Abstract
Retrieval of episodic memories is a multi-component act that relies on numerous operations ranging from processing the retrieval cue, evaluating retrieved information, and selecting the appropriate response given the demands of the task. Motivated by a rich functional neuroimaging literature, recent theorizing about various computations at retrieval has focused on the role of posterior parietal cortex (PPC). In a potentially promising line of research, recent neuroimaging findings suggest that different subregions of dorsal PPC respond distinctly to different aspects of retrieval decisions, suggesting that better understanding of their contributions might shed light on the component processes of retrieval. In an attempt to understand the basic operations performed by dorsal PPC, we used functional MRI and functional connectivity analyses to examine how activation in, and connectivity between, dorsal PPC and ventral temporal regions representing retrieval cues varies as a function of retrieval decision uncertainty. Specifically, participants made a five-point recognition confidence judgment for a series of old and new visually presented words. Consistent with prior studies, memory-related activity patterns dissociated across left dorsal PPC subregions, with activity in the lateral IPS tracking the degree to which participants perceived an item to be old, whereas activity in the SPL increased as a function of decision uncertainty. Importantly, whole-brain functional connectivity analyses further revealed that SPL activity was more strongly correlated with that in the visual word-form area during uncertain relative to certain decisions. These data suggest that the involvement of SPL during episodic retrieval reflects, at least in part, the processing of the retrieval cue, perhaps in service of attempts to increase the mnemonic evidence elicited by the cue.
Keywords: recognition memory, declarative memory, decision-making, fMRI, parietal old/new effect
1. Introduction
Conscious memory for individual events from the past—episodic memory—is a powerful source for informing present decisions, large and small. The ability to incorporate information from past life episodes into an ongoing decision is critical for an organism to be able to avoid past mistakes and guide actions toward the optimal outcome. Despite the fundamental utility of retrieving episodic information from the past, much is still unsettled about the component cognitive and neurobiological operations that give rise to remembering.
One aspect of the cognitive neuroscience of remembering that has given rise to recent debate is how to interpret functional neuroimaging results that suggest that left posterior parietal cortex (PPC) is robustly engaged during episodic memory retrieval (Wagner et al., 2005). Specifically, numerous functional magnetic resonance imaging (fMRI) studies indicate that activity in multiple subregions of left lateral PPC is greater during the correct recognition of previously encountered items as old (i.e., hits) versus correct classification of novel items as new (i.e., correct rejections; for review, see Wagner et al., 2005; Cabeza, 2008; Cabeza et al., 2008; Vilberg and Rugg, 2008; Olson and Berryhill, 2009). At a coarse anatomical level, it has been argued that activity in more dorsal PPC regions –– the superior parietal lobe (SPL) and intra-parietal sulcus (IPS) –– tracks the degree to which a memory probe is perceived as old (perhaps tracking perceived item familiarity, e.g. Henson et al., 1999; Sharot et al., 2004; Wheeler and Buckner, 2004), whereas activity in ventral PPC –– specifically, angular gyrus (AnG) –– tracks the degree to which additional contextual details from the study episode are remembered (perhaps tracking recollection, e.g Eldridge et al., 2000; Cansino et al., 2002; Kahn et al., 2004; Sharot et al., 2004; Wheeler and Buckner, 2004; Woodruff et al., 2005; Kensinger and Schacter, 2006; Montaldi et al., 2006).
While much initial interest focused on characterizing functional distinctions between dorsal and ventral PPC responses during episodic retrieval, recent findings suggest that within these coarse anatomical subdivisions, further functional distinctions are present. Of particular interest for the current study is the observation that retrieval activity in SPL is functionally dissociable from that in lateral IPS (Hutchinson et al., 2009; Nelson et al., 2010; Sestieri et al., 2010; Hutchinson et al., 2014). In particular, activity in SPL (and medial IPS) appears to vary with retrieval decision uncertainty, with elevated activity during slower or less confident memory decisions (Cabeza et al., 2008; Sestieri et al., 2010; Hutchinson et al., 2014), whereas activity in regions along the fundus and lateral bank of the IPS appears to increase in relation to the perceived oldness of the memory probe (e.g., Daselaar et al., 2006; Hutchinson et al., 2014).
Concurrent with the emergence of neuroimaging evidence for the multiple roles of dorsal PPC at retrieval has been a growing debate over how to best interpret these findings. This debate is complicated by the fact that on one hand dorsal PPC displays varied and meaningful sensitivity to key internal variables such as subjective memory strength and decision confidence during retrieval, but on the other hand it is also robustly engaged across a wide range of tasks designed to explore perception- and motor- related processes (e.g. Culham and Valyear, 2006; Silver and Kastner, 2009). Thus, many interpretations of the region’s mechanistic role at retrieval have focused on its position at the intersection of internal and external processing. For example, some have posited that the region might serve to ‘accumulate’ mnemonic evidence (internal) in order to guide a particular decision (Wagner et al., 2005). Another interpretation posits that dorsal PPC performs similar operations of goal-directed (‘top-down’) attention across both internal (e.g., the retrieved contents of memory) and external (e.g., the perceptual cue used to probe memory) information (e.g. Cabeza, 2008; Cabeza et al., 2008; Ciaramelli et al., 2008).
Across both of these interpretations, there is a relatively unexplored common question concerning the nature of the representations with which dorsal PPC might interact during retrieval. That is, do the operations performed by dorsal PPC concern interactions with the external environment through, e.g., the perception of the retrieval probe or do they concern the processing of internal mnemonic signals more tightly linked to episodic retrieval? Moreover, are these operations differentially performed by different subregions of dorsal PPC?
The current experiment sought to further delineate the multi-faceted contributions of dorsal PPC to episodic retrieval by assessing a) the response profiles of dorsal PPC subregions in the face of decision uncertainty, and b) which other regions of the brain respond in a similar fashion and functionally interact with dorsal PPC subregions. In particular, decision certainty during a recognition memory task was measured by having subjects make five-point confidence judgments about the old/new status of test cues. Critically, a series of fMRI analyses were performed to 1) further test whether SPL and lateral IPS demonstrate functionally dissociable activity profiles during recognition memory decisions, and 2) explore the degree to which dorsal PPC contributions to episodic memory might reflect internal or external processing. In particular, insofar as engagement of SPL reflects, at least in part, increased processing of the retrieval cue under uncertain retrieval decisions, we predicted that SPL would demonstrate increased functional coupling with regions in visual cortex that code for the visual aspects of the retrieval cue (also see Dobbins and Wagner, 2005).
2. Materials and Methods
2.1 Participants
Thirty-five healthy adults participated in the study. Participants were right- handed, native English speakers, with no history of neurological disease or contraindications for MR imaging. Data from two participants were excluded due to imaging artifacts; data were also excluded from two participants due to excessive movement, and from five additional participants due to poor recognition memory (average d’ < 0.4 across old/new and confidence judgment tasks) or insufficient number of trials in conditions of interest (5 or fewer trials). Accordingly, a total of 26 participants were included in the final data set (8 female, ages 19–28 yrs). Participants were paid $20/hr for the experiment, which lasted approximately 3.5 hrs. All participants gave informed, written consent in accordance with procedures approved by the institutional review board at Stanford University.
2.2 Materials
Stimuli consisted of 620 visually presented adjectives, taken from a corpus used in several previous fMRI studies (Davachi et al., 2003; Kahn et al., 2004; Hutchinson et al., 2014). The adjectives ranged in length from 3 to 10 letters (mean = 6.93). Twenty adjectives were used during a practice session. Of the 600 remaining items, 300 were presented during an encoding phase and served as old items during the retrieval phases, and 300 served as new items (foils) during retrieval. Old and new items were split evenly between two retrieval tasks (old/new and confidence judgment). Trial order was pseudo-randomized so as to not contain more than three consecutive trials of a given condition. The order of conditions was determined using an optimal sequencing algorithm that maximized the efficiency of the event-related design (OptSeq; Dale, 1999). The algorithm also determined the duration and frequency of null (fixation) events, which accounted for approximately 1/3 of trials. Across participants, stimuli were counterbalanced to be both studied and novel items at retrieval.
Stimulus presentation and collection of behavioral responses were implemented in Matlab, using the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997) running on an Apple MacBookPro laptop. During the non-scanned encoding phase, stimuli were centrally presented on the laptop monitor and responses (button presses) were made on the laptop keyboard. During the scanned memory retrieval phases, stimuli were projected onto a screen and viewed through a mirror on the head coil, and responses (button presses) were made using an MR-compatible response box. All responses in the experiment were made with the right hand.
2.3 Procedure
The experiment consisted of two phases: an incidental study phase administered outside of the scanner, and a test phase conducted during fMRI. The test phase consisted of two different tasks: a recognition confidence task followed by an old/new recognition task. The latter task (old/new recognition) was administered for a purpose irrelevant to the current study and will not be discussed further. Both study and test phases were preceded by a brief practice round containing a set of trials with identical structures to the actual task. The recognition confidence task was additionally preceded by a response training session, wherein participants practiced making the five responses that would be used during that task.
