Abstract
Parastomal hernia is a prevalent problem and treatment can pose difficulties due to significant rates of recurrence and morbidities of the repair. The current standard of care is to perform parastomal hernia repair with mesh whenever possible. There exist multiple options for mesh reinforcement (biologic and synthetic) as well as surgical techniques, to include type of repair (keyhole and Sugarbaker) and position of mesh placement (onlay, sublay, or intraperitoneal). The sublay and intraperitoneal positions have been shown to be superior with a lower incidence of recurrence. This procedure may be performed open or laparoscopically, both having similar recurrence and morbidity results. Prophylactic mesh placement at the time of stoma formation has been shown to significantly decrease the rates of parastomal hernia formation.
Keywords: parastomal hernia, biologic mesh, synthetic mesh, Sugarbaker technique, keyhole technique
CME Objectives: After reading this article, the reader should be able to
Identify the key steps involved with parastomal hernia repair
Describe the Sugarbaker and keyhole approaches to parastomal hernia repair
Understand the effectiveness and use of mesh in the approach to parastomal hernia repair
Understand the relative risks and advantages of synthetic versus biologic meshes in the repair of parastomal hernia
Understand the various anatomic options for mesh placement in parastomal hernia repair
Parastomal hernia is the most frequent complication associated with the creation of an ileostomy or colostomy.1 It is defined as an incisional hernia that occurs at or adjacent to the stoma.2 It is almost an inevitable complication of an ostomy creation if left in place long enough, with occurrence rates reported up to 56%.1 2 3 4 5 The variation in reported occurrence rates can be attributed to the lack of uniform definition of a parastomal hernia, different types of ostomies, as well as different lengths of follow-up time. In general, parastomal hernias occur more frequently with colostomies than ileostomies.6 End colostomies have the highest incidence of hernia formation, ranging from 4 to 48%, followed by loop colostomy (0–30.8%) and end ileostomies (1.8–28.3%), with loop ileostomies (0–6.2%) being the most infrequent.4 This is likely due to not only the larger trephine size usually required for a colostomy, but also the much higher rate of closure of loop ileostomies compared with colostomies, both end and loop, which have only a 40 to 67% rate of reversal.7
Most hernias occur within the first two years after stoma creation.1 Patient factors which have been associated with an increased risk of parastomal hernia include advanced age, obesity, weight gain after ostomy formation, malnutrition, chronic increased intra-abdominal pressure, steroid use, creation of ostomy in emergency setting, and a history of malignancy or inflammatory bowel disease.2 4 8 9 10 Obesity, defined as either BMI > 30 kg/m2 or waist circumference >100 cm, is most commonly associated with parastomal hernia and is the patient factor best supported by the clinical evidence.8 11 It has been shown that hernia prevalence among patients with a BMI of 30 or higher is 59.1% compared with 25.8% in patients with a BMI of less than 30.12 In another study, De Raet and colleagues identified waist circumference over 100 cm to be associated with a 75% chance of parastomal hernia formation.8
Optimal surgical technique is critical. The technical factors which have been shown to decrease the risk of formation of a parastomal hernia include stoma placement in the rectus abdominis muscle, minimizing the size of the transabdominal hole, and preoperative marking by an enterostomal nurse.13 14 It has been well documented that the larger the stomal opening, the greater the chance of hernia formation. In a prospective study looking at predictive factors in parastomal hernia development, Pilgrim et al found that aperture size was an independent predictor of hernia formation. It was concluded that for every additional 1-mm increase in abdominal wall opening size, there was a 10% increase in risk of hernia formation.6 Another study looking at patients with permanent end ostomies found that patients with a trephine <25 mm developed no abdominal hernias in 26-month follow-up.13 Although the exact etiology of the hernia formation is not well understood, it is believed that the hernia forms when tangential forces are applied to the circumference of the trephine, with stronger forces correlating to the larger opening, continually stretching the abdominal wall defect.15 From a pathophysiologic perspective, there has been increasing evidence that the herniation is a result of a shift in the collagen ratio and metabolism during wound repair.10 16 17 18
There are several classification systems for parastomal hernias that are based on size, location, contents, and radiologic findings associated with the hernia; however, none of these have much bearing on the clinical diagnosis or management strategy.19 20 21 22 23 Most patients with parastomal hernias are asymptomatic and diagnosis is typically based on physical exam. The most common presentation is a bulge at the site or adjacent to the site of the stoma. Other symptoms include mild abdominal discomfort, intermittent cramping, distention, nausea, vomiting, diarrhea, and constipation. Nonoperative management is a reasonable initial strategy because of the potentially high recurrence rate after a parastomal hernia repair. Most patients can successfully be managed with patient education, weight loss, and an ostomy hernia belt.24 However, it is estimated that 30 to 56% of patients with a parastomal hernia will ultimately require surgical repair.25 26 A strangulated or incarcerated hernia is an indication for urgent/emergent surgical repair because of the risk of ischemic bowel. Indications for elective repair include chronic obstruction, pain, appliance leakage, discomfort from an ill-fitting appliance, or peristomal skin breakdown.
Treatment Options
The best approach to repair parastomal hernia is, of course, closure of the stoma primarily. However, assuming this is not a clinically appropriate scenario, the hernia must be repaired. The “preferred” surgical approach to managing parastomal hernia repair has evolved over time, spurred by experience and the development of new adjunctive options. These options for treatment of parastomal hernia include primary repair, re-siting of the ostomy, and reinforced repair utilizing prosthetic or biologic mesh. Primary repair and re-siting are, for the most part, historical options, as the gold standard is now a repair with a prosthetic mesh. Primary repair involves reduction of the hernia, excision of the hernia sac as well as the attenuated and scar tissue, and the reapproximation of healthy fascia with suture. This technique has largely been abandoned due to unacceptably high recurrence rates. These have been reported to range between 50 and 100%. This, in combination with a rate of surgical site infection of 12%, has limited its current use.5 27 28 29 30 31 Re-siting of the ostomy is the most recently abandoned dogma, largely because increasing experience showed that creation of a new ostomy at a new location is associated with the same high risk of formation of a primary parastomal hernia at the new stoma site.5 28 In addition, the operation itself confers additional morbidity with a recurrence rate of approximately 36% and complication rate is as high as 88%.5 28 32 Furthermore, the patient is often at risk of developing an incisional hernia at the previous ostomy site.28
Therefore, reinforced repairs are now the most common and accepted methods of parastomal hernia repair and currently the standard of care. The use of synthetic mesh has substantially decreased the recurrence rate of parastomal hernias; however, the rate of local failure is still noteworthy, ranging from 7 to 18%.31 While the complication rates for mesh repairs are lower than for previously used techniques, they do introduce the possibility of complications not seen with other repairs, notably including mesh infection and fistula formation, which can have a significant impact on the patient's recovery and quality of life. It is also important to consider that the majority of the data showing success with mesh repair success have come from nonrandomized studies with small numbers of patients, nonuniform techniques, and wide variability in follow-up times. Nonetheless, it is clear that reinforced repairs offer clear superiority, and new products with hopefully improved efficacy and safety profiles continue to be developed. When performing a mesh repair, there are many different options that must be considered to include: type of mesh (synthetic versus biologic versus hybrid), positioning of mesh relative to the abdominal wall layers, technique of repair, and whether to perform the repair open or laparoscopically.
