Abstract
Growth in teleosts is controlled in large part by the activities of the growth hormone (Gh)/insulin-like growth factor (Igf) system. In this study, we initially identified igf-binding protein (bp)1b, -2b, -4, -5a and -6b transcripts in a tilapia EST library. In Mozambique tilapia (Oreochromis mossambicus), tissue expression profiling of igfbps revealed that igfbp1b and -2b had the highest levels of expression in liver while igfbp4, -5a and -6b were expressed at comparable levels in most other tissues. We compared changes in hepatic igfbp1b, -2b and -5a expression during catabolic conditions (28 days of fasting) along with key components of the Gh/Igf system, including plasma Gh and Igf1 and hepatic gh receptor (ghr2), igf1 and igf2 expression. In parallel with elevated plasma Gh and decreased Igf1 levels, we found that hepatic igfbp1b increased substantially in fasted animals. We then tested whether systemic Gh could direct the expression of igfbps in liver. A single intraperitoneal injection of ovine Gh into hypophysectomized tilapia specifically stimulated liver igfbp2b expression along with plasma Igf1 and hepatic ghr2 levels. Our collective data suggest that hepatic endocrine signaling during fasting may involve post-translational regulation of plasma Igf1 via a shift towards the expression of igfbp1b. Thus, Igfbp1b may operate as a molecular switch to restrict Igf1 signaling in tilapia; furthermore, we provide new details regarding isoform-specific regulation of igfbp expression by Gh.
Keywords: Growth hormone, Insulin-like growth factor, Binding proteins, Receptor, 56 Tilapia, Fasting
1. Introduction
Growth in vertebrates is largely controlled by the coordinated activities of the growth hormone (Gh)/insulin-like growth factor (Igf) system. In teleost fishes, the regulation of growth performance by the Gh/Igf system also seems to be highly conserved (Duan, 1997; Wood et al., 2005). Growth performance is often used as an indicator of the status of individuals and populations in culture and the wild, and therefore, major effort has been applied towards garnering a more comprehensive understanding of how multiple components of the Gh/Igf system interact to control growth and metabolism (Picha et al., 2008; Beckman, 2011).
Gh is a member of the Gh/prolactin/somatolactin family of pituitary hormones that regulate numerous physiological processes that include somatic growth, immune function, osmoregulation, lipid and protein metabolism and feeding behavior (Duan, 1997; Kawauchi and Sower, 2006). The physiological actions of Gh on target tissues are mediated via transmembrane receptors that activate the Jak/Stat signaling pathway Bole-Feysot et al., 1998). Genes encoding Gh receptors (ghrs) have been cloned from several teleost species and display a wide distribution of expression across tissues in accord with the pleiotropic actions of Gh (Reindl and Sheridan, 2012). We have identified two ghr sequences in Mozambique tilapia (Oreochromis mossambicus), denoted ghr1 and ghr2 (Kajimura et al., 2004; Pierce et al., 2007). Phylogenetic analyses, tissue expression patterns, and regulation by Gh of these two ghrs suggest that ghr2 encodes the primary Gh receptor (Kajimura et al., 2004; Pierce et al., 2007; Pierce et al., 2012). Further evidence of divergent physiological roles for these two receptors comes from observations that ghr1 and ghr2 transcript expression is differentially responsive to fasting (Saera-Vila et al., 2005; Uchida et al., 2009; Fox et al., 2010), temperature (Gabillard et al., 2006), stressors (Saera-Vila et al., 2009), metabolic hormones (Reindl et al., 2009; Pierce et al., 2012), xenobiotics (Davis et al., 2009), and salinity (Pierce et al., 2007; Breves et al., 2011). Dynamic ghr expression within key metabolic tissues following alterations in nutritional, osmoregulatory and endocrine states occurs across teleosts (Reinecke, 2010; Reindl and Sheridan, 2012). Thus, the capacity to modulate ghr expression appears to serve as a fundamental mechanism to regulate the sensitivity of target tissues to Gh.
In liver and muscle, Gh can act directly by stimulating mitosis and differentiation among other cellular behaviors, and acts indirectly by initiating the production and release of Igfs (Wood et al., 2005; Duan et al., 2010). In mammals, Igf1 is regarded as the primary somatomedin during postnatal life. Igf2 exhibits minimal dependence upon endocrine Gh and its actions have been largely associated with fetal growth and development (Daughaday and Rotwein, 1989; Constancia et al., 2002). In a subset of teleosts, however, growing evidence supports the operation of Igf2, in addition to Igf1, as a somatomedin throughout the life cycle. For example, hepatic igf2 expression and plasma Igf2 levels are stimulated by Gh both in vivo and in vitro (reviewed by Reindl and Sheridan, 2012) and Igf2 administration stimulates growth in juvenile tilapia (Chen et al., 2000). These findings underscore the importance of considering both Igfs when characterizing the actions of Gh in teleosts.
Igf1 and Igf2 interact with a family of binding proteins, termed Igf binding proteins (Igfbps); the specific character of these interactions determines how the biological actions of Igfs are expressed because Igfbps affect hormone availability, transport and receptor binding (Duan et al., 2010). As in mammals, six Igfbps have been identified in fishes (Daza et al., 2011). Although the mechanisms of action are poorly understood, there is good evidence that Igfbps also exhibit ligand-independent activities (Firth and Baxter, 2002). Gh is an important regulator of igfbp expression and protein secretion in mammals (Yamada and Lee, 2009). This link in fishes, however, has only been investigated in a restricted number of salmonid species (Cheng et al., 2002; Pierce et al., 2006), with few studies aimed at characterizing the effects of Gh on igfbp expression in vivo.
We have previously characterized the responses of the Gh/Igf system to changes in metabolic status in Mozambique tilapia with particular attention to plasma Gh and Igf1 levels and the expression of hepatic ghr2, igf1 and igf2 transcripts (Uchida et al., 2003; Fox et al., 2006; Pierce et al., 2007; Peddu et al., 2009; Fox et al., 2010). In turn, the Mozambique tilapia is positioned as a key in vivo model from which to advance our understanding of how the Gh/Igf system, via the activities of Igfbps, responds to nutritional status. In this study, we identify igfbp1b, -2b and -5a as highly expressed hepatic transcripts and assess their regulation by nutrient restriction and Gh, and therefore contribute new details on the physiology of Igfbps in a widely cultured teleost.
2. Materials and methods
2.1. Animals
Male Mozambique tilapia (O. mossambicus) were maintained in re-circulating fresh water (FW) under artificial photoperiod (14 h light, 10 h dark) at the Department of Biological Sciences (University of Arkansas, Fayetteville, AK). Fish were fed Aquamax Starter Fingerling 300 (PMI Nutrition International, Brentwood, MO) and water temperatures were maintained between 20 and 22 °C. The Institutional Animal Care and Use Committee of the University of Arkansas approved all housing and experimental protocols.
2.2 igfbp sequences
Sequences for igfbp1b (Acc. No. XM_003438121), igfbp2b (Acc. No. XM_005450484), igfbp4 (Acc. No. XM_003454633), igfbp5a (Acc. No. XM_003443250.2) and igfbp6b (Acc. No. XM_003441337) were identified in the Nile tilapia (O. niloticus) transcriptome (Lee et al., 2010) by searching with tBLASTn at the NCBI web resource using the relevant sequences known from rainbow trout (Oncorhynchus mykiss) (Kamangar et al., 2006). The sequence similarities of rainbow trout and Nile tilapia igfbp1b, -2b, -4, -5a and -6b were 79, 75, 76, 70 and 84%, respectively. Nucleotide similarities between Nile and Mozambique tilapia igfbp sequences were ≥98%.
2.3. Fasting experiment
Tilapia (40–60 g) were distributed into 6 tanks representing two treatment groups (3 fed and 3 fasted). Fish were allowed to acclimate to the experimental tanks for 4 weeks prior to the beginning of the experiment. Following this initial acclimation period, food was withheld from 3 tanks while the animals contained in the other 3 tanks were fed at ~5% of their body weight twice daily.