In the study phase, each trial began with presentation of a task cue (750 ms), followed by an adjective (750 ms), a delay (3000 ms), and a response period (1500 ms; Figure 1). One of four task cues prompted participants to covertly generate an instance of one of four types of referents best described by the subsequently presented adjective: the task cues “Person – Male” and “Person – Female” prompted covert generation of the name of a male or female celebrity, respectively, whereas the cues “Scene – Indoor” and “Scene – Outdoor” prompted generation of a mental image of an indoor or outdoor scene, respectively. The subsequent stimulus period displayed an adjective in capitalized black letters on a white background. Participants used the stimulus and delay periods to generate a specific scene or person (3750ms total). During the delay period, a black fixation cross replaced the adjective, and then turned to red to instruct participants to make a response reflecting their generation success for that trial. Participants made one of four button presses describing their success: successful with ease, successful with effort, partially successful, or completely unsuccessful.
Figure 1.
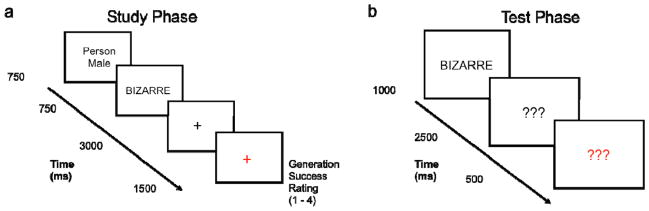
Task Design (a) Schematic of a single trial during the unscanned encoding phase. Participants were cued to generate an instance of a male or female celebrity (person generation task) or an indoor or outdoor scene (scene generation task). At the end of each trial, a red fixation signaled participants to report their success in generating a person or scene described by the adjective. (b) Schematic of a single trial during the scanned retrieval phase. Participants were presented with previously studied and novel words and made a recognition confidence judgment (see text for details).
Following the study phase, participants were informed that their memory for the studied words would be tested during scanning, and were then given instructions for the response training session and test phase. Participants performed the response training session while anatomical MR images were acquired. In the training session, labels (numbers from one to five) of the responses required during the test phase (see below) were visually presented one at a time at fixation, cycling through all five possible responses in random order. Labels were presented for 2000 ms each. On each trial, participants were to make a button press that mapped to the appropriate response (see below). The session continued until the participant responded to the entire response set twice and performance was monitored to ensure that the subject understood the response mappings. After completion of the training session, the test phase began. Across participants, approximately 20 to 30 min lapsed between the end of the study phase and the beginning of the test phase.
The recognition confidence test phase was subdivided into 5 separate scanning runs. Subjects were randomly assigned to one of two groups: the Memory Detection group or the Novelty Detection group (n = 13 for each group). Subjects in both groups were told that they would see old and new items, and that they would be making a five-point confidence judgment for each item. Members of the Memory Detection group were told that they should indicate how confident they were that the item was old (i.e., that it had been encountered during the study phase of the experiment) using a one to five scale. Those in the Novelty Detection group were told to indicate how confident they were that the item was novel (i.e., that it had not been encountered in the study phase of the experiment) using a one to five scale. Both groups were instructed that the scale ranged from ‘most confident’ to ‘least confident’ with intermediate responses indicating intermediate degrees of confidence along the scale, but with no explicit labeling. As we describe below (see Results), there were no significant behavioral differences observed between the Memory Detection and Novelty Detection groups. Accordingly, for condition labeling purposes below, responses were organized with 1 indicating high confidence that the item is old (‘most confident’ responses for the Memory Detection group and ‘least confident’ for the Novelty Detection group), 5 indicating high confidence that the item is new (‘least confident’ responses for the Memory Detection group and ‘most confident’ for the Novelty Detection group) and 2 through 4 indicating the intermediate increments of confidence.
For each trial, an old or new word was centrally presented in black text on a white background for 1000 ms, followed by a set of black question marks for 2500 ms (Figure 1). The end of the trial was signaled by the black question marks turning red for 500 ms and participants were allowed to respond at any point during the trial. Participants were instructed to make the response that best characterized their memory for the item. Inter-trial intervals (null events) consisted of a black fixation cross on a white background.
2.4 fMRI data acquisition
All anatomical and functional data were acquired using a 3.0T Signa MRI system (GE Medical Systems). Four of the participants were scanned at a separate scanner (identical field strength and make) than the others, with slightly different acquisition parameters. For these four participants, the first anatomical series was collected using a T2-weighted flow-compensated spin-echo pulse sequence. The anatomical images were acquired with identical prescription to the functional images (TR = 4.5 s; TE = 80 ms; 30 contiguous 4-mm-thick slices parallel to the AC-PC plane). The second anatomical series was a T1-weighted high-resolution acquisition of the entire brain (TR = 8.368 ms; TE = 1.784 ms; flip angle = 15; FOV = 22 cm; 256 x 256 voxels; 124 contiguous 1.5-mm-thick slices). Functional images were collected using a T2*-weighted two-dimensional gradient echo spiral-in/out pulse sequence (TR = 2s; TE = 30 ms; 1 interleave; flip angle = 75; FOV = 22 cm; 64 x 64 voxels). For the remaining 22 participants, there was a different set of parameters for the T2-weighted anatomical (TR = 3 s; TE = 72 ms; 30 contiguous 4-mm-thick slices parallel to the AC-PC plane) and the T2*-weighted functional images (TR = 2s; TE = 30 ms; 1 interleave; flip angle = 75; FOV = 21 cm; 64 x 64 voxels).
2.5 fMRI data analysis
2.5.1 General analysis procedures
Data were analyzed using SPM5 (Wellcome Department of Cognitive Neurology, London). The first six TRs were discarded and functional volumes were corrected for slice acquisition timing differences and then motion corrected. For each participant, the high-resolution structural volume was coregistered to an average of the functional volumes, and then segmented into cortical grey matter, white matter, and cerebrospinal fluid. The grey matter volume was normalized to the MNI grey matter template, and the normalization parameters were applied to all the functional volumes. Functional volumes were resampled into 3mm3 voxels and smoothed with an 8-mm full-width half-maximum isotropic Gaussian kernel.
Statistical analyses were performed using the general linear model (GLM) across a total of four different models. For all four models, each trial was modeled as a single event at stimulus onset (i.e., the appearance of the word), and convolved with a canonical hemodynamic response function (HRF), and in all cases except the parametric modulation analysis (see below) the temporal and dispersion derivatives were modeled as well (Friston et al., 1998). With the exception of one model assessing the impact of the veridical old/new status of the memory probes, trials were grouped into conditions based on the response of the subject, collapsing across veridical old/new status of items, thus producing five critical regressors of interest (one for each confidence judgment along the five point scale). To accommodate the remainder of the known variance, events of no interest (i.e., when the participant did not make a response) were modeled with a separate regressor. All scanning sessions were concatenated and the resulting functions were entered into a GLM with session and movement parameters treated as covariates. The timeseries was high-pass filtered to remove low-frequency noise (1/128 Hz and below). First-level linear contrasts, as well as quadratic and parametric contrasts (see below), were calculated to produce estimates of effects for each participant. The estimates were then entered into a second-level analysis, using participant as a random effect. One-sample t-tests against a contrast value of zero were performed at each voxel.
2.5.2 Statistical models
A total of four models were run: the first model explored the key condition-wise effects of interest, the second examined how veridical old/new status interacted with confidence ratings, the third assessed how neural activity varied with reaction time, and the fourth characterized how between-region correlations in the BOLD signal during the confidence judgment task varied as a function of condition. Our primary analyses targeted two a priori regions of interest (ROIs) in dorsal PPC (SPL and lateral IPS; see below for details), and entailed two stages—examination of the observed effects in the a priori ROIs (using the first three models) and then exploratory voxel-level statistical tests (using the first and fourth models). Finally, these primary analyses were followed by exploratory ROI analysis, wherein several prefrontal cortical regions were examined to further assess their response properties as a function of retrieval decision confidence (using the first model). We first delineate each model, and then detail the ROIs examined.
The first model was designed to establish the response within ROIs across confidence ratings, as well as to explore three contrasts of interest at the whole-brain level. Specifically, contrasts were created to reveal regions that 1) displayed a positive linear relationship with perceived memory strength, 2) displayed a positive relationship with decision uncertainty, or 3) displayed a positive relationship with decision certainty. A positive linear relationship with ‘perceived memory strength’ used the weights of 2, 1, 0, -1, and -2 across response conditions (with beta estimates of the most confident old response condition being multiplied by 2; low confidence old being multiplied by 1, etc.). Relationships with decision uncertainty/certainty were measured using quadratic contrast weights. That is, weighting coefficients of −2, 1, 2, 1, and −2 across the five response conditions were used to assess which regions positively tracked decision uncertainty, and the inverse (2, −1, −2, −1, 2) for assessing which positively tracked decision certainty.
A second model was created to assess how veridical old/new status modulated activity across confidence responses. Specifically, for each confidence response, trials were separated into whether they had actually been encountered during the study phase (i.e., were Old) or not (i.e., were New). Due to the small number of trials in particular bins, the data were collapsed such that New items receiving the top two responses on the ‘old’ side of the response scale were grouped (1 and 2 responses in Table 1), and Old items receiving the top two responses on the ‘new’ side of the scale were grouped (4 and 5 responses in Table 1). Thus, the modeled conditions were: Old 1, Old 2, Old 3, Old 4/5, New 1/2, New 3, New 4, and New 5. Even with collapsed bins, two subjects were excluded from these analyses due to small bin sizes (fewer than 5 trials in a bin). To perform ANOVAs on these data, the resulting beta coefficients were combined so as to roughly equate confidence ratings across the veridical old and new conditions. That is, ANOVAs consisted of two factors: veridical old/new status (two levels: Old and New) and confidence level [three levels: Possibly Old (average of Old 1 and Old 2 or New 1/2), Unsure (Old 3 or New 3), and Possibly New (Old 4/5 or average of New 4 and New 5)].