Mesh Options
The introduction of tissue reinforcement using mesh in hernia repairs has revolutionized the treatment of inguinal, ventral, and incisional hernias, and now repairs utilizing mesh have become the gold standard for parastomal hernias as well. Mesh options now include many different types of prosthetic and biologic variations (Table 1).
Table 1. Types of mesh available for parastomal hernia repairs.
| Type of mesh | Material | Pore size | Absorbable | Weight |
|---|---|---|---|---|
| Vicryl (Ethicon Endo-Surgery, Inc. Cincinnati, OH) | Polyglactin | Small (0.4 mm) | Yes (60–90 d) | Medium weight: 56 g/m2 |
| Gore-Tex (W.L. Gore & Associates, Inc. Newark, DE) | e-PTFE | Microscopic (3 μm) | No | Heavyweight |
| Marlex (C.R. Bard, Inc. Murray Hill, NJ) | Polypropylene | Small–medium (0.8 mm) | No | Heavyweight: 80–100 g/m2 |
| 3D Max (Davol, A BARD Company, Warwick, RI) | Polypropylene | |||
| Polysoft (Davol) | Polypropylene | |||
| Prolene (Ethicon) | Polypropylene | |||
| Surgipro (Covidien, Mansfield, MA) | Polypropylene | |||
| Prolite (Atrium Medical Corp. Hudson, NH) | Polypropylene | |||
| Trelex (Meadox Medicals Inc. Oakland, NJ) | Polypropylene | |||
| Atrium (Atrium) | Polypropylene | |||
| Premilene (B. Braun Medical Inc. Bethlehem, PA) | Polypropylene | |||
| Parietene (Covidien) | Polypropylene | |||
| Parietene Light (Covidien) | Polypropylene | Large (1.0–3.6 mm) | Light/medium weight: 36–48 g/m2 | |
| Optilene (B-Braun) | Polypropylene | |||
| Mersilene (Ethicon) | Polyester | Large (1–2 mm) | No | Medium weight: ∼40 g/m2 |
| Safil (B-Braun) | Polyglycolic | |||
| Dexon (Syneture (Covidien), Norwalk CT) | Polyglycolic | Medium (0.75 mm) | Yes (60–90 d) | |
| Composite mesh | ||||
| Parietex (Covidien) | Polyester/collagen | Large (> 3 mm) | Partially (20 d) | Medium weight: 75 g/m2 |
| Gore-tex Dual Mesh & Dual Mesh Plus (WL Gore) | e-PTFE | Microscopic (3/22 μm) | No | Heavyweight |
| Vypro, Vypro II (Ethicon) | Polypropylene/PG910 | Large (> 3 mm) | Partially (42 d) | Light weight: 25–30 g/m2 |
| Composix EX, Dulex (Davol) | Polypropylene/e-PTFE | Medium (0.8 mm) | No | Light weight |
| Proceed (Ethicon) | Polypropylene/cellulose (ORC) | Large | Partially (<30 d) | Light weight: 45 g/m2 |
| Dynamesh IPOM (FEG Textilteknik, Aachen, Germany) | Polypropylene/PVDF | Large (1–2 mm) | Partially | Medium weight: 60 g/m2 |
| Sepramesh (Genzyme Corp. Cambridge, MA) | Polypropylene/sodium hyaluronate | Large (1–2 mm) | Partially (<30 d) | Heavyweight: 102 g/m2 |
| Ultrapro (Ethicon) | Polypropylene/polyglecaprone (Monocryl) | Large (> 3 mm) | Partially (< 140 d) | Light weight: 28 g/m2 |
| Ti-mesh (Pfm Medical, Inc. Cologne, Germany) | Polypropylene/titanium | Large (> 1 mm) | No | Light and extra-light: 16–35 g/m2 |
| C-Qur (Atrium) | Polypropylene/omega 3 | Large (> 1 mm) | Partially (∼120 d) | Medium weight: 50 g/m2 |
| Biologic meshes | Source tissue | |||
| Surgisis (Cook Biotech, West Lafayette, IN) | Porcine (small intestine submucosa) | |||
| Fortagen (Organogenesis Inc. Canton, MA) | Porcine (small intestine submucosa) | |||
| Alloderm (Lifecell Corp. Bridgewater, NJ) | Human acellular dermis | |||
| Flex HD (J&J New Brunswick, NJ) | Human acellular dermis | |||
| AlloMax (Davol) | Human acellular dermis | |||
| Collamend (Davol) | Xenogenic acellular dermis (porcine/bovine) | |||
| Strattice (LifeCell) | Xenogenic acellular dermis (porcine/bovine) | |||
| Permacol (Tissue Science Laboratories (Covidien) Hampshire, UK) | Xenogenic acellular dermis (porcine/bovine) | |||
| XenMatriX (Davol) | Xenogenic acellular dermis (porcine/bovine) | |||
| SurgiMend (TEI Biosciences Inc. Waltham, MA) | Xenogenic acellular dermis (porcine/bovine) |
Synthetic Mesh
The most common type of mesh initially used was polypropylene. Polypropylene is an entirely synthetic mesh, whose macroporous structure allows it to affix well to the adjacent tissue due to the ingrowth of fibrocollagenous tissue.31 It has the ability to be incorporated into the native tissue, is permanent, and possesses high tensile strength, thus decreasing the recurrence of the hernia. However, this ingrowth can also lead to a significant inflammatory response causing severe adhesions and possible erosion that may cause complications in future operations.33 This type of mesh is cheap and strong; however, the magnitude of the potential complications has limited its use.34 35
Expanded polytetrafluoroethylene (e-PTFE) is an alternative synthetic that has also been commonly used for parastomal hernia repair. Unlike polypropylene, e-PTFE has a microporous make-up that does not allow tissue ingrowth into the prosthesis. While this characteristic helps to decrease the formation of adhesions, it may also lead to the increase in risk of re-herniation, because the mesh will be anchored to the abdominal wall solely by the sutures placed by the surgeon and encapsulation.36 37 In addition, the microporous composition makes it more susceptible to infection and if it becomes infected it must be removed.37 However, e-PTFE is very soft, allowing it to be better tolerated in the abdominal wall and less likely to erode into surrounding organs.31
There are now multiple types of composite mesh commonly made of polypropylene and e-PTFE, although polyvinylidene fluoride, cellulose, and omega-3 fatty acid–coated synthetic meshes have also been used. Composite mesh combines the advantages of the durability of the polypropylene with the safety of the e-PTFE. The mesh surface against the abdominal wall is the nonabsorbable polypropylene mesh that promotes ingrowth and incorporation, and the mesh surface containing e-PTFE, or similar material that is nonreactive and thus causes fewer adhesions, faces the abdominal contents. However, these composite meshes are not without potential problems. There are reports that adhesions may be prevented in the short term, but not necessarily in the long term.38
It has been well recognized in the recent hernia literature that infectious complications can be devastating. Mesh infection with synthetic mesh often requires mesh explantation, a difficult and potentially very morbid procedure. In addition, erosion of mesh into bowel can be disastrous, causing enterocutaneous fistulas and risk of sepsis or worse. When considering parastomal hernia repair, we are, almost by definition, placing the mesh in contact with bowel, and thus, for these repairs, the choice of an entirely synthetic mesh may be hazardous.37 39 40
One of the additional risks associated with synthetic mesh relates to morphologic change over time. Synthetic mesh tends to contract over time. This results in “pulling away” from the periphery, thus decreasing the effective area of reinforcement. In parastomal repair, where the bowel traverses a hole cut through the mesh, this trephine may also enlarge, thus widening the hole and leaving the bowel susceptible to recurrent herniation against a relatively rigid barrier and creating a “buttonhole” hernia, putting herniated bowel at risk for obstruction or erosion.41 42
Biologic Mesh
Biologic meshes have emerged as an alternative to synthetic reinforcement material. Collagen-based biologic grafts were first introduced in the 1980s.43 They are typically made up of an acellular collagen matrix that is slowly degraded and replaced by the tissue of the host.44 These are based on the premise that healing augmented by a biologic mesh will be more durable than primary repair alone, as well as avoiding the safety pitfalls associated with synthetic mesh and the risks of erosion. Examples of these grafts are derived from human dermis, porcine dermis, porcine small intestinal submucosa, and bovine pericardium. Various types of treatments of the mesh, such as fixing or cross-linking, are designed to increase strength, and durability can affect their strength and rate of bioactivity. In theory, due to their biocompatibility, once implanted they are vascularized and result in migration of host cells, therefore theoretically making them less prone to infection.44 Although biologic mesh was expected to have lower infection rates, it seems that rates of complications have been similar to synthetic mesh. It has been found that cross-linking and chemical treatments that strengthen these biologic meshes also decrease their bioactivity. A retrospective review, including four studies with a combined enrollment of 57 patients who underwent parastomal hernia repair utilizing biologic mesh, found a recurrence rate of 15.7% and a wound-related complication rate of 26.2% with no graft infections reported.44 In general, biologics are soft and pliable which may decrease the chance of discomfort and erosion. One of the most significant barriers to wider use of the biologics is cost: one piece can cost several thousands of dollars.44 In today's tight healthcare market, the logistic issues with obtaining a biologic, which may be 5 to 10 times more expensive than a similar synthetic, may be insurmountable.
