Assessment of different ADRB assays to determine their suitability for the evaluation of neutrophil stimulation by antimalarial antibodies.
Keywords: cellular immune response, malaria, polymorphonuclear neutrophil granulocyte
Abstract
Semi-immunity against Pf malaria is based on a combination of cellular and humoral immune responses. PMNs and IgGs are considered important components of this process, but the underlying mechanisms are unclear. We investigated the neutrophilic ADRB by analyzing the production of ROS in response to Pf antigen-specific IgGs bound to solid-phase immobilized antigens (sADRB) or whole merozoites (mADRB). We found that the PMN stimulations in each assay were based on different underlying mechanisms, demonstrating the importance of the assay set-up for the evaluation of antibody-triggered PMN responses. In the sADRB assay, ROS were produced externally, and by specific blocking of CD32(a)/FcγRII(a), the immediate neutrophilic response was abolished, whereas the removal of CD16(b)/FcγRIII(b) had no substantial effect. The key role of CD32(a) was confirmed using CD16(b)-deficient PMNs, in which similar changes of neutrophilic ADRB profiles were recorded after treatment. In the mADRB assay, ROS were produced almost exclusively within the cell, suggesting that the underlying mechanism was phagocytosis. This was confirmed using an additional phagocytosis assay, in which PMNs specifically ingested merozoites opsonized with Ghanaian plasma IgGs, seven times more often than merozoites opsonized with European plasma IgGs (P<0.001). Our data show that assay set-ups used to evaluate the responses of PMNs and perhaps other effector cells must be chosen carefully to evaluate the appropriate cellular responses. Our robust, stable, and well-characterized methods could therefore be useful in malaria vaccine studies to analyze the antimalarial effector function of antibodies.
Introduction
Malaria is an insect-transmitted parasitic disease that is responsible for ∼627,000 deaths/year. It is one of the most widespread infectious diseases in the world, but there is no available vaccine, and resistance toward common antimalarial drugs is increasing. One major, current research objective is to gain insight into the phenomenon of semi-immunity against malaria, which would facilitate the development of much-needed efficient vaccination and treatment strategies [1–5]. It is therefore necessary to develop and optimize assays that determine the functionality of malaria vaccine candidates in terms of the induced protective immune responses. However, it is unclear which characteristics of malaria blood-stage vaccines induce protective responses, and the contribution of antibodies to the human immune response against malaria is not fully understood [6, 7].
The ability of antibodies to reduce the asexual parasite load has been demonstrated by the passive transfer of IgG from semi-immune adults to infected patients [8, 9]. Antibody-mediated, antiparasitic immune responses are considered to be based on two general functional modes of action: the direct inhibition of merozoite invasion [10] and ADCI [11, 12]. The inhibition of merozoite invasion by antibodies does not, in isolation, correlate with protection against the disease [7]. However, the interaction of antibodies (especially cytophilic IgG1 and IgG3) with monocytes is commonly accepted as the basis of monocyte-mediated ADCI, although the underlying mechanism is not fully understood [13], and the assay remains to be reproducible [6]. Furthermore, the hyperactivation of monocyte erythrophagocytosis in nonimmune malaria patients is considered to be responsible, in part, besides intravascular hemolysis and defective erythropoiesis, for the severe anemia that accompanies malaria [14, 15].
On the other hand, there is early evidence that PMNs may play a role in the human immune defense against malaria. The activity of PMNs against malaria parasites has been demonstrated in vitro [16, 17], and their phagocytic activity has been observed in vivo [18]. The antipathogenic repertoire of PMNs comprises two components: the secretion of regulatory or cytotoxic molecules, such as ROS, and the phagocytosis of target cells [19, 20]. More recently, clinical protection from malaria was shown to correlate with ADRB activity of PMNs [21].
Experimentally, two different assay set-ups have been used to analyze the production of ROS, i.e., the neutrophilic ADRB, in response to malaria-specific antibodies. We distinguish these assays using the terms sADRB (for solid-phase immobilized antigen-based ADRB) and mADRB (for opsonized merozoite-based ADRB) as shown in Supplemental Fig. 1. PMN response profiles differ according to the type of stimulus or the type of immune complex involved [23–25]. Here, we expand these investigations to include neutrophilic, antiparasitic responses. We investigated the mechanism of PMN activation in both assays and characterized the resulting responses using malaria-specific IgG antibodies from the plasma of semi-immune Ghanaian blood donors. The ADRB assays were validated by comparing with ELISAs using the same antigens, i.e., the 11-kDa fragment of MSP-1 (MSP-119), AMA-1, and self-prepared SZ-lysate.