Table 1.
| Response | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Old Item | Mean: SD: |
0.53 0.14 |
0.16 0.08 |
0.11 0.05 |
0.13 0.07 |
0.08 0.06 |
| New Item | Mean: SD: |
0.11 0.08 |
0.11 0.04 |
0.15 0.09 |
0.27 0.10 |
0.36 0.18 |
The third model implemented a parametric modulation analysis designed to assess where activity varied according to trial-by-trial reaction time (RT). Here, each critical trial type was modeled as in the first model (collapsed across veridical old/new status), and orthogonal regressors modulated each of these trial types according to the RT associated with each event (trial) in the regressor. As such, the RT regressors permitted the identification of voxels in which activity varied as a linear function of RT on each trial, for each condition.
For the fourth model, functional connectivity analyses were conducted by submitting seed ROIs to psychophysiological interaction (PPI) analysis to determine whether they showed condition-specific correlation with other regions in the brain (Friston et al., 1997). In this manner, we investigated whether the connectivity between various seed regions and other brain regions differed as a function of decision uncertainty. Seed regions consisted of the SPL and lateral IPS ROIs identified in our prior fMRI study that indicated that SPL tracks retrieval decision uncertainty and lateral IPS activity tracks graded memory strength (Hutchinson et al., 2014; see below). For each subject, the data for each seed region was the principal eigenvariate of all voxels within the ROI. These subject-specific timeseries represented the ‘physiological’ component of the PPI. The timeseries were adjusted for variance associated with effects of no interest, and then a deconvolution of the hemodynamic response was performed. The resultant vector was weighted by a contrast vector representing the relevant ‘psychological’ factor, and then reconvolved with the hemodynamic responses. The outcome of this process formed the ‘psychophysiological interaction’, or PPI regressor. This regressor modeled the between-condition difference in regression slopes between each voxel in the brain and the seed region. The PPI regressor was entered into a GLM, along with regressors modeling main effects of the psychological and physiological factors (i.e., the condition contrast vector and the timeseries, respectively). Voxels that surpassed threshold (p < .001, uncorrected; extent threshold of 5 voxels) in these PPI analyses can be interpreted as showing a significant difference in connectivity with the seed region as a function of the specified contrast. Follow-up analyses revealed that the results in a priori predicted regions survived small volume correction [mask included medial/ventral occipital and temporal cortices, excluding hippocampus (see Kuhl et al., 2011; Gordon et al., 2013 for description); considered significant at FDR corrected, p < .05].
2.5.3 ROI Analyses
As described above, there were two sets of ROI analyses: a primary set of analyses focused on dorsal PPC subregions and a secondary set focused on prefrontal cortical regions. The primary ROI analyses were conducted using a priori functional ROIs in left SPL and lateral IPS, independently defined using data from a separate study of PPC retrieval effects (Hutchinson et al., 2014). In that study, the SPL ROI was argued to track top-down attention during retrieval, as it overlapped with topographic maps of visuospatial attention and its activity was positively related to putative attention demands during retrieval as measured by RT. Activity in the lateral IPS ROI was observed to index perceived/graded memory strength across item and source memory judgments. Finally, for the sake of completeness, we report the results from the two ventral PPC ROIs discussed in Hutchinson et al. (2014): left angular gyrus (AnG) and left temporo-parietal junction (TPJ). In the current study, the mean beta estimate (across all voxels within the ROI) for each condition, as estimated in the first three GLMs described above, was extracted for each ROI and submitted to further analysis.
To situate the present PPC findings within a broader neural context, a secondary set of exploratory ROI analyses were performed to assess the functional profile of regions in prefrontal cortex. First, in an analogous approach as for the a priori parietal ROIs described above, three representative prefrontal cortex ROIs were chosen from the Hutchinson et al. (2014) study. They were: 1) a left superior frontal sulcus (SFS) ROI based on the same contrast producing the SPL ROI above, 2) a left middle frontal gyrus (MFG) ROI based on the same contrast producing the IPS ROI above, and 3) a left inferior frontal gyrus (IFG) ROI based on the same contrast producing the AnG ROI above. As results from the TPJ ROI suggest that this region did not reliably vary with retrieval confidence (see Results), the corresponding frontal ROI (right MFG) was not examined. Second, to better understand the response profile of the various prefrontal subregions observed in our voxelwise analyses, we extracted and plotted parameter estimates for several prefrontal ROIs defined on the linear and quadratic contrasts from this study (all significant voxels within an 8-mm radius from a peak coordinate). As these estimates are non-independent from the ROI definition, they are simply plotted for illustrative purposes and no statistical analyses were performed on them.
Within ROIs, linear and quadratic patterns of activity during the confidence judgment task were tested by weighting beta estimates for each condition in the first model above by an appropriate coefficient. That is, for each participant and for each ROI, there were five different beta estimates (one for each condition). Each beta estimate was multiplied by a coefficient with the sum of the coefficients equaling zero. This produced a single value for each participant for each ROI, which could then be compared to zero using a t-test (note that this procedure is largely the same as that used to test for voxelwise effects at the group level).
2.5.4 Bootstrap analyses of key effects of interest
In addition to the analyses outlined above, the critical subset of the statistical comparisons made was subjected to additional non-parametric bootstrap analyses to further assess the reliability of the effects. Specifically, one set of analyses was conducted to assess the reliability across subjects of the linear and quadratic patterns of activity during the confidence judgment task and a second set of analyses was conducted to assess the reliability of the findings from the PPI analyses.
First, to assess the reliability of the primary ROI results across subjects, we compared the observed weighted beta estimates to a bootstrapped null distribution. That is, as described above, each participant had a single weighted beta estimate for either the linear or the quadratic relationship across confidence conditions. For each ROI, the observed mean across participants was compared to a null distribution of sample means generated by multiplying a random subset of participants’ estimates by -1 across 5000 iterations. In terms of statistical significance, the p-value was then taken to be the proportion of the 5000 iterations that had a more extreme value than the observed mean.
Second, to assess the reliability of the PPI analyses, we performed a bootstrap analysis wherein trial identities were shuffled and a null distribution of the average PPI estimate across participants was used to assess significance. As performing this analysis in a voxelwise manner using the GLM was computationally burdensome, a more targeted ROI-based approach was used. Specifically, based on the outcome of the two PPI analyses using SPL and IPS as seed regions (see Results), there was evidence for task-based correlation between both seed regions and the left visual word form area (VWFA). Accordingly, we sought to assess the reliability between SPL and the VWFA (the latter defined independently as all significant voxels within 8-mm of the VWFA peak from the voxelwise IPS PPI analysis) and IPS and VWFA (defined from the voxelwise SPL PPI analysis). The PPI estimate was derived by generating the PPI regressor in SPM5 for the seed ROI (SPL or IPS) in the same manner as described above and then performing a partial correlation with the timecourse of the VWFA ROI, removing the influence of other regressors as would be done in the PPI GLM analysis (i.e., the timecourse of the seed ROI, the convolved task regressor, and session and movement regressors). For each participant this PPI estimate was calculated, as well as 100 null estimates wherein the trial identity was shuffled, but everything else was kept the same. To assess the significance of the observed sample mean, it was compared to a null distribution wherein 1 of the 100 null values for each participant was randomly selected and averaged to produce a null sample mean value across 1000 iterations. As before, the p-value was then taken to be the proportion of the 1000 iterations that had a more extreme value than the observed mean.
3 Results
3.1 Behavioral results
3.1.1 Study phase
Participants were successful at generating names/scenes for the word cues. The mean proportion of responses across subjects were: ‘successful with ease’ 47.5% (SD = 16.4%), ‘successful with effort’ 22.2% (SD = 9.5%), ‘partially successful’ 17.9% (SD = 9.1%), and ‘completely unsuccessful’ 12.5% (SD = 11.1%).
3.1.2 Test phase
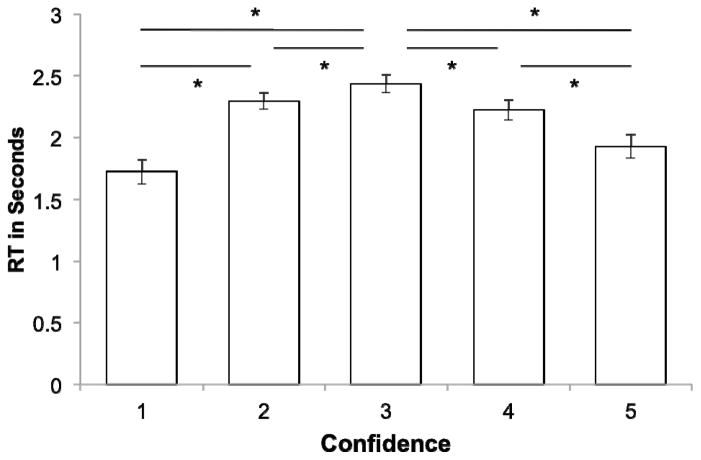
For the confidence judgment task, a measure of recognition accuracy, d’, was calculated for each participant based on their responses to old and new items. Specifically, confidence-based receiver operating characteristics were fit using minimization of the sum of squared errors in order to produce the d’ parameter. Participants had an average d’ of 1.30 (SD = .33), which was significantly greater than zero (p < .001; also see Table 1 for responses by old/new status). Median RTs varied significantly with recognition confidence in a quadratic, inverted U-shaped pattern [Figure 2; test of quadratic pattern (using same approach as for ROI beta estimates; see Methods), p < .001].