Body weight and standard length were measured at each sampling point for calculation of condition factor: (body weight, g)/(standard length, cm)3 × 100. At sampling, all fish were anesthetized in 2-phenoxyethanol (2-PE; 0.3 ml/l; Sigma, St. Louis, MO) and blood was collected from the caudal vasculature by a needle and syringe treated with ammonium heparin (200 U/ml, Sigma). Plasma was separated by centrifugation at 4 °C and stored at −80 °C until analyses for plasma glucose and Igf1. Liver tissue was collected, snap frozen in liquid nitrogen, and stored at −80 °C until RNA isolation.
2.4. Hypophysectomy and Gh injection
A Gh injection experiment was conducted at the Hawaii Institute of Marine Biology, University of Hawaii. Tilapia (70–150 g) were reared in outdoor tanks with a continuous flow of FW under natural photoperiod at 24–26 °C and fed a commercial diet ad libitum (Skretting, Tooele, UT). Hypophysectomy was performed by the transorbital technique (Nishioka, 1994). Briefly, tilapia were anesthetized by immersion in buffered tricaine methanesulfonate (100 mg/l, Argent Chemical Laboratories, Redmond, WA) and 2-PE (0.3 ml/l) in FW. Following removal of the right eye and underlying tissue, a hole was drilled through the neurocranium, and the pituitary was aspirated with a modified Pasteur pipette. The orbit was then packed with microfibrillar collagen hemostat (Ethicon, Somerville, NJ) and fish were allowed to recover in brackish water (12 ‰) composed of seawater (Kaneohe Bay, Hawaii) diluted with FW. Following recovery, fish were transferred to re-circulating experimental aquaria containing aerated brackish water and treated with kanamycin sulfate (National Fish Pharmaceuticals, Tucson, AZ). Upon transfer to experimental aquaria, food was withheld in all experiments to control for the possibility of confounding effects due to disparate feeding patterns between individuals. Water temperatures were maintained between 24 and 26 °C.
To test the effects of Gh on the Gh/Igf system, hypophysectomized fish maintained in brackish water for 3–4 days following surgery (n = 6–9) were administered ovine Gh (oGh; 5 µg/g body weight) or saline vehicle by a single intraperitoneal (IP) injection. oGh was obtained from the National Hormone and Peptide Program (NIDDK-oGH-15) and delivered in saline vehicle (0.9% NaCl; 1.0 µl/g body weight). Fish were lightly anaesthetized with 2-PE prior to injection. After injection, fish were returned to aquaria and left undisturbed for 12 h, after which time plasma and liver samples were collected as described above. At the time of sampling, completeness of hypophysectomy was confirmed by post-mortem inspection of the hypothalamic region. The Institutional Animal Care and Use Committee of the University of Hawaii approved all housing, surgical and experimental protocols.
2.5. Plasma measurements
Plasma glucose concentrations were assayed by the hexokinase method using a commercially available kit (Glucose Assay Kit, GAHK-20, Sigma) modified for a microplate reader (SpectraCount, Packard, Meriden, CT). Plasma Gh was measured by homologous radioimmunoassay as described by Yada et al. (1994). Total plasma Igf1 levels were measured in 25 µl of plasma extracted with 100 µl of acid-ethanol (87.5% ethanol and 12.5% 2 N HCl v/v) by heterologous radioimmunoassay using recombinant salmon Igf1 as the standard and anti-barramundi Igf1 (GroPep, Adelaide, Australia) (Shimizu et al., 1999; Kajimura et al., 2002).
2.6. RNA extraction, cDNA synthesis and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from tissue by the TRI Reagent procedure (MRC, Cincinnati, OH) according to the manufacturer’s protocols. RNA concentration and purity were assessed by spectrophotometric absorbance (Nanodrop 1000, Thermo Scientific, Wilmington, DE). First strand cDNA was synthesized with a High Capacity cDNA Reverse Transcription Kit (Life Technologies, Carlsbad, CA). Relative mRNA expression was determined by qRT-PCR using the StepOnePlus real-time PCR system (Life Technologies, Carlsbad, CA). Primer pairs for ghr1, ghr2, igf1, igf2 and ef1αare previously described (Pierce et al., 2007; Breves et al., 2011; Pierce et al., 2011; Pierce et al., 2012). Additional primer pairs used were (5'-3'): igfbp1b (forward, CCTTCCCTTTGATCACCAAG; reverse, GTGTGACATGGACCCTGTTG; amplicon size = 102 nt), igfbp2b (forward, CCGACTTCCCTTTACAGCAG; reverse, TCAGTCCCATGCACCTCATA; amplicon size = 112 nt), igfbp4 (forward, ATCCCCATACCCAACTGTGA; reverse, TGATCCACACACCAGCATTT; amplicon size = 98 nt), igfbp5a (forward, AACTGGACGGGATCATTCAG; reverse, GCACTGTTTGCGTTTGAAGA; amplicon size = 107 nt), and igfbp6b (forward, TCCTACCTGCAGAGGAAAGC; reverse, CGCAGCTCAGAGTGTAGACG; amplicon size = 104 nt).
The qRT-PCR reactions were setup as previously described (Pierce et al., 2007). Briefly, 200 nM of each primer, 3 µl cDNA and 12 µl of SYBR Green PCR Master Mix (Life Technologies) were added to a 15 µl final reaction volume. The following cycling parameters were employed: 2 min at 50 °C, 10 min at 95 °C followed by 40 cycles at 95 °C for 15 sec and 60 °C for 1 min. After verification that expression did not vary across treatments, ef1αexpression levels were used to normalize target genes. Reference and target genes were calculated by the relative quantification method with PCR efficiency correction (Pfaffl, 2001). Standard curves were prepared from serial dilutions of untreated liver cDNA and included on each plate to calculate the PCR efficiencies for target and normalization genes. Relative gene expression ratios between groups are reported as a fold-change from controls.
2.7. Statistics
Tissue expression profiles were analyzed by one-way ANOVA followed by Tukey’s HSD. The fasting experiment was analyzed by two-way ANOVA. When the interaction between main effects (treatment and time) was significant, Dunn’s test was employed at each time point. Otherwise, significant main effects are indicated in the figure. Group comparisons in the Gh injection experiment were analyzed by Student’s t-test. When necessary, data were transformed to satisfy homogeneity of variance requirements. If transformation was not sufficient, data were analyzed with a nonparametric Kruskal-Wallis analysis. Significance for all tests was set at P < 0.05 and asterisks in the figures indicate significance levels: *P < 0.05, **P < 0.01 and ***P < 0.001. All tests were performed using GraphPad Prism 5.0 (San Diego, CA).
