman AL, Szabo C. Beneficial effects of mercaptoethylguanidine, an inhibitor of the inducible isoform of nitric oxide synthase and a scavenger of peroxynitrite, in a porcine model of delayed hemorrhagic shock. Crit Care Med. 1999;27:1343–1350. doi: 10.1097/00003246-199907000-00027. [DOI] [PubMed] [Google Scholar]
- 180.Ploner F, Radermacher P, Theisen M, et al. Effects of combined selective iNOS inhibition and peroxynitrite blockade during endotoxemia in pigs. Shock. 2001;16:130–136. doi: 10.1097/00024382-200116020-00008. [DOI] [PubMed] [Google Scholar]
- 181.Liaudet L, Szabo A, Soriano FG, Zingarelli B, Szabo C, Salzman AL. Poly (ADP-ribose) synthetase mediates intestinal mucosal barrier dysfunction after mesenteric ischemia. Shock. 2000;14:134–141. doi: 10.1097/00024382-200014020-00010. [DOI] [PubMed] [Google Scholar]
- 182.Cuzzocrea S, Mazzon E, Dugo L, et al. Inducible nitric oxide synthase-deficient mice exhibit resistance to the acute pancreatitis induced by cerulein. Shock. 2002;17:416–422. doi: 10.1097/00024382-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 183.el-Dwairi Q, Comtois A, Guo Y, Hussain SN. Endotoxin-induced skeletal muscle contractile dysfunction: contribution of nitric oxide synthases. Am J Physiol. 1998;274:C770–C779. doi: 10.1152/ajpcell.1998.274.3.C770. [DOI] [PubMed] [Google Scholar]
- 184.Alvarez S, Boveris A. Mitochondrial nitric oxide metabolism in rat muscle during endotoxemia. Free Radic Biol Med. 2004;37:1472–1478. doi: 10.1016/j.freeradbiomed.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 185.Salvemini D, Riley DP, Lennon PJ, et al. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br J Pharmacol. 1999;127:685–692. doi: 10.1038/sj.bjp.0702604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stefanutti G, Pierro A, Smith VV, Klein NJ, Eaton S. Peroxynitrite decomposition catalyst FeTMPyP provides partial protection against intestinal ischemia and reperfusion injury in infant rats. Pediatr Res. 2007;62:43–48. doi: 10.1203/PDR.0b013e31806790c0. [DOI] [PubMed] [Google Scholar]
- 187.Cuzzocrea S, Misko TP, Costantino G, et al. Beneficial effects of peroxynitrite decomposition catalyst in a rat model of splanchnic artery occlusion and reperfusion. FASEB J. 2000;14:1061–1072. doi: 10.1096/fasebj.14.9.1061. [DOI] [PubMed] [Google Scholar]
- 188.Cuzzocrea S, Costantino G, Mazzon E, De Sarro A, Caputi AP. Beneficial effects of Mn(III)tetrakis (4-benzoic acid) porphyrin (MnTBAP), a superoxide dismutase mimetic, in zymosan-induced shock. Br J Pharmacol. 1999;128:1241–1251. doi: 10.1038/sj.bjp.0702826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nin N, Cassina A, Boggia J, et al. Septic diaphragmatic dysfunction is prevented by Mn(III)porphyrin therapy and inducible nitric oxide synthase inhibition. Intensive Care Med. 2004;30:2271–2278. doi: 10.1007/s00134-004-2427-x. [DOI] [PubMed] [Google Scholar]