Figure 2.
Reaction Times for the Recognition Confidence Judgment Task. Reaction time, in seconds, for each response type. * p < .05. Error bars are standard error of the mean (across subjects) for that response type.
There was no evidence that the instructional manipulation (i.e., Memory Detection vs. Novelty Detection) had an impact on behavioral performance. A 3-way mixed ANOVA of recognition confidence, with factors of group (Memory Detection/Novelty Detection), response (highest to lowest confidence old, with the scale inverted for the ND group), and test probe status (old/new), revealed the absence of a 3-way interaction (p > .4) suggesting no difference in recognition accuracy nor confidence between groups. Similarly, a 3-way mixed ANOVA of RT revealed no interaction (p > .9).
3.2 fMRI analyses
Given the emerging literature demonstrating that: 1) distinct dorsal PPC regions demonstrate functionally dissociable activity patterns during episodic retrieval (Nelson et al., 2010; Sestieri et al., 2010; Hutchinson et al., 2014) and 2) SPL is specifically implicated in both perceptual attention and retrieval-related processes, our analyses focused on the SPL and lateral IPS ROIs (selected from Hutchinson et al., 2014).
Analyses of the fMRI data consisted of three main components. First, each of the parietal ROIs from Hutchinson et al. (2014) was analyzed to determine its profile of activity across recognition confidence; we further tested for functional dissociations across the ROIs, and we conducted a whole-brain voxelwise analysis to further reveal other neural regions with similar activity profiles. Second, the ROIs were further probed for their sensitivity to within-condition RT. Finally, a series of PPI analyses were performed to assess whether SPL displayed differential connectivity with extra-parietal cortex as a function of the uncertainty of the retrieval decision, with the goal of testing whether the SPL ROI differentially functionally couples with the area of visual cortex that putatively represents the retrieval cues (i.e., the visual word-form area; VWFA) or with other extra-parietal cortical areas. Notably, we also performed a two-way mixed ANOVA using confidence condition and instructional manipulation group as factors. Across all of the significantly modulated a priori ROIs (as well as the VWFA and lateral occipital ROIs resulting from the PPI analyses) discussed below, there were no interactions of condition and group (all ps > .1). Taken with the lack of behavioral differences described above, these results suggest that the instruction framing did not have a meaningful impact, and thus all fMRI analyses collapsed across group.
3.2.1 IPS and perceived memory strength
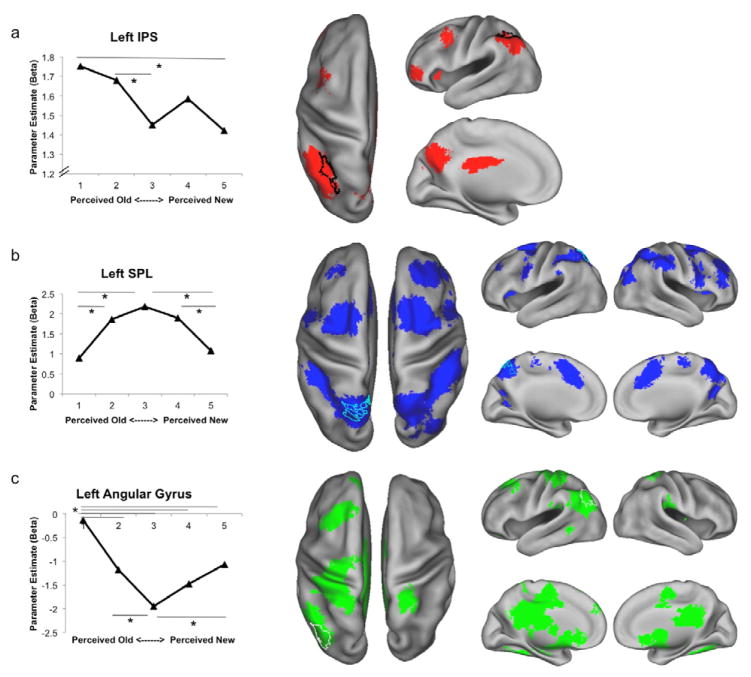
Data were extracted from left lateral IPS (peak coordinates of −30, −54, 39; see Methods) to explore the response profile of the region as a function of recognition confidence. Activity in this region qualitatively displayed a positive linear relationship with perceived memory strength (Figure 3a), which was confirmed (p < .005; bootstrapped p < .001) by a formal test for a linear relationship, multiplying the betas in each condition by the appropriate weighting coefficients (i.e., 2, 1, 0, −1, −2 condition weighting; see Methods). This relationship was not significantly modulated by veridical old/new status, as neither the main effect of old/new status (p > .6) nor the interaction of old/new status and confidence rating (p > .05) reached significance.
Figure 3.
Results from ROI (left panels) and Voxelwise (right panels) Analyses. a) ROI and voxelwise analyses revealed that left IPS (outlined in black) displayed a positive relationship with perceived memory strength, with other regions beyond IPS displaying a similar pattern of activity (shown in red; no regions in the right hemisphere were significantly activated). b) ROI and voxelwise analyses revealed that left SPL (outlined in cyan) displayed greater activity during uncertain retrieval decisions (inverted U-shaped pattern), with other regions displaying similar patterns shown in blue. c) ROI and voxelwise analyses revealed that left AnG (outlined in white) showed the opposite pattern as SPL, displaying increased activity for more certain retrieval decisions, with regions displaying similar patterns shown in green. * p values < .05, all voxelwise comparisons p < .001 uncorrected, 5 voxel extent threshold.
A whole-brain voxelwise contrast targeting effects of perceived memory strength (2, 1, 0, −1, −2; Figure 3a, Table 2) further confirmed this pattern in left lateral IPS/intraparietal lobule, and revealed similar patterns in multiple additional left hemisphere foci, including precuneus, middle frontal gyrus (MFG), insula, and anterior prefrontal cortex (aPFC).
Table 2.
| Positive linear relationship with memory strength: | |||||||
|---|---|---|---|---|---|---|---|
| Anatomical Area | ~BA | Hemi. | x | y | z | Z Score | Size (mm3) |
| Precuneus / Posterior Cingulate | 7/31 | L | −12 | −66 | 36 | 4.78 | 3186 |
| Intraparietal sulcus / Inferior parietal lobule | 7/39/40 | L | −39 | −54 | 45 | 4.5 | 4968 |
| Cingulate cortex | 23 | L | −6 | −24 | 27 | 4.1 | 1134 |
| " | −6 | −12 | 27 | 4.03 | |||
| Middle Frontal Gyrus | 9 | L | −39 | 12 | 42 | 3.61 | 864 |
| Inferior Frontal Gyrus | 47 | L | −42 | 48 | −6 | 3.58 | 837 |
| Inferior Frontal Gyrus | 47 | L | −33 | 21 | −3 | 3.41 | 162 |
| Decision uncertainty (inverted U-shaped pattern): | |||||||
|---|---|---|---|---|---|---|---|
| Anatomical Area | ~BA | Hemi. | x | y | z | Z Score | Size (mm3) |
| Superior Parietal Lobe | 7 | R | 15 | −69 | 57 | 6.37 | 29322 |
| Superior Parietal Lobe | 7 | L | −9 | −72 | 57 | 5.74 | |
| Supramarginal Gyrus | 40 | −42 | −39 | 42 | 5.71 | ||
| Superior Frontal Sulcus | 6/8 | L | −24 | 0 | 57 | 6.29 | 26892 |
| Superior Frontal Sulcus | 6/8 | R | 27 | 6 | 54 | 6.23 | |
| " | 18 | 6 | 60 | 5.91 | |||
| Middle Frontal Gyrus | 9/46 | R | 33 | 36 | 39 | 4.84 | 5967 |
| " | 42 | 39 | 24 | 4.02 | |||
| " | 42 | 42 | 15 | 3.84 | |||
| Inferior Frontal Gyrus | 6/44 | R | 54 | 9 | 21 | 4.83 | 2403 |
| Parieto-Occipital Sulcus | 18/19 | R | 21 | −63 | 24 | 4.49 | 1404 |
| Insula | - | R | 36 | 15 | 9 | 4.31 | 1053 |
| " | L | −33 | 18 | 9 | 4.1 | 864 | |
| Angular Gyrus | 19/39 | R | 39 | −78 | 33 | 3.94 | 2268 |
| Precentral Gyrus | 6 | L | −51 | 0 | 33 | 3.76 | 837 |
| Superior Frontal Sulcus | 9 | L | −30 | 39 | 36 | 3.68 | 459 |
| Precentral Gyrus | 4 | R | 3 | −30 | 63 | 3.58 | 486 |
| Parieto-Occipital Sulcus | 18/19 | L | −21 | −63 | 24 | 3.28 | 189 |