3. Results
3.1. Tissue distribution
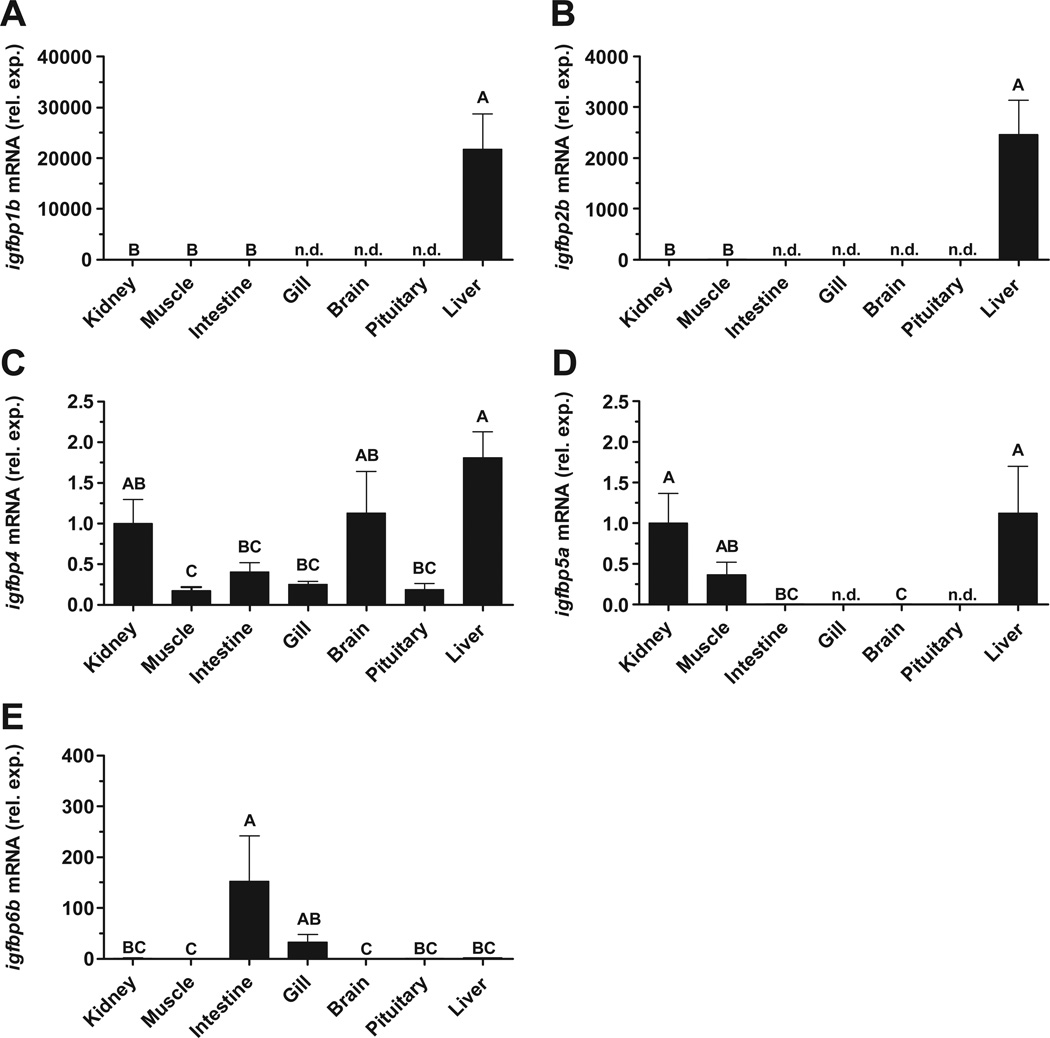
The distribution of igfbp1b, -2b, -4, -5a and -6b expression was analyzed in tissues of fed, FW-acclimated, tilapia. igfbp1b and -2b were expressed at greater than 4- and 3-orders of magnitude higher, respectively, in liver than other examined tissues (Fig. 1A,B). igfbp4 expression was highest in kidney, brain and liver, with comparable expression in other tissues (Fig. 1C). igfbp5a exhibited the highest expression levels in kidney, muscle and liver with low levels found elsewhere (Fig. 1D). igfbp6b expression was highest in intestine, with low levels in other examined tissues (Fig. 1E).
Fig. 1.
Expression of igfbp1b (A), igfbp2b (B), igfbp4 (C), igfbp5a (D) and igfbp6b (E) mRNA in kidney, skeletal muscle, anterior intestine, gill, brain, pituitary and liver of fed, freshwater-acclimated, tilapia. Data were normalized using ef1αas a reference gene and are presented relative to the amount of mRNA in kidney. n.d.; no detection. Groups not sharing the same letter are significantly different (ANOVA, Tukey’s HSD, P < 0.05). Means ± SEM (n = 6–8).
3.2. Effects of fasting on the Gh/Igf system
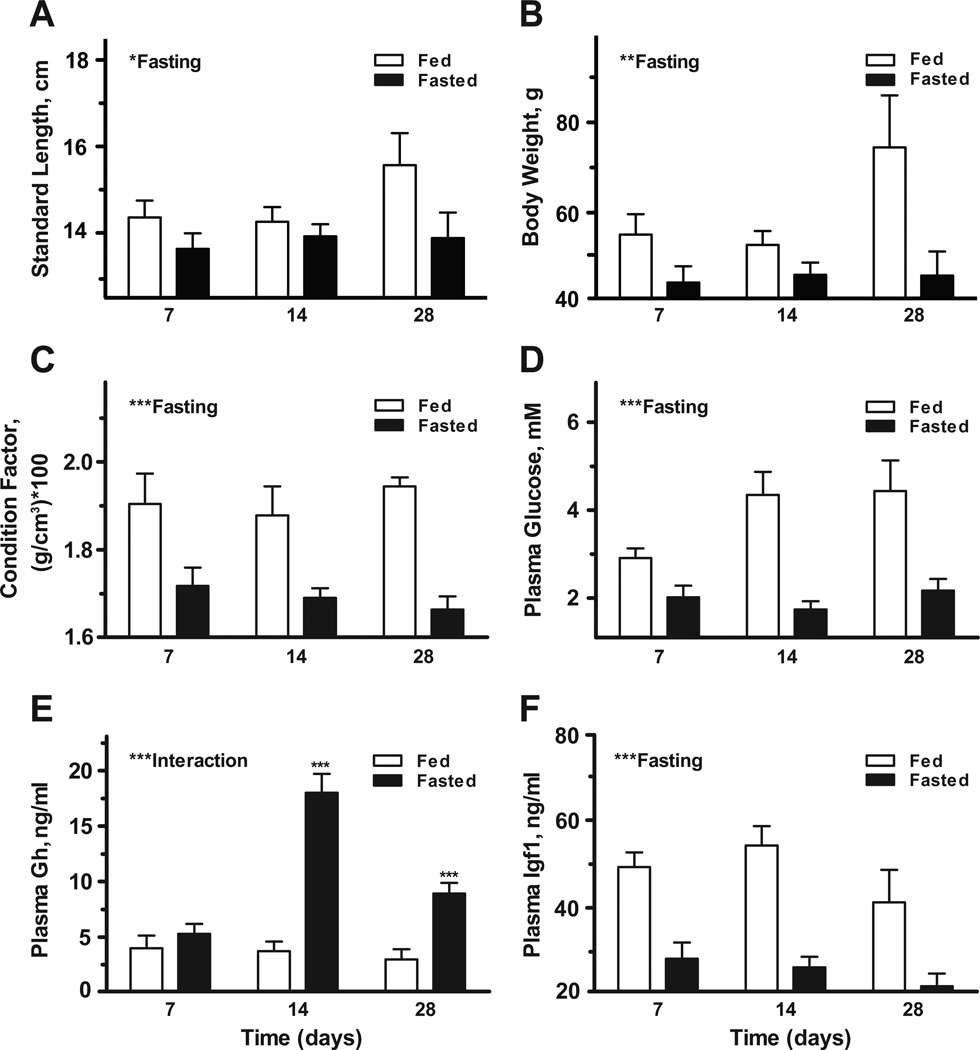
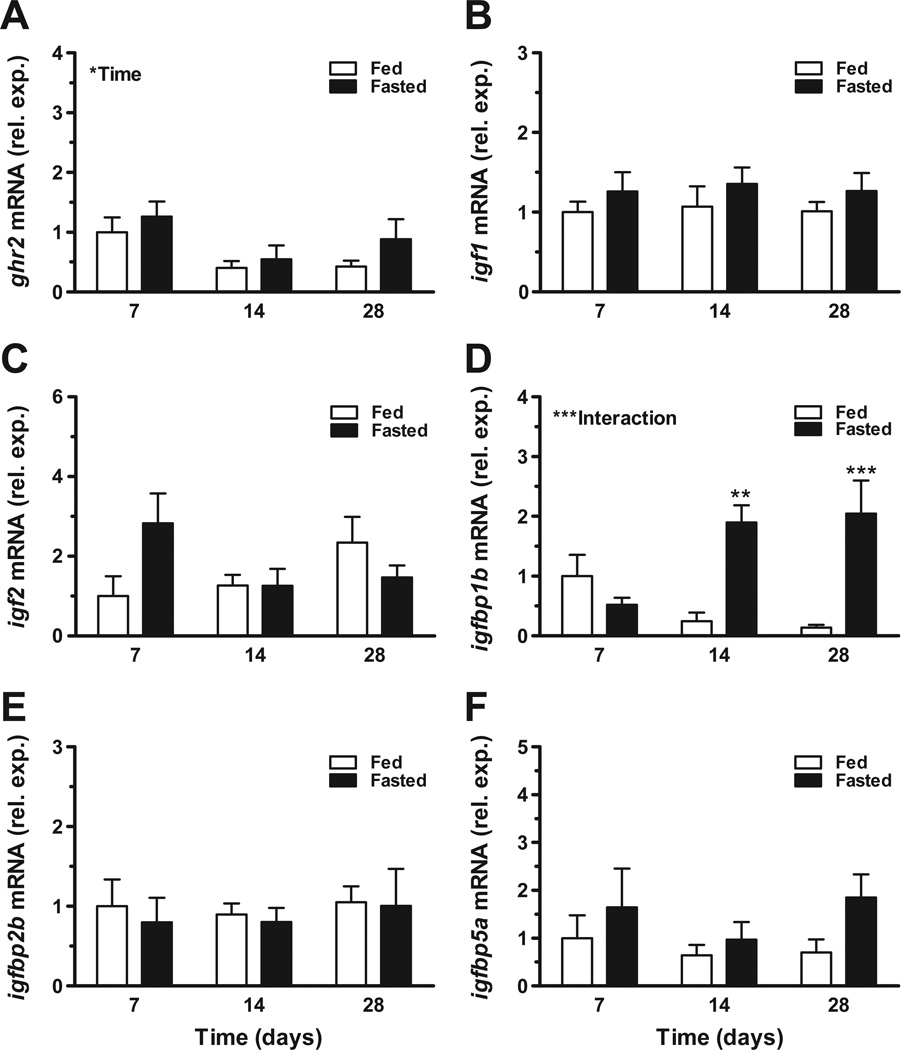
We described responses of the Gh/Igf system to catabolic conditions by fasting tilapia for 7, 14 and 28 days and assaying systemic hormone levels and hepatic gene expression. There was a significant main effect of fasting on standard length (Fig. 2A), body weight (Fig. 2B), condition factor (Fig. 2C), and plasma glucose (Fig. 2D). There was a significant interaction between time and fasting on plasma Gh levels. Plasma Gh was significantly elevated from fed controls after 14 and 28 days of fasting (Fig. 2E). Similarly, there was a significant effect of fasting on plasma Igf1 levels (Fig. 2F). With respect to hepatic gene expression, there were no clear effects of fasting on ghr2, igf1 or igf2 expression (Fig. 3A–C). There was a significant interaction between time and fasting on igfbp1b levels, with elevated expression observed after 14 and 28 days of fasting (Fig. 3D); alternatively, igfbp2b and -5a were not responsive the fasting (Fig. 3E–F).
Fig. 2.
Effects of fasting on standard length (A), body weight (B), condition factor (C), and plasma glucose (D), Gh (E) and Igf1 (F). Tilapia were exposed to continuous feeding (open bars) or fasting (solid bars) and sampled at 7, 14 and 28 days. Significant effects of fasting or interaction between time and fasting are indicated in respective panels. When there was a significant interaction between time and fasting, post-hoc comparisons were made between fed and fasted groups at each time point. *P < 0.05, **P < 0.01 and ***P < 0.001. Means ± SEM (n = 9–10).
Fig. 3.