In our practice, the net positives with regard to safety profile and efficacy have made the biologic options our first choice in parastomal hernia repair. Our preference is to use the non–cross-linked options which better combine the advantages of increased strength of an augmented repair, while allowing effective biologic remodeling.
Mesh Placement Options
There are different options for placement of the mesh relative to the abdominal wall fascia. These include placement over the fascia (onlay technique), below the anterior fascia and muscular levels, but above the posterior sheath/peritoneum, referred to as a sublay, or underlay, or the intraperitoneal technique in which the mesh is placed below all fascial levels. In all cases of parastomal hernia repair, the basic tenets involve reduction of the hernia, excision of the hernia sac, reapproximation of the hernia defect around the bowel, and placement of mesh to support the repair.
The onlay repair involves the placement of mesh over a primary fascial repair. The theoretical advantage of this technique is that it is a local repair that may avoid the morbidity of a prolonged open abdominal surgery. Patients do not need to undergo the extensive abdominal wall dissection to create planes in which to place the mesh that are required for the other techniques. In addition, patients have a quicker recovery and, because there may be no need for another abdominal incision, they are not at risk of an incisional hernia. A disadvantage is that intra-abdominal pressure may displace the mesh potentially explaining its higher recurrence rates, reported as high as 18.6%.31 Another disadvantage of this repair is that it theoretically has an increased risk of infection as it is in close proximity to the contaminated ostomy opening; however, data suggest that its infection rates are similar to other mesh repairs.31
The most common mesh repairs are done in the sublay and intraperitoneal positions that place the mesh below the anterior fascia. The advantage of a sublay repair is that it is performed in a sterile environment with a decreased risk of wound infection. Sublay and intraperitoneal placement of the mesh provides more biomechanical support due to the abdominal pressure further securing the mesh to the abdominal wall. While the sublay repair protects the mesh from interaction with abdominal organs, the intraperitoneal position poses an increased risk for bowel erosion and adhesion formation. In the intraperitoneal repair, care must be taken to maximize tissue apposition between the mesh and the abdominal wall to minimize the formation of seroma. This includes liberal use of closed suction drains placed between the mesh and the abdominal wall. Hansson and colleagues performed a systematic review of surgical techniques for parastomal hernia repair that involved a total of 35 studies, in which they found that, although not statistically significant, the onlay technique had the highest recurrence rate and the intraperitoneal had the lowest.31
Technique: Intraperitoneal Mesh Repair
There have been two primary ways described for intraperitoneal mesh repair, the “Sugarbaker” technique and the “keyhole” technique. The Sugarbaker technique was first described in 1985. A laparotomy was performed, and after the hernia was reduced, the sac resected, and the stoma trephine reduced to appropriate size (enough to just admit the surgeon's finger), the ostomy opening is covered with an intraperitoneally placed prosthetic mesh that is sutured to the fascia. The bowel is lateralized and secured between the mesh and the peritoneum, thereby lateralizing the forces which press the bowel ventrally onto the abdominal wall, shifting them from pushing up toward the defect and causing these forces to press ventrally against an intact abdominal wall (Fig. 1). In the seminal paper describing this technique, there were six recurrent and one primary parastomal hernias repaired, with no recurrences reported with a 5-year follow-up.45 In another, slightly larger study, 20 open parastomal hernia repairs with the Sugarbaker technique using a mesh with an overlap of at least 5 cm were reviewed retrospectively. There was a 15% recurrence rate with a mean follow-up of 42 months. Complications of the procedure included bowel obstruction secondary to dense adhesions, wound infection, seroma formation, and pain at the site of transfascial sutures.46 Initial use of this technique may cause anxiety due to the sharp angle created in the large bowel conduit (Fig. 1b). Surgeons should be reassured that with appropriate technique, this will not result in obstruction. If biologic mesh is used, eventually native tissue ingrowth results in, essentially, an extraperitoneal-type colostomy. In general, this approach is not used for small bowel stomas.
Fig. 1.
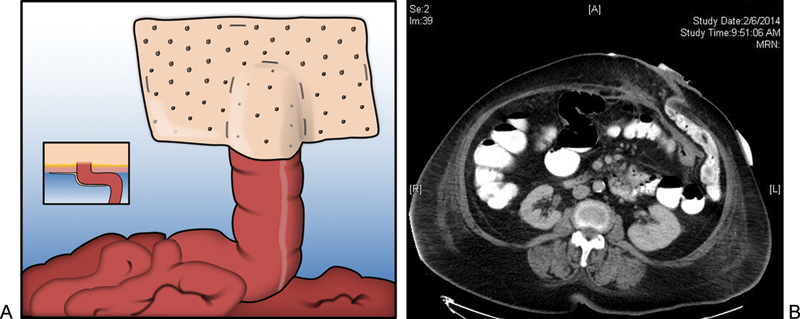
(a) Depiction of the Sugarbaker repair. Inset depicts axial view of lateralized bowel traversing abdominal wall with mesh placement relative to bowel and abdominal wall. (b) Postoperative CT scan showing axial view of lateralized bowel traversing the abdominal wall. Note the lateral most portion of bowel as it enters between the biologic mesh and the anterior abdominal wall. Contrast flows freely indicating lack of obstruction.