PMNs constitutively express two types of FcγR, namely CD16(b)/FcγRIII(b) and CD32(a)/FcγRII(a), but they can also express CD64/FcγRI after stimulation [26]. The role of the FcγRs in PMN activation remains unclear [25, 27]. At least in murine models, FcγRs are known to control Plasmodium berghei and Plasmodium chabaudi infections [28, 29]. Therefore, we also determined which FcγR was responsible for the stimulation of human PMNs in the sADRB and mADRB assays. Finally, we localized the ROS after stimulation, representing the site of many other neutrophil-derived, antipathogenic compounds, and showed that PMNs phagocytose and do not secrete ROS toward extracellular-opsonized merozoites in vitro.
MATERIALS AND METHODS
Ethics statement and collection of SIP samples
Plasma samples were obtained in accordance with the Helsinki Declaration on Scientific Research, and study approval was received from the Regional Committee on Human Research Publication and Ethics of The Kwame Nkrumah University of Science and Technology (Kumasi, Ghana). All study participants declared written, informed consent after the aims and procedures had been explained to them. All participants were clinically examined for acute infection, pregnancy, nursing, and/or anemia, which were disqualifying criteria. As a prognostic marker for the semi-immunity of the study population, the study participants had been living in the holoendemic region of central Ghana without acute malaria infections for at least 2 years. In total, samples from 31 adult blood donors were collected, including eight females and 23 males. The mean age of the study group was 31 (±6) years.
Cultivation of Pf and the preparation of merozoites and SZ-lysate
Pf 3D7A (MRA-151) and D10 ACP(transit)-GFP (MRA-569; D10 with cytosolic expression of the GFP) [30] were cultivated routinely, as described previously [31]. Briefly, parasites were maintained at 5% hematocrit in 0+ erythrocyte pools from 16 blood donors from the regional blood bank. Parasites were synchronized when necessary using 5% sorbitol [32]. After the enrichment of late-stage parasites by MACS (Miltenyi Biotec, Bergisch Gladbach, Germany) [33] or 70% Percoll-gradient centrifugation [34], the resulting enriched schizonts were allowed to mature for 8 h in the presence of 10 μM E64, as described previously [35].
The SZ-lysate was prepared by pelleting E64-treated schizonts for 10 min at 640 g, resuspending them in sterile PBS (Life Technologies, Darmstadt, Germany), and freezing at −80°C. After thawing, schizonts were lysed by sonication.
Free merozoites were prepared by pelleting E64-treated schizonts for 10 min at 1900 g. The pellets were resuspended in 5 ml complete medium, and the schizonts were ruptured by passage through a 1.2-μm Acrodisc syringe filter (Pall, Dreieich, Germany) [35]. Merozoites were stored in RPMI 1640 (PAA Laboratories, Coelbe, Germany) in aliquots and frozen immediately at −80°C for use in the ADRB assays. As described previously [21], freeze-thawed, opsonized merozoites retain their ability to induce ADRB activity, although they are not viable any more. Merozoites for flow cytometry were used immediately after filtration.
Determination of plasma IgG antigen reactivities
The reactivities of 31 Ghanaian SIP samples toward 100 ng MSP-119 (Pf 3D7A), 50 ng AMA-1 (DiCo1–3) [36], or 500 ng SZ-lysate (Pf 3D7A)/well were determined by ELISA [37]. Antigens were coated onto the surface of 96-well, high-binding plates (Greiner Bio-One, Solingen, Germany). Samples were applied in three, 1:5 serial dilutions, starting from 1:100. A SIP-pool was applied in seven, twofold dilutions. Based on the reactivity of the positive control, a standard curve was fitted with a four-parameter logistic model, using the open-source software “R” for statistical computing [38]. Sample reactivity is indicated as relative reactivity to the SIP-pool.
Antigen-bound human IgG was detected with a goat anti-human IgGFcAP antibody (Jackson ImmunoResearch, West Grove, PA, USA). Sample positivity was defined as the reactivity of a NIP control plus two sds.