| Calcarine Sulcus | 19 | L | −21 | −66 | 6 | 3.14 | 189 |
| Decision certainty (U-shaped pattern): | |||||||
|---|---|---|---|---|---|---|---|
| Anatomical Area | ~BA | Hemi. | x | y | z | Z Score | Size (mm3) |
| Cerebellum | - | R | 3 | −63 | −15 | 5.38 | 21141 |
| " | 27 | −45 | −27 | 5.37 | |||
| Cerebellum | - | L | −30 | −45 | −27 | 5.08 | |
| Angular Gyrus | 39 | L | −42 | −72 | 39 | 5.05 | 9639 |
| " | −39 | −48 | 27 | 3.62 | |||
| Postcentral Gyrus | 2 | L | −24 | −39 | 63 | 4.89 | 61695 |
| Superior Frontal Gyrus | 6 | −9 | −12 | 57 | 4.76 | ||
| Post/Precentral Gyrus | 3/4 | −33 | −33 | 66 | 4.73 | ||
| Middle/Superior Frontal | |||||||
| Gyrus | 8/9 | L | −33 | 24 | 57 | 4.24 | 2295 |
| " | −21 | 33 | 51 | 3.48 | |||
| " | −39 | 15 | 48 | 3.19 | |||
| Postcentral Gyrus Postcentral |
2 | R | 24 | −45 | 72 | 4.16 | 1377 |
| Gyrus/Supramarginal | |||||||
| Gyrus | 2/40 | R | 54 | −30 | 24 | 4 | 1620 |
| " | " | L | −54 | −33 | 24 | 3.67 | 351 |
| Superior Frontal Gyrus | 9/10 | L | −12 | 57 | 27 | 3.58 | 459 |
| Inferior Frontal Gyrus | 6 | R | 48 | 0 | 9 | 3.51 | 189 |
| Inferior Frontal Gyrus | 47 | L | −33 | 36 | −9 | 3.45 | 297 |
| Fusiform Gyrus | 20/36 | L | −36 | −9 | −27 | 3.41 | 135 |
| Middle Temporal Gyrus | 21/37 | L | −63 | −54 | −3 | 3.31 | 162 |
| PPI analysis: Functional coupling with SPL during uncertain decisions: | |||||||
|---|---|---|---|---|---|---|---|
| Anatomical Area | ~BA | Hemi. | x | y | z | Z Score | Size (mm3) |
| Cerebellum | - | R | 33 | −48 | −24 | 4.33 | 3105 |
| Cerebellum | - | 12 | −69 | −24 | 3.91 | ||
| Inferior Occipital Gyrus | 37 | L | −45 | −60 | −12 | 3.86 | 2646 |
| Fusiform Gyrus | 37 | −36 | −54 | −21 | 3.78 | ||
| Inferior Occipital Gyrus | 19 | R | 36 | −84 | −9 | 3.86 | 2349 |
| Middle Occipital Gyrus | 18 | 33 | −93 | 0 | 3.85 | ||
| Precentral Gyrus | 6 | L | −42 | 3 | 30 | 3.79 | 567 |
| Middle Occipital Gyrus | 18/19 | L | −36 | −87 | −3 | 3.54 | 1242 |
| " | −30 | −90 | −12 | 3.28 | |||
| Precentral Gyrus/Anterior | |||||||
| Cingulate | 6/32 | R | 12 | 9 | 51 | 3.46 | 162 |
| Central Sulcus | 3/4 | L | −36 | −30 | 69 | 3.41 | 702 |
| " | −33 | −21 | 69 | 3.39 | |||
| White matter | - | L | −27 | −78 | 6 | 3.4 | 135 |
| Midbrain | L | −6 | −18 | −12 | 3.34 | 162 | |
| PPI analysis: Functional coupling with IPS during uncertain decisions: | |||||||
|---|---|---|---|---|---|---|---|
| Anatomical Area | ~BA | Hemi. | x | y | z | Z Score | Size (mm3) |
| Fusiform Gyrus | 37 | L | −36 | −60 | −18 | 4.53 | 2673 |
| " | −48 | −69 | −12 | 3.4 | |||
| Cerebellum | - | R | 12 | −51 | −12 | 4.4 | 7371 |
| " | 36 | −51 | −24 | 4.36 | |||
| " | 27 | −57 | −24 | 4.17 | |||
| Precentral Gyrus | 4/6 | L | −36 | −21 | 69 | 3.72 | 1512 |
| Intraparietal Sulcus | 7 | L | −24 | −66 | 48 | 3.51 | 324 |
| Superior Frontal Sulcus | 6 | L | −30 | 0 | 63 | 3.44 | 675 |
| " | −27 | −3 | 54 | 3.37 | |||
| Superior Frontal Gyrus | 6 | R | 24 | 3 | 69 | 3.42 | 351 |
| Inferior Occipital Gyrus | 19 | R | 36 | −81 | −12 | 3.4 | 216 |
| Superior Parietal Lobe | 7 | L | −12 | −66 | 60 | 3.28 | 162 |
3.2.2 SPL and decision uncertainty
In contrast to lateral IPS, data extracted from the left SPL ROI (peak coordinates of −12, −63, 60) displayed an inverted U-shaped pattern across recognition confidence (p < .001 for −2, 1, 2, 1, −2 condition weighting; bootstrapped p < .001) revealing that SPL activity increased during uncertain retrieval decisions (Figure 3b). This decision uncertainty BOLD effect mirrors the pattern of RTs across conditions, suggesting a positive relationship between the two (see below). Importantly, the pattern of activity significantly differed between left SPL and left lateral IPS (Region x Condition, p < .001), indicating that these regions functionally dissociate. Finally, SPL was insensitive to the veridical old/new status of the item (main effect of status and interaction of status and confidence, ps > .5).
A whole-brain voxelwise contrast targeting effects of decision uncertainty (−2, 1, 2, 1, −2; Figure 3b, Table 2) confirmed the decision uncertainty effect in bilateral SPL, and revealed similar patterns in multiple additional foci, including bilateral SFS, more anterior and medial regions along the IPS, and right MFG.
3.2.3 Effects of decision RT
The preceding analyses revealed that the two dorsal PPC ROIs displayed sensitivity to decision uncertainty (SPL) or perceived memory strength (lateral IPS). To further characterize the degree to which these ROIs tracked decision uncertainty, we examined whether activity in each ROI co-varied with decision RT. To quantify the relationship between evoked activity in each ROI and decision RT, we performed a parametric modulation analysis of the BOLD response according to within-condition, trial-by-trial RT. This analysis revealed that SPL showed significant (positive) modulation by RT in 3 out of the 5 retrieval conditions whereas IPS did not display any significant modulations. Taken with the above findings, these results strongly suggest that activity in SPL, but not in lateral IPS, tracks decision uncertainty, further documenting the functional distinction between SPL and lateral IPS activity at retrieval.
3.2.4 Functional connectivity during decision uncertainty
Given the aims of the study, a targeted connectivity analysis was performed to shed light on the processes taking place during uncertain decisions. Specifically, it was hypothesized that insofar as uncertain mnemonic decisions prompt increased processing of relevant forms of information, then regions that are more functionally coupled with dorsal PPC might provide insight into the nature of the operations performed by it. Thus, connectivity analyses were performed using the SPL ROI, and for exploratory purposes the lateral IPS ROI, as a seed region (PPI analysis, see Methods section for details). This analysis revealed regions that have greater connectivity with each seed ROI for trials where subjects made a response indicating uncertainty (the middle response ‘3’ on the five-point scale) versus trials where they reported being certain (‘1’ and ‘5’ responses). Importantly, regions in visual cortex, including foci falling at or near the VWFA and the lateral occipital cortex (Figure 4, Table 2), were significantly more correlated with SPL and IPS during uncertain decisions, suggesting the engagement of perceptual processes during difficult mnemonic judgments. Further, additional bootstrap analyses (see Methods) suggested that this task-based correlation was reliable between both SPL and VWFA (p < .001) and IPS and VWFA (p < .01)
Figure 4.