The other primary option for surgical repair is the “keyhole” technique. In the keyhole technique, a cut-out of mesh is made to circumferentially surround the ostomy and cover the entire hernia defect.34 47 48 One of the tricks of this technique is to not make the keyhole too small so as to cause a bowel obstruction, but to not make it so large as to increase the risk of herniation (Fig. 2).
Fig. 2.
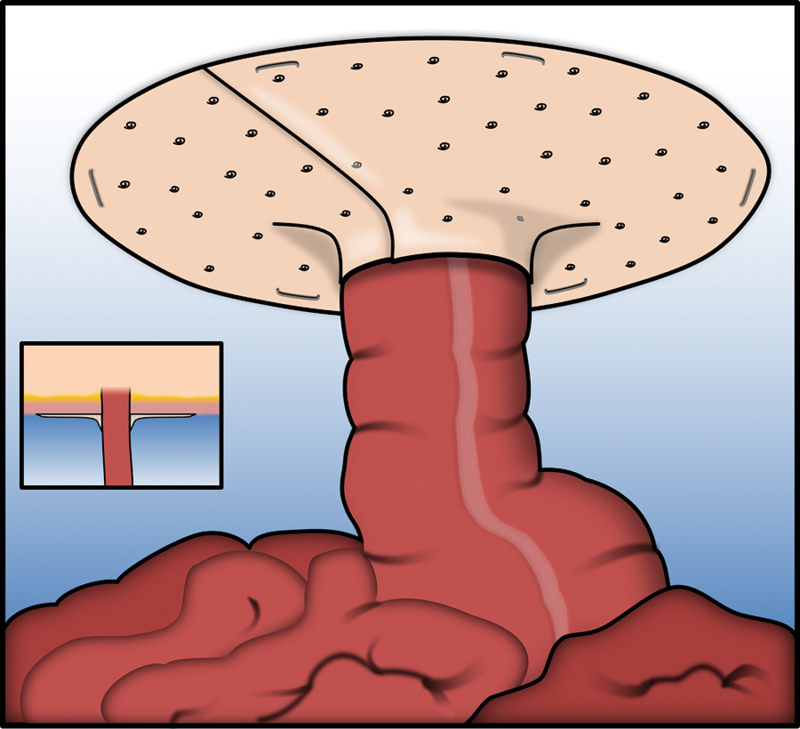
Depiction of the “Keyhole” repair. Inset depicts axial view of bowel traversing abdominal wall with mesh surrounding limb of stoma relative to bowel and abdominal wall.
The different studies using open mesh repair of parastomal hernias can be seen in Table 1.
Laparoscopic versus Open Repair
The laparoscopic approach has been increasingly adopted over the last two decades. The utility of laparoscopy for the repair of parastomal hernia, although now becoming a commonly used technique, has lagged behind the use of laparoscopy in other areas of colorectal surgery. In a recent retrospective study by Halabi and colleagues, using ACS-NSQIP data, records of patients who underwent parastomal hernia repair from 2005 to 2011 were systematically examined. Only 10.4% of the 2,167 patients in the study were treated laparoscopically. They hypothesized this was due to the fact that parastomal hernia repair cases are often associated with dense adhesions making laparoscopy more difficult or unsafe. Another possible explanation of the low utilization of laparoscopy that was offered is the lack of the strong clinical evidence demonstrating that laparoscopic parastomal hernia repairs are superior to open repairs, unlike the evidence that exists supporting the use of laparoscopy for ventral hernia repairs.49
However, there are multiple theoretical advantages to the use of laparoscopy when treating parastomal hernias. First, it avoids another large incision and potential hernia site in the abdominal wound and allows faster postoperative recovery. It also provides a better view of the defect, allowing a more precise repair and reinforcement with mesh and greater overlap of the defect.16 50 Unfortunately, there have been variable levels of success reported in the literature (see Table 2). Most studies demonstrate low infection rates (0–5%) and conversion to an open procedure is infrequent. In Hansson and colleagues' review, they looked at 363 laparoscopic repairs and found a conversion to open rate of 3.6%.31 The most common reasons for conversion include inadvertent enterotomy and dense adhesions (Table 3).49
Table 2. Outcomes of open mesh repairs of parastomal hernias.
| Study | Year | Number of repairs | Mesh type | Mesh position | Type of repair | Recurrence (%) | Infection (%) | Follow-up (mo) (mean) |
|---|---|---|---|---|---|---|---|---|
| Sugarbaker45 | 1980 | 7 | Polypropylene | Intraperitoneal | SB | 0 | 0 | 48–84 |
| Byers et al33 | 1992 | 9 | Polypropylene | Intraperitoneal | KH | 0 | 11.1 | (13.4) |
| Hofstetter et al48 | 1998 | 13 | PTFE | Intraperitoneal | KH | 0 | 0 | Over 96 |
| Morris-Stiff and Hughes34 | 1998 | 7 | Polypropylene | Intraperitoneal | KH | 28.6 | 14.3 | 60–89 (81) |
| Kasperk et al67 | 2000 | 7 | Polypropylene | Sublay | KH | 28.6 | 0 | 4–36 |
| Stelzner et al46 | 2004 | 20 | PTFE | Intraperitoneal | SB | 15 | 5 | 3–84 (42) |
| van Sprundel et al47 | 2005 | 15 | PTFE | Intraperitoneal | KH | 13.3 | 0 | 5–52 (29) |
| Longman and Thompson68 | 2005 | 10 | Polypropylene | Sublay | KH | 0 | 0 | 2–40 (30) |
| Ballas et al69 | 2006 | 2 | PTFE | Intraperitoneal | 0 | 0 | 24–60 (42) | |
| Guzmán-Valdivia et al70 | 2005 | 25 | Polypropylene | Sublay | KH | 8 | 8 | 8–24 (12) |
Abbreviations: KH, keyhole; SB, Sugarbaker.
Table 3. Outcomes of laparoscopic parastomal hernia repair.
| Study | Year | Technique | Mesh type | Number of repairs | Conversion (%) | Recurrence (%) | Infection (%) | Follow-up (mo) (mean) |
|---|---|---|---|---|---|---|---|---|
| Safadi71 | 2004 | KH | PTFE | 9 | 0 | 44.4 | 0 | 6–33 (24) |
| LeBlanc et al72 | 2005 | KH/SB | PTFE | 12 | 0 | 8.3 | 0 | 3–39 (20) |
| Muysoms et al73 | 2008 | KH/SB | Polyester/PTFE/PP | 24 | 0 | 41.7 | 0 | 4–54 (21.2) |
| Berger and Bientzle74 | 2007 | SB/SW | PVDF/PP | 66 | 1.5 | 12 | 4.5 | 3–72 (median = 24) |
| Mancini et al75 | 2007 | SB | PTFE | 25 | 0 | 4 | 4 | 2–38 (median = 19) |
| McLemore et al76 | 2007 | KH/SB | PTFE | 19 | – | 10.5 | 2 | (20) |
| Craft et al77 | 2008 | KH/SB | PTFE | 21 | 0 | 4.7 | 4.8 | 3–36 (14) |
| Pastor et al53 | 2009 | KH/SB | PTFE | 12 | 8.3 | 33.3 | 16.6 | 12–72 (13.9) |
| Hansson et al50 | 2009 | KH | PTFE | 54 | 14.5 | 37 | 1.8 | 12–72 (median = 36) |
| Wara and Andersen78 | 2011 | KH | PP/PTFE | 66 | 4 | 3 | 4.5 | 6–132 (median = 36) |
| Mizrahi et al79 | 2012 | KH | PP/PTFE | 29 | 6.9 | 46.4 | 3.4 | 12–53 (median = 30) |
Abbreviations: KH, keyhole; PP, polypropylene; PTFE, polytetrafluorethylene; PVDF, polyvinylidene fluoride; SB, Sugarbaker; SW, sandwich.