Purification of plasma IgG
Plasma IgG was purified from 5 ml plasma (0.45 μm prefiltered) by Protein G affinity chromatography (1-ml HiTrap Protein G column, equilibrated with 0.2 M Tris-HCl, pH 9.0), using the ÄKTA purifier HPLC system (GE Healthcare, Uppsala, Sweden) and Unicorn software version 5.10. The IgG fraction was eluted in 0.1 M glycine (pH 2.7), neutralized immediately with 1 M Tris-HCl (pH 9.0), dialyzed against PBS, and stored at −80°C.
PMN isolation, FcγR treatment, and flow cytometry
PMNs were obtained from healthy, malaria-naive European blood donors. Each experiment was performed at least twice, using PMNs from two donors in technical duplicates, except for the experiments using CD16(b)-deficient PMNs, which were isolated from one single donor.
PMNs were isolated by dextran sedimentation and Ficoll-gradient centrifugation, as described previously [39], with minor modifications. The PMNs were kept sterile at 4°C throughout the procedure. Purified PMNs were resuspended in HBSS (E15-009; PAA Laboratories) without Ca2+, Mg2+, or phenol red, which was used throughout the investigation. The cells were counted in a CASY cell counter (Scharfe System, Reutlingen, Germany), viability was confirmed using the trypan blue exclusion method, and purity was confirmed by Giemsa staining.
The dependence of ROS production on FcγR was determined by removing CD16(b) or blocking CD32(a). CD16(b) was removed by adding 1 U/ml Pi-PLC (Molecular Probes, Darmstadt, Germany) to 2 × 106 PMNs/ml for 30 min at 37°C [25]. CD32(a) was blocked specifically by adding 0.5 μg of a specific blocking antibody (eBioscience, Frankfurt, Germany) to 105 PMNs for 1 h at 4°C [40].
FcγR availability was monitored by flow cytometry on a BD FACSVerse (BD Biosciences, Heidelberg, Germany) using the following specific fluorophore-conjugated antibodies: mouse anti-human CD16FITC (Immunostep, Salamanca, Spain), mouse anti-human CD32Alexa Fluor 647 (Abd Serotec, Duesseldorf, Germany), and mouse anti-human CD64FITC (Abd Serotec). PMNs were gated in the FSC and SSC channels, and 10,000 cells were counted/sample.
ADRB assays
The ADRB assays were carried out based on previously described methods [21, 41, 42]. We set up alternative assays to determine the sADRB and mADRB (Supplemental Fig. 1).
In the sADRB assay, opaque, 96-well, high-binding plates (Greiner Bio-One) were coated at 4°C overnight. The IgG reactivity of the 31 SIP samples was screened in a final assay volume of 150 μl (with 750 ng coated single antigen or 1.5 μg coated SZ-lysate), whereas PMN responses (location of ROS and dependence on FcγRs) were characterized in a final assay volume of 75 μl (with 75 ng coated single antigen). The following steps were carried out at room temperature, unless otherwise stated, and the wells were washed three times in PBS containing 0.1% Tween-20 between steps. The wells were blocked for 1 h with 2% BSA in PBS, followed by incubation with 1:5-diluted decomplemented plasma or purified IgG for 2 h. We used 1:5-diluted, purified IgG for the determination of plasma IgG reactivity or 1:5-diluted plasma for the characterization experiments. Chemiluminescence was detected in HBSS using 83.3 μM luminol and appropriate numbers of PMNs at 37°C for 1 h (3×105 PMNs in the screening experiments; 1.5×105 PMNs to characterize the PMN response), with readings taken at 2-min intervals using a multiplate reader (Synergy HT; BioTek, Bad Friedrichshall, Germany; Genios Pro, Tecan, Männedorf, Switzerland). PMNs were added in the dark, immediately before readings were initiated. ROS were localized by adding 2000 U/ml CAT (Sigma-Aldrich, Taufkirchen, Germany) and 50 U/ml SOD (Sigma-Aldrich) before adding the PMNs to scavenge extracellular ROS. Control experiments were set up using the less membrane-permeable luminescence amplifier isoluminol [43–45]. In preliminary titration experiments, an isoluminol concentration of 83.3 μM was shown to be saturated.
For the mADRB assay, 1.25 × 106 merozoites were incubated with 50 μl purified IgG or plasma (native or decomplemented) for 2 h. The opsonized merozoites were pelleted (20 min, 1500 g), resuspended in HBSS (50 μl for IgG reactivity assays; 25 μl for PMN response assays), and then transferred to a previously blocked well of an opaque, 96-well, high-binding plate (Greiner Bio-One) for the measurement of chemiluminescence, as stated above.