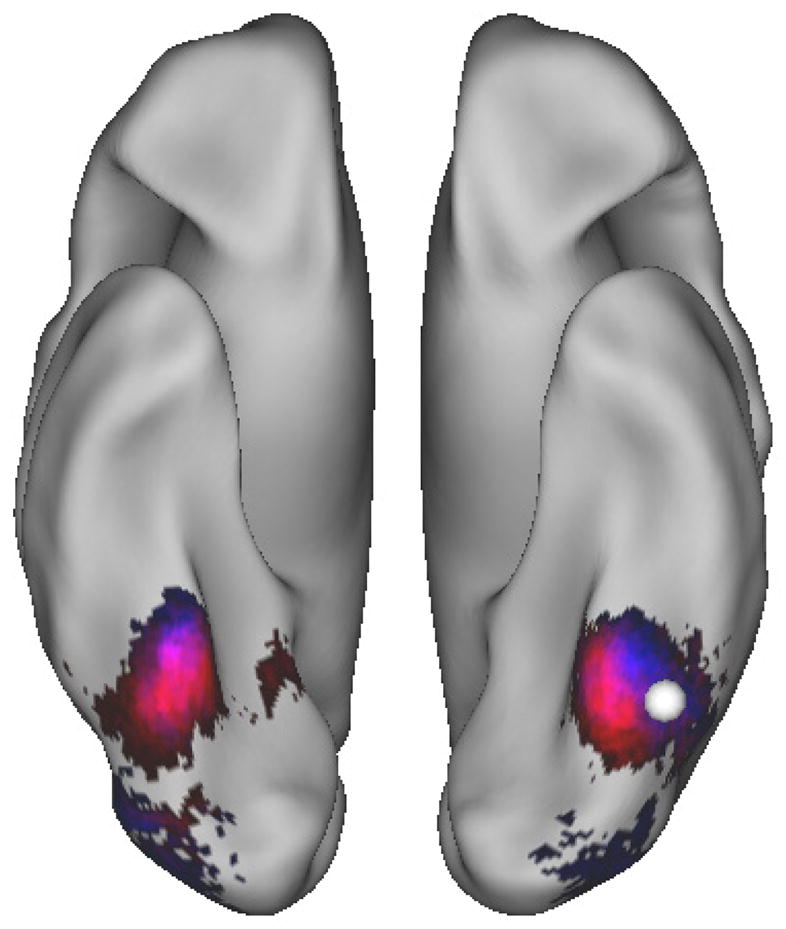
Regions in Visual Cortex Display Uncertainty-modulated Connectivity with SPL and IPS. PPI analyses revealed regions in both the left visual word-form area [compare localization with white VWFA focus (MNI coordinates −44, −58, −15) taken from Jobard et al. (2003); (also see Wandell et al., 2012)] and lateral occipital cortex demonstrated a stronger correlation with SPL (blue regions) and IPS (red regions; overlap in purple) during uncertain versus certain decisions.
3.2.5 Response profiles in ventral PPC
Given the debate over the degree of heterogeneity within ventral PPC during episodic retrieval (Cabeza, 2008; Ciaramelli et al., 2008; Hutchinson et al., 2009; Nelson et al., 2010; Cabeza et al., 2012b; Cabeza et al., 2012a; Nelson et al., 2012), we examined activity from the left temporo-parietal junction (TPJ; peak coordinates of -66, −36, 33; which displayed a pattern of activity consistent with its role in bottom-up attention) and left angular gyrus (AnG; peak coordinates of −39, −72, 42; activity tracked the degree of recollection during retrieval) observed to functionally dissociate in Hutchinson et al. (2014).
First, to assess if these regions were differentially active as a function of recognition confidence in the current experiment, one-way ANOVAs were performed on their evoked activity with recognition confidence as a factor. These analyses revealed that only activity in AnG varied with confidence (p < .001; TPJ: p > .15). Accordingly, all subsequent analysis was confined to AnG.
Data extracted from the AnG ROI revealed a significant U-shaped pattern across recognition confidence (p < .001 for 2, −1, −2, −1, 2 condition weighting; bootstrapped p < .001), revealing that activity positively varied with decision certainty (Figure 3c). It is notable that this effect was asymmetric, such that the increase in activity for items perceived as ‘old’ with high confidence was greater than that for items perceived as ‘new’ with high confidence (p < .05). The pattern of activity in AnG significantly differed from that in SPL (p < .001) and lateral IPS (p < .001), further documenting rich functional heterogeneity across lateral PPC during retrieval. AnG was insensitive to the veridical old/new status of the item (main effect of status and interaction of status and confidence ps > .2).
A whole-brain voxelwise contrast targeting effects of decision certainty (2, −1, −2, −1, 2; Figure 3c, Table 2) confirmed this pattern in left AnG, and revealed numerous additional foci in frontal and subcortical regions, including MFG, aPFC, insula, and striatum.
3.2.6 Response profiles in prefrontal cortex
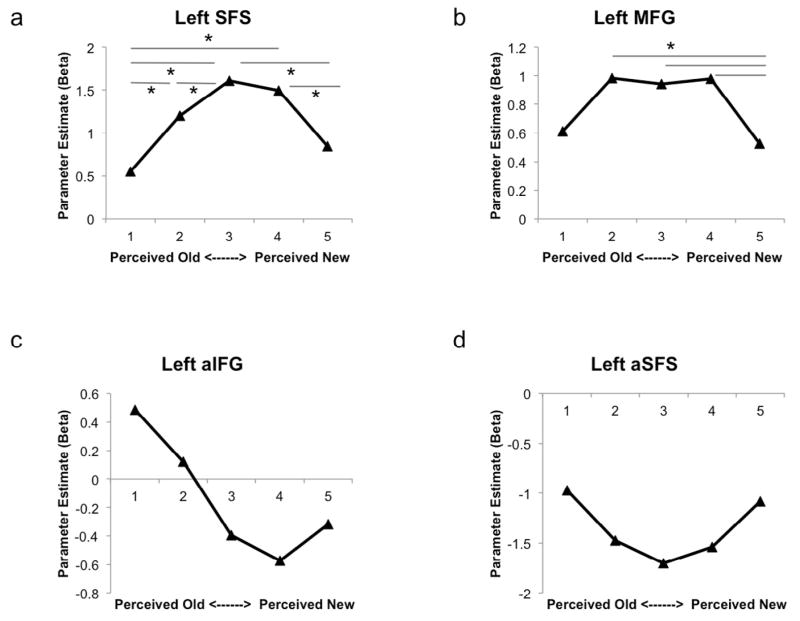
Next, to better understand how prefrontal regions, including those identified in the various voxelwise analyses above, might additionally contribute to decision-related processes during retrieval, we conducted exploratory analyses that examined the response profile across several prefrontal ROIs (see Figure 5). This was done for three a priori ROIs from Hutchinson et al. (2014; SFS, MFG, and IFG, see Methods for details) as well as for two functional ROIs defined off of the linear and quadratic contrasts described above (anterior SFS based on the positive linear ‘memory strength’ contrast and anterior IFG based on the U-shaped ‘decision certainty’ contrast).
Figure 5.
Results from Prefrontal ROI Analyses. Analysis of a priori ROIs taken from Hutchinson et al. (2014) in a) left SFS and b) MFG revealed that both regions displayed increased activation during uncertain recognition decisions, similar to SPL. Functional ROIs in c) left aIFG taken from the positive linear ‘memory strength’ contrast and d) the U-shaped ‘decision certainty’ contrast illustrate the activation in these regions across the confidence judgment task (shown for illustrative purposes only). * p values < .05.
For the a priori ROIs, we first performed a one-way ANOVA to assess if these regions were differentially active across conditions in the current experiment. Although SFS (based on peak coordinates of −21, 6, 66) and MFG (based on peak coordinates of −48, 30, 36) ROIs significantly varied by condition (ps < .05), the left IFG (based on peak coordinates of −45, 40, −3) ROI did not (p > .5), and thus, follow up analyses were limited to SFS and MFG (Figure 5). The left SFS ROI displayed an inverted U-shaped pattern across recognition confidence (p < .001 for −2, 1, 2, 1, −2 condition weighting), revealing a pattern of activity very similar to that of left SPL. The left MFG ROI interestingly also displayed an inverted U-shaped pattern across recognition confidence (p < .05) despite originating from a contrast which also elicited IPS activity in the prior study (Hutchinson et al., 2014).
Next, to further articulate the activity of prefrontal regions involved in recognition confidence judgments, we extracted the parameter estimates for functional ROIs in anterior IFG (centered on the peak coordinate of −42, 48, −6 from the positive linear contrast) and anterior SFS (centered on the peak coordinate of −33, 24, 57 from the U-shaped quadratic contrast). As statistical tests on these estimates would be biased by their definition criteria, the data are plotted for illustrative purposes only (Figure 5).
4. Discussion
The current experiment sought to better understand the role that dorsal PPC plays during episodic retrieval. To this end, we examined the response profile of two left PPC subregions –– SPL and lateral IPS –– as subjects made recognition memory decisions using a five-point confidence scale. These a priori regions of interest displayed dissociable patterns of activity, with SPL activity tracking decision uncertainty and lateral IPS activity tracking perceived memory strength. These findings complement recent findings documenting the functional richness of dorsal PPC during retrieval (Nelson et al., 2010; Sestieri et al., 2010; Nelson et al., 2013; Hutchinson et al., 2014). Moreover, SPL activity varied with decision reaction time on a trial-by-trial basis during retrieval attempts. Finally, as hypothesized, psychophysiological interaction (PPI) analysis demonstrated that during uncertain decisions SPL displays increased correlation with posterior visual areas that may subserve the representation of retrieval cues; this finding is consistent with the interpretation that SPL-mediated processing is directed to the memory probe when decisions are uncertain (possibly due to ambiguous memory signals). Unexpectedly, PPI also showed a similar uncertainty-modulated coupling between lateral IPS and visual cortex. We first discuss the response profiles of lateral IPS and SPL, along with the whole-brain networks and prefrontal components that were similarly active during retrieval. Subsequently, we consider the implications of the SPL and IPS connectivity analyses. Finally, we briefly discuss the results from ventral PPC.
4.1 Recognition confidence within dorsal PPC
Memory strength in lateral IPS and beyond
Left lateral IPS activity positively varied with the degree to which participants perceived an item as having been encountered before (as expressed through recognition confidence judgments). This activity profile is consistent with the hypothesis that the region tracks the subjective sense of test probe familiarity, and is qualitatively similar to results from prior studies reporting a parametric increase in activity in dorsal PPC regions as a function of recognition confidence (Yonelinas et al., 2005; Daselaar et al., 2006; Montaldi et al., 2006).