Laparoscopically, one may repair the defect via a keyhole repair, a modified Sugarbaker, or a “sandwich” technique. The modified Sugarbaker is the same as described for the open repair, but with these key technical points: the surgeon must achieve a minimum overlap of 5 cm past the defect, transabdominal suture fixation with permanent suture at 3–5 cm intervals, and placement of transabdominal suture on either side of the lateralized bowel.51 Hansson et al found that the laparoscopic keyhole technique had higher rates of recurrence than laparoscopic Sugarbaker repairs, 34.6 versus 11.6%, respectively.31 It appears that using a solid piece of mesh rather than a cut piece of mesh provides a lower recurrence rate as well as shorter operative times.
The sandwich technique has also been described for laparoscopic repairs. This is a combination of both the keyhole and Sugarbaker techniques, using a piece of mesh in the intraperitoneal position as in the keyhole technique and then lateralizing the bowel and covering this with another piece of mesh using the Sugarbaker technique. This technique does result in an area of mesh overlapping with mesh, which is generally avoided. There is only one study looking at this technique, performed by Berger and colleagues, that includes 42 patients with only a 2.1% rate of hernia recurrence.52 This technique, although only studied in a small group of people, did have the lowest recurrence rate for laparoscopic repairs.31
In an overall comparison made between open and laparoscopic cases using NSQIP data from 2005 to 2011, it was determined the laparoscopic approach is associated with better short-term results than open surgery, to include a 3-day reduction in length of hospital stay, a shorter operative time, and a 58% reduction in morbidity and 65% reduction in the odds of a superficial skin infection.49 However, in this study it was noted that patients who underwent laparoscopic repair were likely to be in better overall health than those who underwent open repairs.49 In Hansson's review, it was determined that laparoscopy had no advantage over open repair in regard to morbidity, mortality, and recurrence.31 There is only one study to specifically compare open to laparoscopic cases in a nonrandomized retrospective fashion. There was again no difference in morbidity, mortality, or recurrence, but there was a nearly significant difference in length of hospital stay (3 vs. 5 days).53
Prophylactic Use of Mesh When Creating the Initial Stoma
Due to the high rate of a parastomal hernia formation and the lack of a truly effective or superior way of treating parastomal hernias, there has been considerable interest into effective prophylaxis against the development of these hernias—thus, the idea of initial placement of a prophylactic mesh around the ostomy site at the time of ostomy creation. This idea was first introduced in 1986 and subsequently reported to be safe and effective in observational studies.54 Different surgical techniques have been described in placing mesh at the time of primary stoma formation, very similar to the different techniques of parastomal hernia repair. The onlay technique involves positioning of the mesh on the external rectus fascia.55 This was initially described by Bayer et al and Gogenur et al, who each used different types of mesh placed over the fascia with the bowel coming up through it.54 56 Bayer reported on 36 patients in which no patients had parastomal hernias in 1 to 4 years of follow-up, 8% had infections, and 3% had the need for re-operation for stomal narrowing.54 Similarly, Gogenur reported on 24 patients followed with a mean follow-up of 12 months. There were no immediate complications reported, but two (8%) patients did report migration of the mesh through the skin requiring local excision and two additional (8%) patients developed symptomatic recurrences.56
The sublay technique has been the most commonly used and the one most studied showing promising results. Multiple studies have examined this technique with several variations to include different types of fixation techniques and mesh.25 56 57 58 In the last 10 years, there have been three randomized controlled trials which have shown that implantation of mesh at the time of ostomy formation is associated with a decreased risk of parastomal hernia as compared with when no mesh is used.59 60 61 A meta-analysis was performed looking at these three prospective studies. The rate of hernia in the nonmesh group was 54.7% compared with the hernia rate of 12.3% in the mesh group, with a risk reduction of 77%. The need for surgical intervention for the parastomal hernia was 13% in the nonmesh group compared with 0% in the mesh group. There was also no difference in morbidity when compared with the group that did not receive implant of mesh at the time of ostomy formation.62
Questions that are raised with the use of prophylactic placement of mesh revolve around the technique as well as type and size of mesh, what size hole, and how should it best be secured in place. From a technique perspective, this is driven primarily by experience and comfort. Most of the existing literature is based on a keyhole reinforcement, although in theory a Sugarbaker approach would work equally well. Our decision to use the keyhole technique for prophylaxis centers around the ease and efficiency of its use. Adding this to the existing operation and stoma creation takes little extra time, whereas an appropriately placed Sugarbaker reinforcement may add 45 minutes to an hour, to an already long case. The concerns with the placement of synthetic mesh in this situation is that according to the general consensus in hernia literature, synthetic mesh is contraindicated in clean-contaminated, contaminated, and dirty surgical fields because of high complication rates.37 39 40 63 Interestingly, as with all dogma, it is eventually called into question; Carbonell and colleagues, citing the “questionable long-term durability” of biologic meshes, conducted a study looking at outcomes using synthetic mesh in contaminated ventral hernia repairs. Stoma revisions or creations comprised about half of the contaminated cases. In a retrospective analysis of 100 patients, they found that surgical site occurrence rates were reasonable (26.2% in clean-contaminated cases and 34% in contaminated cases), with a 7% recurrence rate and mesh explantation required in only 4 patients, with a mean follow-up of almost 11 months (10.4 ± 9.9).64 Nevertheless, despite the favorable outcomes of this small study, the Ventral Hernia Working Group recommendations still currently contraindicates synthetic mesh in such cases, recommending bioprosthetics.65 Therefore, biologic mesh has also been applied prophylactically at the time of ostomy creation and also been found to reduce the incidence of parastomal hernia similar to synthetic mesh.59 Recently, there has been a cost analysis performed that found the use of prophylactic mesh to prevent parastomal hernia formation was less expensive and more effective when compared with no prophylactic mesh.66
Conclusion
Parastomal hernia is a massively prevalent problem. In reality, almost every stoma will ultimately result in some degree of parastomal hernia if followed for long enough. The complications of hernia range from asymptomatic to potentially life-threatening. The traditional paradigm of direct repair and stoma re-siting has largely been abandoned due to unacceptable recurrence rates at the initial site as well as the new site. The current standard of care is to perform an appropriate repair of the hernia in situ, with augmented repair using mesh. The current trend, and our recommendation and practice, is to use biologic mesh due to the efficacy and favorable safety profile. The sublay or intra-abdominal approach offers the lowest recurrence rate, and is our recommendation. The decision whether to approach the repair laparoscopically or open is based on the surgeon's level of experience and comfort. Finally, due to the known likely development of parastomal hernia in the majority of cases, we recommend prophylactic parastomal reinforcement at the time of permanent stoma creation. Given the increased use of laparoscopy at the time of many colectomies, as well as the ease of placement, a sublay or intraperitoneal technique in these cases is favored.