Effects of fasting on hepatic gene expression of ghr2 (A), igf1 (B), igf2 (C), igfbp1b (D), igfbp2b (E) and igfbp5a (F). Tilapia were exposed to continuous feeding (open bars) or fasting (solid bars) and sampled at 7, 14 and 28 days. Significant effects of time or interaction between time and fasting are indicated in respective panels. When there was a significant interaction between time and fasting, post-hoc comparisons were made between fed and fasted groups at each time point. *P < 0.05, **P < 0.01 and ***P < 0.001. Means ± SEM (n = 7–8).
3.3. Effects of Gh injection
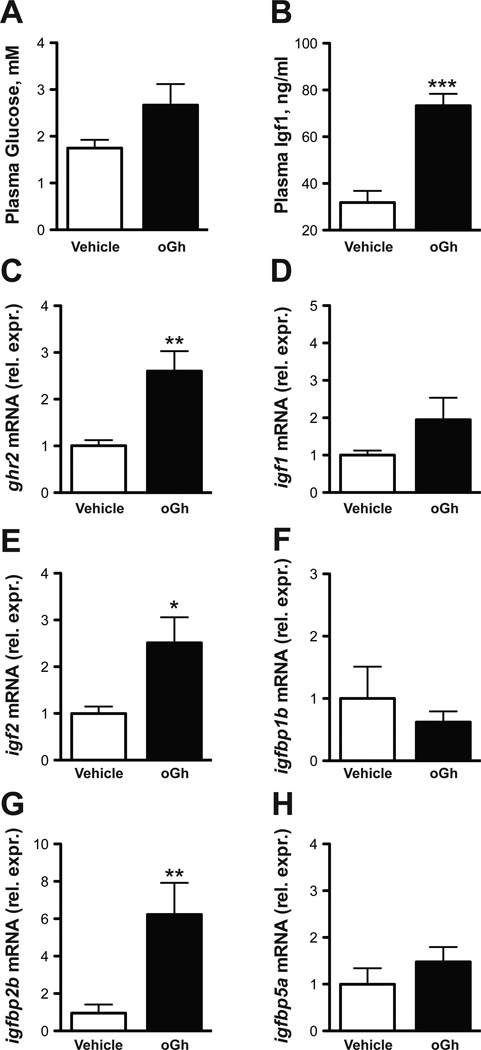
To examine whether systemic Gh could affect the expression of igfbp transcripts, we injected hypophysectomized tilapia with purified oGh and quantified hepatic gene expression at 12 h post injection. oGh injection did not impact plasma glucose (Fig. 4A). Plasma Igf1 roughly doubled following oGh injection (Fig. 4B). oGh injection stimulated hepatic ghr2 by 2.6-fold from the levels of vehicle-injected fish (Fig. 4C). There was no effect of oGh on ghr1 expression (data not shown). While igf1 expression was not significantly increased by oGh (Fig. 4D), oGh stimulated igf2 expression by 2.5-fold (Fig. 4E). There were no effects of oGh on the expression of igfbp1b or -5a (Figs. 4F,H). Alternatively, oGh stimulated igfbp2b levels by 6.2-fold from the levels of vehicle-injected fish (Fig. 4G).
Fig. 4.
Effects of ovine Gh (oGh) on plasma glucose (A) and Igf1 (B) and hepatic gene expression of ghr2 (C), igf1 (D), igf2 (E), igfbp1b (F), igfbp2b (G) and igfbp5a (H). Hypophysectomized animals were sampled 12 h after a single IP-injection of oGh (5 μg/g body weight). Group differences were analyzed by Student’s t-test. *P < 0.05, **P < 0.01 and ***P < 0.001. Means ± SEM (n = 6–9).
4. Discussion
This study aimed to provide new details on how nutritional conditions and endocrine Gh drive activities within the Gh/Igf system of Mozambique tilapia, with special attention to igfbps expressed in liver. Following the identification of five igfbp transcripts in a tilapia EST library (Lee et al., 2010), we investigated expression patterns across multiple tissues and found that igfbp1b, -2b and -5a were robustly, and nearly exclusively, expressed in liver (Fig. 1A,B,D). Currently, it is not clear how these transcripts correspond to Igfbps of different sizes (20–40 kDa) previously described by Park et al. (2000) and Kajimura et al. (2003). However, the high levels of expression for igfbp1b and -2b in liver is similar to patterns reported in other teleosts, including chinook salmon (Oncorhynchus tshawytscha), gilthead sea bream (Sparus aurata), yellowtail (Seriola quinqueradiata), fine flounder (Paralichthys adspersus) and zebrafish (Danio rerio) (Funkenstein et al., 2002; Kamei et al., 2008; Zhou et al., 2008; Pedroso et al., 2009; Shimizu et al., 2011a,b; Safian et al., 2012). With igfbp1b, -2b and -5a identified as transcripts with robust expression in liver, we first turned to characterizing their expression dynamics under a nutrient restriction paradigm.