Beyond parietal cortex, regions displaying a similar activity profile included left MFG, aPFC (including aIFG; see Figure 5c), insula, and precuneus. Extant neuroimaging studies have also observed medial parietal, anterior and lateral frontal activations in contrasts thought to index item familiarity or graded memory strength during recognition (McDermott et al., 2000; Kahn et al., 2004; Henson et al., 2005; Yonelinas et al., 2005; Daselaar et al., 2006; Montaldi et al., 2006). It is also worth noting, however, that because the recognition confidence scale used here did not explicitly distinguish between recollection- vs. familiarity-based decisions, it is possible that the observed graded memory strength effect in these foci may not be specific to item familiarity processes. For example, the involvement of left lateral PFC here might additionally reflect the engagement of recollection-related processes (Nolde et al., 1998; Dobbins et al., 2002; Yonelinas, 2002; Dobbins and Wagner, 2005).
Decision uncertainty in SPL and beyond
SPL activity functionally dissociated from that in lateral IPS, as SPL displayed increased activity as decision uncertainty increased. This was true at the condition level (i.e., as a function of subjectively reported decision confidence) as well as at the trial level, as revealed by a trial-by-trial correlation between activity and reaction time. One account for this set of findings is that activity in SPL tracks the deployment of goal-directed attention, which is increased during a difficult recognition judgment. Support for this account also comes from the whole-brain contrast indexing decision uncertainty, which revealed effects not only in bilateral SPL, but also in bilateral SFS. Indeed, an a priori left SFS ROI taken from Hutchinson et al. (2014) displayed a strikingly similar pattern of activity as SPL (see Figure 5a). The topography of this dorsal frontal-parietal finding bears striking similarity to the top-down attention network advanced by Corbetta and Shulman (2002; Corbetta et al., 2008). Moreover, in addition to this dorsal network, there was a cluster of activation in the right MFG. Numerous other studies have reported right MFG activity in contrasts that potentially index decision uncertainty during retrieval (Henson et al., 1999; Wheeler and Buckner, 2004; Fleck et al., 2006) and the current results extend the literature in this regard. Moreover, an a priori ROI centered in left MFG displayed a similar pattern of activity (see Figure 5b) despite being defined on a contrast thought to index item memory (Hutchinson et al., 2014).
A notable alternative to an attention account of SPL activity is that it may reflect ‘mnemonic accumulation’ computations. That is, during perceptual decisions, it is thought that PPC regions integrate or accumulate sensory evidence across the possible choices until a decision threshold is reached for a given option (e.g., Shadlen and Newsome, 2001; Usher and McClelland, 2001; Smith and Ratcliff, 2004; Heekeren et al., 2006; Gold and Shadlen, 2007; Ratcliff and McKoon, 2008). Some have argued that perceptual accumulation computations result in a greater fMRI BOLD response when evidence is weaker (Ho et al., 2009; Kayser et al., 2010), and fMRI studies of perceptual decision making have observed greater SPL and medial IPS activity under such conditions (for discussion, see Uncapher et al., in press). By extension, the present SPL activity could reflect mnemonic accumulation computations, though such an account does not readily accommodate the connectivity profiles found in the current study.
4.2 Connectivity profiles of dorsal PPC
The PPI connectivity analyses provide important new insight into the interactions between dorsal parietal areas and the rest of the brain during uncertain recognition decisions. Specifically, left SPL, and unexpectedly left lateral IPS, displayed increased functional connectivity with areas of occipotemporal cortex (including the putative visual word-form area and lateral occipital cortex) during uncertain relative to certain decisions. At least for the SPL, this result complements those from a recent study which reported resting-state functional connectivity between SPL and the visual word-form area (Vogel et al., 2012), suggesting that uncertain decisions might engage pathways that are otherwise intrinsically connected.
Mechanistically, this uncertainty-modulated connectivity is broadly consistent with models in which dorsal attention regions modulate activity in lower-level perceptual areas (e.g., Kastner and Ungerleider, 2000; Reynolds and Chelazzi, 2004; Lauritzen et al., 2009; Miller and Buschman, 2013; Squire et al., 2013). In the context of the present experiment, these results suggest increased top-down attention to the retrieval cues (here a visual word) during uncertain decisions. One possibility is that when mnemonic evidence is farther from the highest confident ‘old’ or highest confident ‘new’ decision bounds, participants increase their attention to the retrieval cue in an attempt to trigger additional mnemonic evidence that may ultimately guide the decision. Future studies that exploit high-temporal resolution methods, such as intracranial electroencephalography (i.e., electrocorticography), promise to shed light on whether such SPL-mediated attention to retrieval cue representations is more sustained, is stronger but not temporally extended, or entails additional shifts of attention back to the cue as the retrieval attempt unfolds.
Interestingly, in contrast to the uncertainty-modulated functional coupling between SPL and visual cortical areas, PPI analysis did not reveal significantly enhanced coupling between the SPL and regions that are typically implicated as the source of mnemonic evidence (e.g., the MTL). While a null result, this finding tentatively suggests that insofar as coupling with SPL reflects involvement of top-down attention during retrieval, then such attention may be guided, at least in part, toward the retrieval cue, rather than to the internal byproducts (mnemonic evidence) of retrieval. Again, we stress that this conclusion is tentative as it rests on a null result; future studies are needed to further test the attention to mnemonic evidence hypothesis.
Counter to expectations as well as to the present observation that lateral IPS activity does not track decision uncertainty, this dorsal PPC region nevertheless also displayed increased coupling with visual regions during uncertain decisions. Given the functionally dissociable evoked activity patterns in SPL and lateral IPS, interpretation of this IPS effect is challenging and inherently speculative. Based on the proximity of lateral IPS to parietal regions implicated in action representation (e.g., Grafton and Hamilton, 2007), a speculative possibility is that SPL, IPS and posterior visual regions collectively serve to guide attention to or processing of stimulus-response mappings (here, in the context of a recognition task). That is, insofar as an uncertain decision puts a greater demand on the stimulus-response mapping network (e.g., by requiring the visual cue to be maintained for longer or activating multiple candidate response representations), then there might be greater connectivity between regions coding for the stimulus (VWFA), the response (motor cortex, see Table 2), and their dynamic mapping (SPL and IPS). In this interpretation, SPL might play a role in the visual aspects of these mappings [thus, the overlap with retinotopic regions in Hutchinson et al. (2014)], and IPS in the motoric component. Alternatively, insofar as an uncertain decision puts a greater demand specifically on motoric selection (e.g., by activating multiple candidate response representations), then there might be greater connectivity between IPS and regions coding for the response (motor cortex). In this interpretation, because IPS-motor cortex coupling covaries in a similar manner as SPL and VWFA, then there is an apparent correlation between IPS and VWFA as well. Both of these speculative interpretations require future, targeted assessment in order to assess their viability.
4.3 Evoked activity in ventral PPC
AnG displayed an asymmetric decision certainty effect, where activity was greater for high relative to low confidence responses, but with high confidence ‘old’ responses eliciting greater activity than high confidence ‘new’ responses. Although studies using source memory paradigms have reported selective increases in AnG activity when event (source) details can be recollected (Cansino et al., 2002; Kahn et al., 2004; Kensinger and Schacter, 2006; Hutchinson et al., 2014), a few prior studies using confidence-related (or otherwise scaled) old/new recognition responses have reported a similar asymmetric decision certainty effect (Yonelinas et al., 2005; Daselaar et al., 2006; Cabeza, 2008). It has been argued that items that are endorsed as novel with high confidence capture attention in a ‘bottom-up’ manner similar to strong memories endorsed with high confidence (Cabeza, 2008). When taken in isolation, this pattern may be viewed consistent with the attention-to-memory account of ventral PPC functioning (also see O'Connor et al., 2010). On the other hand, a bottom-up attention account of AnG activation during retrieval has been strongly challenged by other recent findings (e.g., Vilberg and Rugg, 2007; Nelson et al., 2012; Vilberg and Rugg, 2012; Hutchinson et al., 2014), suggesting alterative interpretations are needed. One possibility is that AnG may serve as a cortical convergence zone that binds event elements, be they elements recollected at retrieval (Shimamura, 2011) or elements that are part of a highly novel event (e.g., Uncapher and Wagner, 2009). From this perspective, greater AnG activity for high confidence ‘old’ trials reflects the role of AnG in recollection whereas greater AnG activity for high confidence ‘new’ trials reflects the role of AnG in encoding highly novel events. Alternatively, AnG activity during retrieval may reflect accumulator computations, insofar as greater accumulation slopes produce increased (rather than decreased) fMRI BOLD activity (Criss et al., 2013), which is a matter of debate (c.f., (Ho et al., 2009; Kayser et al., 2010). Such an interpretation is also complicated by the lack of a consistent correspondence between reaction time and activity in the region on a trial-by-trial basis.
Regions in frontal cortex, anatomically distinct from those that tracked perceived memory strength (see above), showed a similar pattern of activity as AnG, which perhaps provides supporting evidence for accumulator accounts of AnG retrieval-related activity. In particular, the dorsal cluster of activity in left MFG/aSFS (see Figure 5d) found in this contrast is close to the regions argued to index decision certainty (‘DLPFC/SFS’) in several studies of perceptual decision-making (Heekeren et al., 2004; Heekeren et al., 2006), and significant activity in right insula in the current experiment (not shown; MNI coordinates of 48, 0, 12) falls close to a region in insula argued to be related to evidence accumulation in the service of a perceptual decision in another study (Ho et al., 2009) .