Further experience as well as development of effective and safer biologic meshes will continue to provide surgeons with safer, more effective material to use to prevent the development of parastomal hernia.
References
- 1.Londono-Schimmer E E, Leong A P, Phillips R K. Life table analysis of stomal complications following colostomy. Dis Colon Rectum. 1994;37(9):916–920. doi: 10.1007/BF02052598. [DOI] [PubMed] [Google Scholar]
- 2.Pearl R K. Parastomal hernias. World J Surg. 1989;13(5):569–572. doi: 10.1007/BF01658872. [DOI] [PubMed] [Google Scholar]
- 3.Shabbir J, Chaudhary B N, Dawson R. A systematic review on the use of prophylactic mesh during primary stoma formation to prevent parastomal hernia formation. Colorectal Dis. 2012;14(8):931–936. doi: 10.1111/j.1463-1318.2011.02835.x. [DOI] [PubMed] [Google Scholar]
- 4.Carne P W, Robertson G M, Frizelle F A. Parastomal hernia. Br J Surg. 2003;90(7):784–793. doi: 10.1002/bjs.4220. [DOI] [PubMed] [Google Scholar]
- 5.Cheung M T, Chia N H, Chiu W Y. Surgical treatment of parastomal hernia complicating sigmoid colostomies. Dis Colon Rectum. 2001;44(2):266–270. doi: 10.1007/BF02234303. [DOI] [PubMed] [Google Scholar]
- 6.Pilgrim C H, McIntyre R, Bailey M. Prospective audit of parastomal hernia: prevalence and associated comorbidities. Dis Colon Rectum. 2010;53(1):71–76. doi: 10.1007/DCR.0b013e3181bdee8c. [DOI] [PubMed] [Google Scholar]
- 7.Husain S G, Cataldo T E. Late stomal complications. Clin Colon Rectal Surg. 2008;21(1):31–40. doi: 10.1055/s-2008-1055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Raet J, Delvaux G, Haentjens P, Van Nieuwenhove Y. Waist circumference is an independent risk factor for the development of parastomal hernia after permanent colostomy. Dis Colon Rectum. 2008;51(12):1806–1809. doi: 10.1007/s10350-008-9366-5. [DOI] [PubMed] [Google Scholar]
- 9.Bass E M, Del Pino A, Tan A, Pearl R K, Orsay C P, Abcarian H. Does preoperative stoma marking and education by the enterostomal therapist affect outcome? Dis Colon Rectum. 1997;40(4):440–442. doi: 10.1007/BF02258389. [DOI] [PubMed] [Google Scholar]
- 10.Jansen P L, Mertens Pr Pr, Klinge U, Schumpelick V. The biology of hernia formation. Surgery. 2004;136(1):1–4. doi: 10.1016/j.surg.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Arumugam P J, Bevan L, Macdonald L. et al. A prospective audit of stomas—analysis of risk factors and complications and their management. Colorectal Dis. 2003;5(1):49–52. doi: 10.1046/j.1463-1318.2003.00403.x. [DOI] [PubMed] [Google Scholar]
- 12.Schreinemacher M H, Vijgen G H, Dagnelie P C, Bloemen J G, Huizinga B F, Bouvy N D. Incisional hernias in temporary stoma wounds: a cohort study. Arch Surg. 2011;146(1):94–99. doi: 10.1001/archsurg.2010.281. [DOI] [PubMed] [Google Scholar]
- 13.Hotouras A, Murphy J, Power N, Williams N S, Chan C L. Radiological incidence of parastomal herniation in cancer patients with permanent colostomy: what is the ideal size of the surgical aperture? Int J Surg. 2013;11(5):425–427. doi: 10.1016/j.ijsu.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Sjödahl R, Anderberg B, Bolin T. Parastomal hernia in relation to site of the abdominal stoma. Br J Surg. 1988;75(4):339–341. doi: 10.1002/bjs.1800750414. [DOI] [PubMed] [Google Scholar]
- 15.de Ruiter P, Bijnen A B. Successful local repair of paracolostomy hernia with a newly developed prosthetic device. Int J Colorectal Dis. 1992;7(3):132–134. doi: 10.1007/BF00360352. [DOI] [PubMed] [Google Scholar]
- 16.Hansson B M, de Hingh I H, Bleichrodt R P. Laparoscopic parastomal hernia repair is feasible and safe: early results of a prospective clinical study including 55 consecutive patients. Surg Endosc. 2007;21(6):989–993. doi: 10.1007/s00464-007-9244-6. [DOI] [PubMed] [Google Scholar]
- 17.Klinge U, Binnebösel M, Rosch R, Mertens P. Hernia recurrence as a problem of biology and collagen. J Minim Access Surg. 2006;2(3):151–154. doi: 10.4103/0972-9941.27729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Junge K, Klinge U, Rosch R. et al. Decreased collagen type I/III ratio in patients with recurring hernia after implantation of alloplastic prostheses. Langenbecks Arch Surg. 2004;389(1):17–22. doi: 10.1007/s00423-003-0429-8. [DOI] [PubMed] [Google Scholar]
- 19.Devlin H B. London: Butterworths; 1983. Peristomal hernia; p. 441. [Google Scholar]
- 20.Moreno-Matias J, Serra-Aracil X, Darnell-Martin A. et al. The prevalence of parastomal hernia after formation of an end colostomy. A new clinico-radiological classification. Colorectal Dis. 2009;11(2):173–177. doi: 10.1111/j.1463-1318.2008.01564.x. [DOI] [PubMed] [Google Scholar]
- 21.Gil G, Szczepkowski M. A new classification of parastomal hernias—from the experience at Bielański Hospital in Warsaw. Pol Przegl Chir. 2011;83(8):430–437. doi: 10.2478/v10035-011-0067-8. [DOI] [PubMed] [Google Scholar]
- 22.Seo S H, Kim H J, Oh S Y, Lee J H, Suh K W. Computed tomography classification for parastomal hernia. J Korean Surg Soc. 2011;81(2):111–114. doi: 10.4174/jkss.2011.81.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin M, Bailey H. New York: Marcel Dekker, Inc.; 1993. Parastomal hernias; pp. 245–267. [Google Scholar]
- 24.Martin L, Foster G. Parastomal hernia. Ann R Coll Surg Engl. 1996;78(2):81–84. [PMC free article] [PubMed] [Google Scholar]
- 25.Israelsson L A. Preventing and treating parastomal hernia. World J Surg. 2005;29(8):1086–1089. doi: 10.1007/s00268-005-7973-z. [DOI] [PubMed] [Google Scholar]
- 26.Ripoche J, Basurko C, Fabbro-Perray P, Prudhomme M. Parastomal hernia. A study of the French federation of ostomy patients. J Vis Surg. 2011;148(6):e435–e441. doi: 10.1016/j.jviscsurg.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Carne P W, Frye J N, Robertson G M, Frizelle F A. Parastomal hernia following minimally invasive stoma formation. ANZ J Surg. 2003;73(10):843–845. doi: 10.1046/j.1445-2197.2003.02779.x. [DOI] [PubMed] [Google Scholar]