In the current study, we employed a 28-day nutrient restriction paradigm that we expected to induce catabolic conditions in tilapia (Uchida et al., 2003; Fox et al., 2008; Fox et al., 2010). Indeed, accompanying strong reductions in body weight, condition factor and plasma glucose, we observed increased levels of circulating Gh in parallel with marked reductions in plasma Igf1 levels (Fig. 2B–F). The disparate responses of plasma Gh and Igf1 are in strong accord with patterns previously described in fasted tilapia (Uchida et al., 2003; Fox et al., 2006; Fox et al., 2010) and therefore substantiate the efficacy of the experimental paradigm employed in this study. Interestingly, this apparent uncoupling of plasma Gh from hepatic Igf1 synthesis and secretion (termed Gh-resistance”) has been documented during catabolic conditions in a number of teleosts including coho salmon (Oncorhynchus kisutch), chinook salmon and channel catfish (Ictalurus punctatus) (Duan and Plisetskaya, 1993; Pierce et al., 2005; Small and Peterson, 2005). Because it is commonly held that liver is the primary source of circulating Igfs in both mammals and fishes (Reinecke et al., 1997; Ohlsson et al., 2009; Reinecke, 2010; Reindl and Sheridan, 2012), we next turned to describing hepatic transcriptional responses to nutrient restriction. Despite marked changes in plasma Gh and Igf1 levels, we found that ghr2, igf1 and igf2 expression levels were not impacted by fasting (Fig. 3A–C). Nonetheless, of the investigated igfbp transcripts, igfbp1b was markedly changed after 14 and 28 days of fasting (Fig. 3D) suggesting that transcriptional regulation of this particular igfbp isoform plays a key role in the response to nutrient restriction.
While some Igfbps promote Igf1 activity, others are inhibitory, and some exhibit ligand-independent activities; the regulatory systems controlling Igfbp production are complex and remain largely unclear (Duan et al., 2010; Reindl and Sheridan, 2012). A strong association between elevated plasma Igfbp1 and fasting conditions appears to be conserved from teleosts to mammals (Lee et al., 1997; Siharath et al., 1996). In mammals, Igfbp1 inhibits somatic growth and glucose metabolism and is regulated by metabolic hormones, including insulin, glucocorticoids and thyroid hormones (Lee et al., 1997). Recently, Kamei et al. (2008) established that igfbp1b underlies growth and developmental retardation of embryonic zebrafish. In accord with highly conserved activities linked to diminished Igf1 signaling, Igfbp1 responds to nutritional status in a variety of species. For example, a 22 kDa Igfbp in chinook salmon plasma understood to be a homolog of human Igfbp1 was induced by a reduction in feeding ration (Shimizu et al., 2005; Shimizu et al., 2009). Similarly, igfbp1 expression was increased following 30 and 45 days of fasting in channel catfish (Peterson and Weldbieser, 2009) and chronic hypoxia in Atlantic croaker (Micropogonias undulates) (Rahman and Thomas, 2011) Albeit based on a restricted number of species, it appears that Igfbp1 plays a conserved role in the response to fasting in teleosts (Kelley et al., 2001; Shimizu et al., 2005; Shimizu et al., 2006; Wood et al. 2005). With this response now identified in tilapia, we propose that an important hepatic response to fasting in tilapia may involve regulation of plasma Igf1 via a shift towards the expression of igfbp1b to restrict Igf signaling. Future work (i.e., development of a radioimmunoassay for Igfbp1b) should resolve the relationship between the induction of igfbp1b gene expression and circulating levels of this Igfbp isoform.
In teleosts, the regulatory systems controlling igfbp gene expression remain largely unknown (Duan and Xu, 2005; Duan et al., 2010; Reindl and Sheridan, 2012). Since Gh is a known regulator of igfbp expression and protein secretion in mammals Yamada and Lee, 2009), we conducted a Gh injection experiment to address whether Gh is a potential regulator of igfbp expression in tilapia. First, we observed a clear induction of hepatic ghr2 mRNA in hypophysectomized animals following Gh treatment Fig. 4C), a pattern consistent with the proposition that ghr2 encodes the primary Gh receptor in tilapia (Kajimura et al., 2004; Pierce et al., 2007; Pierce et al., 2012). As reported previously (Chen et al., 2007), plasma Igf1 increased following oGh injection Fig. 4B). Following similar patterns reported in rainbow trout, coho salmon, common carp (Cyprinus carpio), channel catfish, gilthead sea bream and Japanese eel (Anguilla japonica) (Shamblott et al., 1995; Tse et al., 2002; Vong et al., 2003; Carnevali et al., 2005; Petersen et al., 2005; Moriyama et al., 2008; Pierce et al., 2010), animals in this study showed markedly elevated hepatic igf2 expression following Gh treatment (Fig. 4E), similar to responses in intact animals (Eppler et al., 2010; Pierce et al., 2011; Shved et al., 2011). While multiple lines of evidence support the operation of Igf1, as opposed to Igf2, as the primary somatomedin in post-embryonic mammals (Le Roith et al., 2001), in light of the demonstrated bioactivity of native recombinant Igf2 to stimulate growth in tilapia (Chen et al., 2000) our data linking Gh with igf2 in hypophysectomized animals suggest that Igf2 should be further investigated as a possible somatomedin in tilapia. In any event, the positive relationship among Gh injection and plasma Igf1 and hepatic igf2 suggests that animals were in an anabolic state at the time of sampling.
Igfbp3, which in mammals may account for up to 90% of Igf1 binding (Baxter, 1994), was reported in tilapia to be highly expressed in liver and inducible by Gh (Cheng et al., 2002). Despite this, however, the prevailing 40–50 kDa Igfbp in circulation in salmonids, and possibly other teleosts, is not Igfbp3 as it is in mammals, but an Igfbp2 paralog (Shimizu et al., 2011a). Here, we found that hepatic igfbp2b expression was strongly stimulated by oGh (Fig. 4G), a pattern consistent with previous reports showing that the 40–50 kDa Igfbps in teleost plasma are responsive to Gh (Shimizu et al., 1999; Shimizu et al., 2003). When considering that igfbp2b expression is subject to Gh regulation in tilapia along with the known regulation of Igfbp3 by Gh in rat (Schmid et al., 1994), the conserved actions of Gh on Igfbps from mammals to fish appear to be more closely related to their prevalence in circulation rather than to their molecular structure per se. Since Igfs are known to directly modulate igfbp expression in mammals (Conover, 1990), Gh-stimulated plasma Igf1 (Fig. 4B) may indirectly link Gh with igfbp2b expression. The potential for direct actions of Gh and Igf1 on hepatic igfbp2b expression should be addressed in future studies employing primary hepatocyte culture (Pierce et al., 2006; Pierce et al., 2011).
In summary, our principal findings include the identification of igfbp1b as a transcript that is highly responsive to nutritional conditions in tilapia. In addition to plasma Igf1 and hepatic igf2, we identified hepatic igfbp2b as a Gh-responsive transcript in hypophysectomized animals. Since the regulation of igfbp expression in teleosts undoubtedly extends beyond Gh to include peripheral signals under pituitary control such as cortisol and thyroid hormones (Kajimura et al., 2003; Pierce et al., 2006), the hypophysectomized tilapia model is well suited to further resolve how the pituitary mediates igfbp transcriptional responses to a variety of physiological challenges (nutrient restriction, salinity challenge, handling stress). Inasmuch as tilapiine species are among the most important aquaculture resources worldwide, they continue to represent key models from which to gain an improved understanding of how the Gh/Igf system coordinates growth and metabolism in teleosts.