Widespread responses were also observed in subcortical regions, including the striatum bilaterally, with activity largely centered on the putamen. An intriguing possibility is that the present data align with a ‘goal-dependent’ account of striatal functioning, wherein activity in the striatum during retrieval tracks the degree to which internal goals are satisfied (Han et al., 2010; also see Schwarze et al., 2013). While speculative, according to such an account, high confidence responses (old and new) may be perceived as intrinsically more rewarding to the participants, even in the absence of any explicit reward structure; as this account rests on reverse inference, further directed testing of this hypothesis is required
5. Conclusions
The present study provides additional compelling evidence that, during mnemonic decisions, distinct regions in PPC display unique activity profiles as a function of recognition confidence. Within dorsal PPC, SPL in particular displays greater evoked activity, a relationship with decision reaction time, and greater connectivity with visual regions that represent the retrieval cue during uncertain decisions. These findings are consistent with the hypothesis that SPL mediates attention to, or perceptual processing of, the visual stimuli used to probe memory. As retrieval attempts are complex neurocognitive acts, we expect that future research will further advance understanding of the rich computational contributions of PPC to memory-guided decision-making.
Acknowledgments
This work was supported by a grant from the National Institute of Mental Health (5R01–MH080309). The authors would also like to thank Jessica Wilson for her assistance in data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brainard DH. The Psychophysics Toolbox. Spatial vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Cabeza R. Role of parietal regions in episodic memory retrieval: The dual attentional processes hypothesis. Neuropsychologia. 2008;46:1813–1827. doi: 10.1016/j.neuropsychologia.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Response to Nelson et al.: ventral parietal subdivisions are not incompatible with an overarching function. Trends Cogn Sci. 2012a;16:400–401. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci. 2012b;16:338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–613. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M. Top-down and bottom-up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia. 2008;46:1828–1851. doi: 10.1016/j.neuropsychologia.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criss AH, Wheeler ME, McClelland JL. A differentiation account of recognition memory: evidence from fMRI. J Cogn Neurosci. 2013;25:421–435. doi: 10.1162/jocn_a_00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol. 2006;16:205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Cabeza R. Triple dissociation in the medial temporal lobes: recollection, familiarity, and novelty. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Wagner AD. Domain-general and domain-sensitive prefrontal mechanisms for recollecting events and detecting novelty. Cereb Cortex. 2005;15:1768–1778. doi: 10.1093/cercor/bhi054. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Fleck MS, Daselaar SM, Dobbins IG, Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb Cortex. 2006;16:1623–1630. doi: 10.1093/cercor/bhj097. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Rissman J, Kiani R, Wagner AD. Cortical Reinstatement Mediates the Relationship Between Content-Specific Encoding Activity and Subsequent Recollection Decisions. Cereb Cortex. 2013 doi: 10.1093/cercor/bht194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Hamilton AF. Evidence for a distributed hierarchy of action representation in the brain. Human movement science. 2007;26:590–616. doi: 10.1016/j.humov.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Huettel SA, Raposo A, Adcock RA, Dobbins IG. Functional significance of striatal responses during episodic decisions: recovery or goal attainment? J Neurosci. 2010;30:4767–4775. doi: 10.1523/JNEUROSCI.3077-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG. A general mechanism for perceptual decision-making in the human brain. Nature. 2004;431:859–862. doi: 10.1038/nature02966. [DOI] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ruff DA, Bandettini PA, Ungerleider LG. Involvement of human left dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc Natl Acad Sci U S A. 2006;103:10023–10028. doi: 10.1073/pnas.0603949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Hornberger M, Rugg MD. Further dissociating the processes involved in recognition memory: an FMRI study. J Cogn Neurosci. 2005;17:1058–1073. doi: 10.1162/0898929054475208. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho TC, Brown S, Serences JT. Domain general mechanisms of perceptual decision making in human cortex. J Neurosci. 2009;29:8675–8687. doi: 10.1523/JNEUROSCI.5984-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn Mem. 2009;16:343–356. doi: 10.1101/lm.919109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, Uncapher MR, Weiner KS, Bressler DW, Silver MA, Preston AR, Wagner AD. Functional Heterogeneity in Posterior Parietal Cortex Across Attention and Episodic Memory Retrieval. Cereb Cortex. 2014;24:49–66. doi: 10.1093/cercor/bhs278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kayser AS, Buchsbaum BR, Erickson DT, D'Esposito M. The functional anatomy of a perceptual decision in the human brain. J Neurophysiol. 2010;103:1179–1194. doi: 10.1152/jn.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes underlying memory attribution on a reality-monitoring task. Cereb Cortex. 2006;16:1126–1133. doi: 10.1093/cercor/bhj054. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Rissman J, Chun MM, Wagner AD. Fidelity of neural reactivation reveals competition between memories. Proc Natl Acad Sci U S A. 2011;108:5903–5908. doi: 10.1073/pnas.1016939108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen TZ, D'Esposito M, Heeger DJ, Silver MA. Top-down flow of visual spatial attention signals from parietal to occipital cortex. Journal of vision. 2009;9(18):11–14. doi: 10.1167/9.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Jones TC, Petersen SE, Lageman SK, Roediger HL. Retrieval success is accompanied by enhanced activation in anterior prefrontal cortex during recognition memory: an event-related fMRI study. J Cogn Neurosci. 2000;12:965–976. doi: 10.1162/08989290051137503. [DOI] [PubMed] [Google Scholar]
- Miller EK, Buschman TJ. Cortical circuits for the control of attention. Curr Opin Neurobiol. 2013;23:216–222. doi: 10.1016/j.conb.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- Nelson SM, McDermott KB, Petersen SE. In favor of a 'fractionation' view of ventral parietal cortex: comment on Cabeza et al. Trends Cogn Sci. 2012;16:399–400. doi: 10.1016/j.tics.2012.06.014. author reply 400–391. [DOI] [PubMed] [Google Scholar]
- Nelson SM, McDermott KB, Wig GS, Schlaggar BL, Petersen SE. The critical roles of localization and physiology for understanding parietal contributions to memory retrieval. Neuroscientist. 2013;19:578–591. doi: 10.1177/1073858413492389. [DOI] [PubMed] [Google Scholar]
- Nelson SM, Cohen AL, Power JD, Wig GS, Miezin FM, Wheeler ME, Velanova K, Donaldson DI, Phillips JS, Schlaggar BL, Petersen SE. A parcellation scheme for human left lateral parietal cortex. Neuron. 2010;67:156–170. doi: 10.1016/j.neuron.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, D'Esposito M. Left prefrontal activation during episodic remembering: an event-related fMRI study. Neuroreport. 1998;9:3509–3514. doi: 10.1097/00001756-199810260-00032. [DOI] [PubMed] [Google Scholar]
- O'Connor AR, Han S, Dobbins IG. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? J Neurosci. 2010;30:2924–2934. doi: 10.1523/JNEUROSCI.4225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Berryhill M. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobio Learn Mem. 2009;91:155–165. doi: 10.1016/j.nlm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spatial vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural computation. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Schwarze U, Bingel U, Badre D, Sommer T. Ventral striatal activity correlates with memory confidence for old- and new-responses in a difficult recognition test. PLoS One. 2013;8:e54324. doi: 10.1371/journal.pone.0054324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C, Shulman GL, Corbetta M. Attention to memory and the environment: functional specialization and dynamic competition in human posterior parietal cortex. J Neurosci. 2010;30:8445–8456. doi: 10.1523/JNEUROSCI.4719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nat Neurosci. 2004;7:1376–1380. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 2011;11:277–291. doi: 10.3758/s13415-011-0031-4. [DOI] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13:488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Ratcliff R. Psychology and neurobiology of simple decisions. Trends Neurosci. 2004;27:161–168. doi: 10.1016/j.tins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Squire RF, Noudoost B, Schafer RJ, Moore T. Prefrontal contributions to visual selective attention. Annu Rev Neurosci. 2013;36:451–466. doi: 10.1146/annurev-neuro-062111-150439. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobio Learn Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uncapher MR, Gordon AM, Wagner AD. Parietal lobe mechanisms subserving episodic memory retrieval. In: Gazzaniga MS, Mangun GR, editors. The Cognitive Neurosciences. 5. Cambridge, MA: MIT Press; (in press) [Google Scholar]
- Usher M, McClelland JL. The time course of perceptual choice: the leaky, competing accumulator model. Psychol Rev. 2001;108:550–592. doi: 10.1037/0033-295x.108.3.550. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–1799. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. The neural correlates of recollection: transient versus sustained FMRI effects. J Neurosci. 2012;32:15679–15687. doi: 10.1523/JNEUROSCI.3065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Miezin FM, Petersen SE, Schlaggar BL. The putative visual word form area is functionally connected to the dorsal attention network. Cereb Cortex. 2012;22:537–549. doi: 10.1093/cercor/bhr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wandell BA, Rauschecker AM, Yeatman JD. Learning to see words. Annu Rev Psychol. 2012;63:31–53. doi: 10.1146/annurev-psych-120710-100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson JD, Uncapher MR, Rugg MD. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43:1022–1032. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Yonelinas A. The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]