- 28.Rubin M S Schoetz D J Jr Matthews J B Parastomal hernia. Is stoma relocation superior to fascial repair? Arch Surg 19941294413–418., discussion 418–419 [DOI] [PubMed] [Google Scholar]
- 29.Rieger N, Moore J, Hewett P, Lee S, Stephens J. Parastomal hernia repair. Colorectal Dis. 2004;6(3):203–205. doi: 10.1111/j.1463-1318.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- 30.Riansuwan W, Hull T L, Millan M M, Hammel J P. Surgery of recurrent parastomal hernia: direct repair or relocation? Colorectal Dis. 2010;12(7):681–686. doi: 10.1111/j.1463-1318.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- 31.Hansson B M, Slater N J, van der Velden A S. et al. Surgical techniques for parastomal hernia repair: a systematic review of the literature. Ann Surg. 2012;255(4):685–695. doi: 10.1097/SLA.0b013e31824b44b1. [DOI] [PubMed] [Google Scholar]
- 32.Tekkis P P, Kocher H M, Payne J G. Parastomal hernia repair: modified thorlakson technique, reinforced by polypropylene mesh. Dis Colon Rectum. 1999;42(11):1505–1508. doi: 10.1007/BF02235057. [DOI] [PubMed] [Google Scholar]
- 33.Byers J M, Steinberg J B, Postier R G. Repair of parastomal hernias using polypropylene mesh. Arch Surg. 1992;127(10):1246–1247. doi: 10.1001/archsurg.1992.01420100112019. [DOI] [PubMed] [Google Scholar]
- 34.Morris-Stiff G, Hughes L E. The continuing challenge of parastomal hernia: failure of a novel polypropylene mesh repair. Ann R Coll Surg Engl. 1998;80(3):184–187. [PMC free article] [PubMed] [Google Scholar]
- 35.Halm J A de Wall L L Steyerberg E W Jeekel J Lange J F Intraperitoneal polypropylene mesh hernia repair complicates subsequent abdominal surgery World J Surg 2007312423–429., discussion 430 [DOI] [PubMed] [Google Scholar]
- 36.Simmermacher R K, Schakenraad J M, Bleichrodt R P. Reherniation after repair of the abdominal wall with expanded polytetrafluoroethylene. J Am Coll Surg. 1994;178(6):613–616. [PubMed] [Google Scholar]
- 37.Bleichrodt R P, Simmermacher R K, van der Lei B, Schakenraad J M. Expanded polytetrafluoroethylene patch versus polypropylene mesh for the repair of contaminated defects of the abdominal wall. Surg Gynecol Obstet. 1993;176(1):18–24. [PubMed] [Google Scholar]
- 38.Schreinemacher M H, Emans P J, Gijbels M J, Greve J W, Beets G L, Bouvy N D. Degradation of mesh coatings and intraperitoneal adhesion formation in an experimental model. Br J Surg. 2009;96(3):305–313. doi: 10.1002/bjs.6446. [DOI] [PubMed] [Google Scholar]
- 39.Trupka A W, Hallfeldt K K, Schmidbauer S, Schweiberer L. Management of complicated incisional hernias with underlay-technique implanted polypropylene mesh. An effective technique in French hernia surgery [in German] Chirurg. 1998;69(7):766–772. doi: 10.1007/s001040050488. [DOI] [PubMed] [Google Scholar]
- 40.Temudom T Siadati M Sarr M G Repair of complex giant or recurrent ventral hernias by using tension-free intraparietal prosthetic mesh (Stoppa technique): lessons learned from our initial experience (fifty patients) Surgery 19961204738–743., discussion 743–744 [DOI] [PubMed] [Google Scholar]
- 41.Cassar K, Munro A. Surgical treatment of incisional hernia. Br J Surg. 2002;89(5):534–545. doi: 10.1046/j.1365-2168.2002.02083.x. [DOI] [PubMed] [Google Scholar]
- 42.Klinge U, Klosterhalfen B, Müller M, Schumpelick V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg. 1999;165(7):665–673. doi: 10.1080/11024159950189726. [DOI] [PubMed] [Google Scholar]
- 43.Decurtins M, Buchmann P. Bovines Perikard Ein neues Material zur plastischen Deckung grosser Bauchwanddehiszenzen (in German) Res Exp Med (Berl) 1982;180(1):11–14. doi: 10.1007/BF01852226. [DOI] [PubMed] [Google Scholar]
- 44.Slater N J, Hansson B M, Buyne O R, Hendriks T, Bleichrodt R P. Repair of parastomal hernias with biologic grafts: a systematic review. J Gastrointest Surg. 2011;15(7):1252–1258. doi: 10.1007/s11605-011-1435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugarbaker P H. Peritoneal approach to prosthetic mesh repair of paraostomy hernias. Ann Surg. 1985;201(3):344–346. doi: 10.1097/00000658-198503000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stelzner S, Hellmich G, Ludwig K. Repair of paracolostomy hernias with a prosthetic mesh in the intraperitoneal onlay position: modified Sugarbaker technique. Dis Colon Rectum. 2004;47(2):185–191. doi: 10.1007/s10350-003-0030-9. [DOI] [PubMed] [Google Scholar]
- 47.van Sprundel T C, Gerritsen van der Hoop A. Modified technique for parastomal hernia repair in patients with intractable stoma-care problems. Colorectal Dis. 2005;7(5):445–449. doi: 10.1111/j.1463-1318.2005.00820.x. [DOI] [PubMed] [Google Scholar]
- 48.Hofstetter W L, Vukasin P, Ortega A E, Anthone G, Beart R W Jr. New technique for mesh repair of paracolostomy hernias. Dis Colon Rectum. 1998;41(8):1054–1055. doi: 10.1007/BF02237400. [DOI] [PubMed] [Google Scholar]
- 49.Halabi W J, Jafari M D, Carmichael J C. et al. Laparoscopic versus open repair of parastomal hernias: an ACS-NSQIP analysis of short-term outcomes. Surg Endosc. 2013;27(11):4067–4072. doi: 10.1007/s00464-013-3062-9. [DOI] [PubMed] [Google Scholar]
- 50.Hansson B M, Bleichrodt R P, de Hingh I H. Laparoscopic parastomal hernia repair using a keyhole technique results in a high recurrence rate. Surg Endosc. 2009;23(7):1456–1459. doi: 10.1007/s00464-008-0253-x. [DOI] [PubMed] [Google Scholar]
- 51.Asif A, Ruiz M, Yetasook A. et al. Laparoscopic modified Sugarbaker technique results in superior recurrence rate. Surg Endosc. 2012;26(12):3430–3434. doi: 10.1007/s00464-012-2358-5. [DOI] [PubMed] [Google Scholar]
- 52.Berger D, Bientzle M. Polyvinylidene fluoride: a suitable mesh material for laparoscopic incisional and parastomal hernia repair! A prospective, observational study with 344 patients. Hernia. 2009;13(2):167–172. doi: 10.1007/s10029-008-0435-4. [DOI] [PubMed] [Google Scholar]
- 53.Pastor D M, Pauli E M, Koltun W A, Haluck R S, Shope T R, Poritz L S. Parastomal hernia repair: a single center experience. JSLS. 2009;13(2):170–175. [PMC free article] [PubMed] [Google Scholar]