Highlights.
▶ igfbp1b, -2b, -4 and -5a are highly expressed in liver ▶ Fasting stimulated plasma Gh and decreased plasma Igf1 ▶ Hepatic igfbp1b was enhanced 60 after 14 and 28 days of fasting ▶ Gh injection stimulated hepatic igfbp2b in hypophysectomized tilapia
Acknowledgments
This work was supported by The Binational Agricultural Research Development (BARD; IS-4296-10) fund, NSF (IOS-1119693), USDA (2008-35206-18785 and -18787), NIH (F32 DK095575), the Arkansas Biosciences Institute and the Edwin W. Pauley Summer Program in Marine Biology (2012).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter RC. Insulin-like growth factor binding proteins in the human circulation: a review. Horm. Res. 1994;42:140–144. doi: 10.1159/000184186. [DOI] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr. Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- Beckman BR. Perspectives on concordant and discordant relations between insulin-like growth factor 1 (IGF1) and growth in fishes. Gen. Comp. Endocrinol. 2011;170:233–252. doi: 10.1016/j.ygcen.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Breves JP, Seale AP, Helms RE, Tipsmark CK, Hirano T, Grau EG. Dynamic gene expression of GH/PRL-family hormone receptors in gill and kidney during freshwater-acclimation of Mozambique tilapia. Comp. Biochem. Physiol. A. 2011;158:194–200. doi: 10.1016/j.cbpa.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Carnevali O, Cardinali M, Maradonna F, Parisi M, Olivotto I, Polzonetti-Magni AM, Mosconi G, Funkenstein B. Hormonal regulation of hepatic IGF-I and IGF-II gene expression in the marine teleost Sparus aurata. Mol. Reprod. Dev. 2005;71:12–18. doi: 10.1002/mrd.20122. [DOI] [PubMed] [Google Scholar]
- Chen JY, Chen JC, Chang CY, Shen SC, Chen MS, Wu JL. Expression of recombinant tilapia insulin-like growth factor-I and stimulation of juvenile tilapia growth by injection of recombinant IGFs polypeptides. Aquaculture. 2000;181:347–360. [Google Scholar]
- Chen MH, Li YH, Chang Y, Hu SY, Gong HY, Lin GH, Chen TT, Wu JL. Co-induction of hepatic IGF-I and progranulin mRNA by growth hormone in tilapia, Oreochromis mossambicus. Gen. Comp. Endocrinol. 2007;150:212–218. doi: 10.1016/j.ygcen.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Cheng R, Chang KM, Wu JL. Different temporal expressions of tilapia (Oreochromis mossambicus) insulin-like growth factor-I and IGF binding protein-3 after growth hormone induction. Mar. Biotechnol. 2002;4:218–225. doi: 10.1007/s10126-002-0014-0. [DOI] [PubMed] [Google Scholar]
- Conover CA, Ronk M, Lombana F, Powell DR. Structural and biological characterization of bovine insulin-like growth factor binding protein-3. Endocrinology. 1990;127:2795–2803. doi: 10.1210/endo-127-6-2795. [DOI] [PubMed] [Google Scholar]
- Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, Stewart F, Kelsey G, Fowden A, Sibley C, Reik W. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature. 2002;417:945–948. doi: 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- Daughaday WH, Rotwein P. Insulin-like growth factors I and II. Peptide, messenger ribonucleic acid and gene structures, serum, and tissue concentrations. Endocr. Rev. 1989;10:68–91. doi: 10.1210/edrv-10-1-68. [DOI] [PubMed] [Google Scholar]
- Davis LK, Visitacion N, Riley LG, Hiramatsu N, Sullivan CV, Hirano T, Grau EG. Effects of o,p'-DDE, heptachlor, and 17beta-estradiol on vitellogenin gene expression and the growth hormone/insulin-like growth factor-I axis in the tilapia, Oreochromis mossambicus. Comp. Biochem. Physiol. C. 2009;149:507–514. doi: 10.1016/j.cbpc.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Daza DO, Sundstrom G, Bergqvist CA, Duan C, Larhammar D. Evolution of the insulin-like growth factor binding protein (IGFBP) family. Endocrinology. 2011;152:2278–2289. doi: 10.1210/en.2011-0047. [DOI] [PubMed] [Google Scholar]
- Duan C. The insulin-like growth factor system and its biological actions in fish. Amer. Zool. 1997;37:491–503. [Google Scholar]
- Duan C, Plisetskaya EM. Nutritional regulation of insulin-like growth factor-I mRNA expression in salmon tissues. J. Endocrinol. 1993;139:243–252. doi: 10.1677/joe.0.1390243. [DOI] [PubMed] [Google Scholar]
- Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 2010;167:344–351. doi: 10.1016/j.ygcen.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Duan C, Xu QJ. Roles of insulin-like growth factor (IGF) binding proteins in regulating IGF actions. Gen. Comp. Endocrinol. 2005;142:44–52. doi: 10.1016/j.ygcen.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Eppler E, Berishvili G, Mazel P, Caelers A, Hwang G, Maclean N, Reinecke M. Distinct organ-specific up- and down-regulation of IGF-I and IGF-II mRNA in various organs of a GH-overexpressing transgenic Nile tilapia. Transgenic Research. 2010;19:231–240. doi: 10.1007/s11248-009-9314-8. [DOI] [PubMed] [Google Scholar]
- Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002;23:824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- Fox BK, Riley LG, Hirano T, Grau EG. Effects of fasting on growth hormone, growth hormone receptor, and insulin-like growth factor-I axis in seawater acclimated tilapia, Oreochromis mossambicus. Gen. Comp. Endocrinol. 2006;148:340–347. doi: 10.1016/j.ygcen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Fox BK, Breves JP, Davis LK, Pierce AL, Hirano T, Grau EG. Tissue-specific regulation of the growth hormone/insulin-like growth factor axis during fasting and re-feeding: importance of muscle expression of IGF-I and IGF-II mRNA in the tilapia. Gen. Comp. Endocrinol. 2010;166:573–580. doi: 10.1016/j.ygcen.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Funkenstein B, Tsai W, Maures T, Duan C. Ontogeny, tissue distribution, and hormonal regulation of insulin-like growth factor binding protein-2 (IGFBP-2) in a marine fish, Sparus aurata. Gen. Comp. Endocrinol. 2002;128:112–122. doi: 10.1016/s0016-6480(02)00059-x. [DOI] [PubMed] [Google Scholar]
- Gabillard JC, Kamangar BB, Montserrat N. Coordinated regulation of the GH/IGF system genes during refeeding in rainbow trout (Oncorhynchus mykiss) J. Endocrinol. 2006;191:15–24. doi: 10.1677/joe.1.06869. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Uchida K, Yada T, Hirano T, Grau EG. Effects of insulin-like growth factors (IGF-I and -II) on growth hormone and prolactin release and gene expression in euryhaline tilapia, Oreochromis mossambicus. Gen. Comp. Endocrinol. 2002;127:223–231. doi: 10.1016/s0016-6480(02)00055-2. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Hirano T, Visitacion N, Moriyama S, Aida K, Grau EG. Dual mode of cortisol action on GH/IGF-I/IGF binding proteins in the tilapia, Oreochromis mossambicus. J. Endocrinol. 2003;178:91–99. doi: 10.1677/joe.0.1780091. [DOI] [PubMed] [Google Scholar]
- Kajimura S, Kawaguchi N, Kaneko T, Kawazoe I, Hirano T, Visitacion N, Grau EG, Aida K. Identification of the growth hormone receptor in an advanced teleost, the tilapia (Oreochromis mossambicus) with special reference to its distinct expression pattern in the ovary. J. Endocrinol. 2004;181:65–76. doi: 10.1677/joe.0.1810065. [DOI] [PubMed] [Google Scholar]
- Kamei H, Lu L, Jiao S, Li Y, Gyrup C, Laursen LS, Oxvig C, Zhou J, Duan C. Duplication and diversification of the hypoxia-inducible IGFBP-1 gene in zebrafish. PLoS One. 2008;3(8):e3091. doi: 10.1371/journal.pone.0003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamangar BB, Gabillard JC, Bobe J. Insulin-like growth factor binding protein (IGFBP) 1, -2, -3, -4, -5, -6 and IGFBP-related protein 1 during rainbow trout postvitellogenesis and oocyte maturation: molecular characterization, expression profiles, and hormonal regulation. Endocrinology. 2006;147:2399–2410. doi: 10.1210/en.2005-1570. [DOI] [PubMed] [Google Scholar]
- Kawauchi H, Sower SA. The dawn and evolution of hormones in the adenohypophysis. Gen. Comp. Endocrinol. 2006;148:3–16. doi: 10.1016/j.ygcen.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Lee PD, Giudice LC, Conover CA, Powell DR. Insulin-like growth factor binding protein-1: recent findings and new directions. Proc. Soc. Exp. Biol. Med. 1997;216:319–357. doi: 10.3181/00379727-216-44182. [DOI] [PubMed] [Google Scholar]
- Lee BY, Howe AE, Conte MA, D’Cotta H, Pepey E, Baroiller JF, di Palma F, Carleton KL, Kocher TD. An EST resource for tilapia based on 17 normalized libraries and assembly of 116,899 sequence tags. BMC Genomics. 2010;11:278. doi: 10.1186/1471-2164-11-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001;22:53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- Moriyama S, Yamaguchi K, Takasawa T, Chiba H, Kawauchi H. Identification of two insulin-like growth factor IIs in the Japanese eel, Anguilla japonica: cloning, tissue distribution, and expression after growth hormone treatment and seawater acclimation. Comp. Biochem. Physiol. B. 2008;149:47–57. doi: 10.1016/j.cbpb.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Nishioka RS. Hypophysectomy of fish. In: Hochachka PW, Mommsen TP, editors. Biochemistry and Molecular Biology of Fishes: Analytical Techniques. New York: Elsevier; 1994. pp. 49–58. [Google Scholar]
- Ohlsson C, Mohan S, Sjögren K, Tivesten A, Isgaard J, Isaksson O, Jansson JO, Svensson J. The role of liver-derived insulin-like growth factor-I. Endocr. Rev. 2009;30:494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R, Shepherd BS, Nishioka RS, Grau EG, Bern HA. Effects of homologous pituitary hormone treatment on serum insulin-like growth-factor-binding proteins (IGFBPs) in hypophysectomized tilapia, Oreochromis mossambicus with special reference to a novel 20-kDa IGFBP. Gen. Comp. Endocrinol. 2000;117:404–412. doi: 10.1006/gcen.1999.7421. [DOI] [PubMed] [Google Scholar]
- Peddu SC, Breves JP, Kaiya H, Grau EG, Riley LG. Pre- and postprandial effects on ghrelin signaling in the brain and on the GH/IGF-I axis in the Mozambique tilapia (Oreochromis mossambicus) Gen. Comp. Endocrinol. 2009;161:412–418. doi: 10.1016/j.ygcen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Pedroso FL, Fukada H, Masumoto T. Molecular characterization, tissue distribution patterns and nutritional regulation of IGFBP-1,-2,-3 and-5 in yellowtail, Seriola quinqueradiata. Gen. Comp. Endocrinol. 2009;161:344–353. doi: 10.1016/j.ygcen.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Peterson BC, Waldbieser GC, Bilodeau L. Effects of recombinant bovine somatotropin on growth and abundance of mRNA for IGF-I and IGF-II in channel catfish (Ictalurus punctatus) J. Anim. Sci. 2005;83:816–824. doi: 10.2527/2005.834816x. [DOI] [PubMed] [Google Scholar]
- Peterson BC, Waldbieser GC. Effects of fasting on IGF-I, IGF-II, and IGF-binding protein mRNA concentrations in channel catfish (Ictalurus punctatus) Dom. Anim. Endocrinol. 2009;37:74–83. doi: 10.1016/j.domaniend.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucl. Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picha ME, Turano MJ, Beckman BR, Borski RJ. Endocrine biomarkers of growth and applications to aquaculture: a minireview of growth hormone, insulin-like growth factor (IGF)-I, and IGF-binding proteins as potential growth indicators in fish. N. Am. J. Aquacult. 2008;70:196–211. [Google Scholar]
- Pierce AL, Shimizu M, Beckman BR, Baker DM, Dickhoff WW. Time course of the GH/IGF axis response to fasting and increased ration in chinook salmon (Oncorhynchus tshawytscha) Gen. Comp. Endocrinol. 2005;140:192–202. doi: 10.1016/j.ygcen.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Pierce AL, Shimizu M, Felli L, Swanson P, Dickhoff WW. Metabolic hormones regulate insulin-like growth factor binding protein-1 mRNA levels in primary cultured salmon hepatocytes; lack of inhibition by insulin. J. Endocrinol. 2006;191:379–386. doi: 10.1677/joe.1.06986. [DOI] [PubMed] [Google Scholar]
- Pierce AL, Fox BK, Davis LK, Visitacion N, Kitashashi T, Hirano T, Grau EG. Prolactin receptor, growth hormone receptor, and putative somatolactin receptor in Mozambique tilapia: tissue specific expression and differential regulation by salinity and fasting. Gen. Comp. Endocrinol. 2007;154:31–40. doi: 10.1016/j.ygcen.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Pierce AL, Dickey JT, Felli L, Swanson P, Dickhoff WW. Metabolic hormones regulate basal and growth hormone-dependent igf2 mRNA level in primary cultured coho salmon hepatocytes: effects of insulin, glucagon, dexamethasone, and triiodothyronine. J. Endocrinol. 2010;204:331–339. doi: 10.1677/JOE-09-0338. [DOI] [PubMed] [Google Scholar]
- Pierce AL, Breves JP, Moriyama S, Hirano T, Grau EG. Differential regulation of Igf1 and Igf2 mRNA levels in tilapia hepatocytes: effects of insulin and cortisol on GH sensitivity. J. Endocrinol. 2011;211:201–210. doi: 10.1530/JOE-10-0456. [DOI] [PubMed] [Google Scholar]
- Pierce AL, Breves JP, Moriyama S, Uchida K, Grau EG. Regulation of growth hormone (GH) receptor (GHR1 and GHR2) mRNA level by GH and metabolic hormones in primary cultured tilapia hepatocytes. Gen. Comp. Endocrinol. 2012;179:22–29. doi: 10.1016/j.ygcen.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Rahman MS, Thomas P. Characterization of three IGFBP mRNAs in Atlantic croaker and their regulation during hypoxic stress: potential mechanisms of their upregulation by hypoxia. Am. J. Physiol. Endocrinol. Metab. 2011;301:E367–E648. doi: 10.1152/ajpendo.00168.2011. [DOI] [PubMed] [Google Scholar]
- Reindl KM, Kittilson JD, Sheridan MA. Differential ligand binding and agonist-induced regulation characteristics of the two rainbow trout GH receptors, Ghr1 and Ghr2, in transfected cells. J. Endocrinol. 2009;202:463–471. doi: 10.1677/JOE-09-0057. [DOI] [PubMed] [Google Scholar]
- Reindl KM, Sheridan MA. Peripheral regulation of the growth hormone-insulin-like growth factor system in fish and other vertebrates. Comp. Biochem. Physiol. A. 2012;163:231–245. doi: 10.1016/j.cbpa.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Reinecke M. Influences of the environment on the endocrine and paracrine fish growth hormone-insulin-like growth factor-I system. J. Fish Biol. 2010;76:1233–1254. doi: 10.1111/j.1095-8649.2010.02605.x. [DOI] [PubMed] [Google Scholar]