- 54.Bayer I, Kyzer S, Chaimoff C. A new approach to primary strengthening of colostomy with Marlex mesh to prevent paracolostomy hernia. Surg Gynecol Obstet. 1986;163(6):579–580. [PubMed] [Google Scholar]
- 55.Helgstrand F, Gögenur I, Rosenberg J. Prevention of parastomal hernia by the placement of a mesh at the primary operation. Hernia. 2008;12(6):577–582. doi: 10.1007/s10029-008-0387-8. [DOI] [PubMed] [Google Scholar]
- 56.Gögenur I, Mortensen J, Harvald T, Rosenberg J, Fischer A. Prevention of parastomal hernia by placement of a polypropylene mesh at the primary operation. Dis Colon Rectum. 2006;49(8):1131–1135. doi: 10.1007/s10350-006-0615-1. [DOI] [PubMed] [Google Scholar]
- 57.Berger D. Prevention of parastomal hernias by prophylactic use of a specially designed intraperitoneal onlay mesh (Dynamesh IPST) Hernia. 2008;12(3):243–246. doi: 10.1007/s10029-007-0318-0. [DOI] [PubMed] [Google Scholar]
- 58.López-Cano M, Lozoya-Trujillo R, Espin-Basany E. Prosthetic mesh in parastomal hernia prevention. Laparoscopic approach. Dis Colon Rectum. 2009;52(5):1006–1007. doi: 10.1007/DCR.0b013e31819a6a58. [DOI] [PubMed] [Google Scholar]
- 59.Hammond T M, Huang A, Prosser K, Frye J N, Williams N S. Parastomal hernia prevention using a novel collagen implant: a randomised controlled phase 1 study. Hernia. 2008;12(5):475–481. doi: 10.1007/s10029-008-0383-z. [DOI] [PubMed] [Google Scholar]
- 60.Jänes A Cengiz Y Israelsson L A Preventing parastomal hernia with a prosthetic mesh: a 5-year follow-up of a randomized study World J Surg 2009331118–121., discussion 122–123 [DOI] [PubMed] [Google Scholar]
- 61.Serra-Aracil X, Bombardo-Junca J, Moreno-Matias J. et al. Randomized, controlled, prospective trial of the use of a mesh to prevent parastomal hernia. Ann Surg. 2009;249(4):583–587. doi: 10.1097/SLA.0b013e31819ec809. [DOI] [PubMed] [Google Scholar]
- 62.Wijeyekoon S P, Gurusamy K, El-Gendy K, Chan C L. Prevention of parastomal herniation with biologic/composite prosthetic mesh: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Surg. 2010;211(5):637–645. doi: 10.1016/j.jamcollsurg.2010.06.111. [DOI] [PubMed] [Google Scholar]
- 63.Voyles C R, Richardson J D, Bland K I, Tobin G R, Flint L M, Polk H C Jr. Emergency abdominal wall reconstruction with polypropylene mesh: short-term benefits versus long-term complications. Ann Surg. 1981;194(2):219–223. doi: 10.1097/00000658-198108000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carbonell A M, Criss C N, Cobb W S, Novitsky Y W, Rosen M J. Outcomes of synthetic mesh in contaminated ventral hernia repairs. J Am Coll Surg. 2013;217(6):991–998. doi: 10.1016/j.jamcollsurg.2013.07.382. [DOI] [PubMed] [Google Scholar]
- 65.Breuing K, Butler C E, Ferzoco S. et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148(3):544–558. doi: 10.1016/j.surg.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 66.Lee L, Saleem A, Landry T, Latimer E, Chaudhury P, Feldman L S. Cost effectiveness of mesh prophylaxis to prevent parastomal hernia in patients undergoing permanent colostomy for rectal cancer. J Am Coll Surg. 2014;218(1):82–91. doi: 10.1016/j.jamcollsurg.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 67.Kasperk R, Klinge U, Schumpelick V. The repair of large parastomal hernias using a midline approach and a prosthetic mesh in the sublay position. Am J Surg. 2000;179(3):186–188. doi: 10.1016/s0002-9610(00)00309-3. [DOI] [PubMed] [Google Scholar]
- 68.Longman R J, Thomson W H. Mesh repair of parastomal hernias—a safety modification. Colorectal Dis. 2005;7(3):292–294. doi: 10.1111/j.1463-1318.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- 69.Ballas K D, Rafailidis S F, Marakis G N, Pavlidis T E, Sakadamis A K. Intraperitoneal ePTFE mesh repair of parastomal hernias. Hernia. 2006;10(4):350–353. doi: 10.1007/s10029-006-0090-6. [DOI] [PubMed] [Google Scholar]
- 70.Guzmán-Valdivia G, Guerrero T S, Laurrabaquio H V. Parastomal hernia-repair using mesh and an open technique. World J Surg. 2008;32(3):465–470. doi: 10.1007/s00268-007-9373-z. [DOI] [PubMed] [Google Scholar]
- 71.Safadi B. Laparoscopic repair of parastomal hernias: early results. Surg Endosc. 2004;18(4):676–680. doi: 10.1007/s00464-003-8518-x. [DOI] [PubMed] [Google Scholar]
- 72.LeBlanc K A, Bellanger D E, Whitaker J M, Hausmann M G. Laparoscopic parastomal hernia repair. Hernia. 2005;9(2):140–144. doi: 10.1007/s10029-004-0295-5. [DOI] [PubMed] [Google Scholar]
- 73.Muysoms E E, Hauters P J, Van Nieuwenhove Y, Huten N, Claeys D A. Laparoscopic repair of parastomal hernias: a multi-centre retrospective review and shift in technique. Acta Chir Belg. 2008;108(4):400–404. doi: 10.1080/00015458.2008.11680249. [DOI] [PubMed] [Google Scholar]
- 74.Berger D, Bientzle M. Laparoscopic repair of parastomal hernias: a single surgeon's experience in 66 patients. Dis Colon Rectum. 2007;50(10):1668–1673. doi: 10.1007/s10350-007-9028-z. [DOI] [PubMed] [Google Scholar]
- 75.Mancini G J, McClusky D A III, Khaitan L. et al. Laparoscopic parastomal hernia repair using a nonslit mesh technique. Surg Endosc. 2007;21(9):1487–1491. doi: 10.1007/s00464-007-9419-1. [DOI] [PubMed] [Google Scholar]
- 76.McLemore E C, Harold K L, Efron J E, Laxa B U, Young-Fadok T M, Heppell J P. Parastomal hernia: short-term outcome after laparoscopic and conventional repairs. Surg Innov. 2007;14(3):199–204. doi: 10.1177/1553350607307275. [DOI] [PubMed] [Google Scholar]
- 77.Craft R O, Huguet K L, McLemore E C, Harold K L. Laparoscopic parastomal hernia repair. Hernia. 2008;12(2):137–140. doi: 10.1007/s10029-007-0299-z. [DOI] [PubMed] [Google Scholar]
- 78.Wara P, Andersen L M. Long-term follow-up of laparoscopic repair of parastomal hernia using a bilayer mesh with a slit. Surg Endosc. 2011;25(2):526–530. doi: 10.1007/s00464-010-1205-9. [DOI] [PubMed] [Google Scholar]
- 79.Mizrahi H, Bhattacharya P, Parker M C. Laparoscopic slit mesh repair of parastomal hernia using a designated mesh: long-term results. Surg Endosc. 2012;26(1):267–270. doi: 10.1007/s00464-011-1866-z. [DOI] [PubMed] [Google Scholar]