- Reinecke M, Schmid A, Ermatinger R, Loffing-Cueni D. Insulin-like growth factor I in the teleost Oreochromis mossambicus the tilapia: gene sequence, tissue expression, and cellular localization. Endocrinology. 1997;138:3613–3619. doi: 10.1210/endo.138.9.5375. [DOI] [PubMed] [Google Scholar]
- Saera-Vila A, Calduch-Giner JA, Perez-Sanchez J. Duplication of growth hormone receptor (GHR) in fish genome: gene organization and transcriptional regulation of GHR type I and II in gilthead sea bream (Sparus aurata) Gen. Comp. Endocrinol. 2005;142:193–203. doi: 10.1016/j.ygcen.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Saera-Vila A, Calduch-Giner JA, Prunet P, Perez-Sanchez J. Dynamics of liver GH/IGF axis and selected stress markers in juvenile gilthead sea bream (Sparus aurata) exposed to acute confinement: differential stress response of growth hormone receptors. Comp. Biochem. Physiol. A. 2009;154:197–203. doi: 10.1016/j.cbpa.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Safian D, Fuentes EN, Valdés JA, Molina A. Dynamic transcriptional regulation of autocrine/paracrine igfbp1, 2, 3, 4, 5, and 6 in the skeletal muscle of the fine flounder during different nutritional statuses. J. Endocrinol. 2012;214:95–108. doi: 10.1530/JOE-12-0057. [DOI] [PubMed] [Google Scholar]
- Schmid C, Schläpfer I, Peter M, Böni-Schnetzler M, Schwander J, Zapf J, Froesch ER. Growth hormone and parathyroid hormone stimulate IGFBP-3 in rat osteoblasts. Am. J. Physiol. 1994;267:E226–E233. doi: 10.1152/ajpendo.1994.267.2.E226. [DOI] [PubMed] [Google Scholar]
- Shamblott MJ, Cheng CM, Bolt D, Chen TT. Appearance of insulin-like growth factor mRNA in the liver and pyloric ceca of a teleost in response to exogenous growth hormone. Proc. Natl. Acad. Sci. USA. 1995;92:6943–6946. doi: 10.1073/pnas.92.15.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, Swanson P, Dickhoff WW. Free and protein-bound insulin-like growth factor-I (IGF-I) and IGF-binding proteins of coho salmon, Oncorhynchus kisutch. Gen. Comp. Endocrinol. 1999;115:398–405. doi: 10.1006/gcen.1999.7328. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Swanson P, Hara A, Dickhoff WW. Purification of a 41-kDa insulin-like growth factor binding protein from serum of chinook salmon, Oncorhynchus tshawytscha. Gen. Comp. Endocrinol. 2003;132:103–111. doi: 10.1016/s0016-6480(03)00052-2. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Dickey JT, Fukada H, Dickhoff WW. Salmon serum 22 kDa insulin-like growth factor-binding protein (IGFBP) is IGFBP-1. J. Endocrinol. 2005;184:267–276. doi: 10.1677/joe.1.05880. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Beckman BR, Hara A, Dickhoff WW. Measurement of circulating salmon IGF binding protein-1: assay development, response to feeding ration and temperature, and relation to growth parameters. J. Endocrinol. 2006;188:101–110. doi: 10.1677/joe.1.06475. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Cooper KA, Dickhoff WW, Beckman BR. Postprandial changes in plasma growth hormone, insulin, insulin-like growth factor (IGF)-I, and IGF-binding proteins in coho salmon fasted for varying periods. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R352–R361. doi: 10.1152/ajpregu.90939.2008. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Suzuki S, Horikoshi M, Hara A, Dickhoff WW. Circulating salmon 41-kDa insulin-like growth factor binding protein (IGFBP) is not IGFBP-3 but an IGFBP-2 subtype. Gen. Comp. Endocrinol. 2011a;171:326–331. doi: 10.1016/j.ygcen.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Kishimoto K, Yamaguchi T, Nakano Y, Hata A, Dickhoff WW. Circulating salmon 28- and 22-kDa insulin-like growth factor binding protein (IGFBPs) are co-rthologs of IGFBP-1. Gen. Comp. Endocrinol. 2011b;174:97–106. doi: 10.1016/j.ygcen.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Shved N, Berishvili G, Mazel P, Baroiller JF, Eppler E. Growth hormone (GH) treatment acts on the endocrine and autocrine/paracrine GH/IGF-axis and on TNF-α expression in bony fish pituitary and immune organs. Fish Shellfish Immunol. 2011;31:944–952. doi: 10.1016/j.fsi.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Siharath K, Kelley KM, Bern HA. A low-molecular-weight (25-kDa) IGF-binding protein is increased with growth inhibition in the fasting striped bass, Morone saxatilis. Gen. Comp. Endocrinol. 1996;102:307–316. doi: 10.1006/gcen.1996.0074. [DOI] [PubMed] [Google Scholar]
- Small BC, Peterson BC. Establishment of a time-resolved fluoroimmunoassay for measuring plasma insulin-like growth factor I (IGF-I) in fish: effect of fasting on plasma concentrations and tissue mRNA expression of IGF-I and growth hormone (GH) in channel catfish (Ictalurus punctatus) Domest. Anim. Endocrinol. 2005;28:202–215. doi: 10.1016/j.domaniend.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Tse MC, Vong QP, Cheng CH, Chan KM. PCR-cloning and gene expression studies in common carp (Cyprinus carpio) insulin-like growth factor-II. Biochim. Biophys. Acta. 2002;1575:63–74. doi: 10.1016/s0167-4781(02)00244-0. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kajimura S, Riley LG, Hirano T, Aida K, Grau EG. Effects of fasting on growth hormone/insulin-like growth factor I axis in the tilapia, Oreochromis mossambicus. Comp. Biochem. Physiol. A. 2003;134:429–439. doi: 10.1016/s1095-6433(02)00318-5. [DOI] [PubMed] [Google Scholar]
- Uchida K, Moriyama S, Breves JP, Fox BK, Pierce AL, Borski RJ, Hirano T, Grau EG. cDNA cloning and isolation of somatolactin in Mozambique tilapia and effects of seawater acclimation, confinement stress, and fasting on its pituitary expression. Gen. Comp. Endocrinol. 2009;161:162–170. doi: 10.1016/j.ygcen.2008.11.031. [DOI] [PubMed] [Google Scholar]
- Vong QP, Chan KM, Cheng CH. Quantification of common carp (Cyprinus carpio) IGF-I and IGF-II mRNA by real-time PCR: differential regulation of expression by GH. J. Endocrinol. 2003;178:513–521. doi: 10.1677/joe.0.1780513. [DOI] [PubMed] [Google Scholar]
- Wood AW, Duan C, Bern HA. Insulin-like growth factor signaling in fish. Int. Rev. Cytol. 2005;243:215–285. doi: 10.1016/S0074-7696(05)43004-1. [DOI] [PubMed] [Google Scholar]
- Yada T, Hirano T, Grau EG. Changes in plasma levels of the two prolactins and growth hormone during adaptation to different salinities in the euryhaline tilapia (Oreochromis mossambicus) Gen. Comp. Endocrinol. 1994;93:214–223. doi: 10.1006/gcen.1994.1025. [DOI] [PubMed] [Google Scholar]
- Yamada PM, Lee KW. Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. Am. J. Physiol. Cell Physiol. 2009;296:C954–C976. doi: 10.1152/ajpcell.00598.2008. [DOI] [PubMed] [Google Scholar]
- Zhou J, Li W, Kamei H, Duan C. Duplication of the IGFBP-2 gene in the teleost fish: protein structure and functionality conservation and gene expression divergence. PLoS One. 2008;3(12):e3926. doi: 10.1371/journal.pone.0003926. [DOI] [PMC free article] [PubMed] [Google Scholar]