The required amounts or dilutions for the plasma samples in the sADRB and mADRB assays were estimated previously by titration experiments. For the sADRB, dilutions of 1:5 and 1:25 were tested. In the case of the mADRB, six, twofold dilutions, starting with 100 μl pure plasma, were used for the opsonization of merozoites.
For plasma IgG screening experiments, samples were standardized according to their reactivity against 167 μg/ml human OZ. The area under the curve was calculated for each kinetics measurement. PBS was used to determine background chemiluminescence, whereas purified IgG from a nonimmune donor was used as a negative control. Sample positivity was defined as the reactivity of the NIP control plus two sds.
PMN responses were characterized as an example using SIP 5 (highly reactive to AMA-1) and a sample pool, containing four highly reactive samples, to rule out individual differences (SIP-pool). Samples were chosen based on their reactivity in the screening of ADRB assays. One NIP was used as a negative control.
Kinetic profiles for the ADRB assays were plotted using GraphPad Prism version 5 (GraphPad Software, La Jolla, CA, USA).
In the characterization experiments, the luminescence intensities of the standard sADRB and mADRB were normalized to the reactivity of SIP 5 in each assay and presented in arbitrary units. The kinetic profiles of the modified sADRB and mADRB assay (FcγR evaluation or identification of the cellular site of the production of ROS) are presented as residual percentages of the response toward the respective samples in the unmodified sADRB or mADRB assay.
Confirmation of phagocytosis by flow cytometry and confocal laser-scanning microscopy
We opsonized 3.6 × 106 Pf D10 ACP(transit)-GFP merozoites with 50 μl decomplemented plasma (31 SIPs and six NIPs) or HBSS, each in duplicate for 2 h at room temperature. The merozoites were pelleted for 20 min at 1500 g and mixed with 1.5 × 105 PMNs. The samples were incubated for 10 min at 37°C and analyzed immediately by flow cytometry on a BD FACSVerse. PMNs were gated in the FSC and SSC channels, and background GFP was determined using PMNs that had not been exposed to merozoites. The percentage of phagocytic PMNs was calculated as the relative number of PMNs with positive GFP/5000 counted cells. A positive percentage of phagocytosis was defined if its number was higher than the percentage induced by the most reactive NIP, plus two sds. Box plots showing the phagocytosis rates for the SIPs and NIPs, respectively, were generated using OriginPro 8.1 G (OriginLab, Northhampton, MA, USA).
After flow cytometry, PMN and merozoite nuclei were counterstained with 100 μg/ml Hoechst 33342 (Sigma-Aldrich). The samples were transferred onto SuperFrost microscope slides (Thermo Fisher Scientific, Braunschweig, Germany), covered with a coverslip, and analyzed immediately by confocal laser-scanning microscopy using a Leica TCS SP8 confocal microscope (Leica Microsystems, Wetzlar, Germany) to distinguish merozoite adherence from phagocytosis.
Statistical analysis
The statistical correlation of the reactivities measured by ELISA and the ADRB assays were assessed by determining the Kendall's rank correlation coefficient τ using the open-source software R. The results were considered significant at P ≤ 0.05.
The statistical significance of the differences in phagocytosis rates between SIP- and NIP-opsonized merozoites was assessed using the Mann-Whitney test in GraphPad Prism version 5.
RESULTS
Characterization of the sADRB and mADRB assay
In titration experiments, optimal plasma amounts were determined for both ADRB assays. The optimal detection of a positive response was seen at a dilution of 1:5 in the sADRB assay and the application of 50 μl plasma in the mADRB assay. We ascertained the specificity of the sADRB and mADRB assays by comparing them with corresponding ELISA-based sample reactivities. The percentage of reactive samples among the 31 Ghanaian SIPs compared with a German NIP control was comparable between the ELISAs and corresponding sADRB assays (Supplemental Figs. 2 and 3 and Table 1). Moreover, there was a statistically significant correlation when comparing the intensities of the ELISA and ADRB assay reactivities (Table 1). In the sADRB assay, these correlations were more significant for individual antigens, whereas in the mADRB assay, the correlations were more significant for the whole SZ-lysate. These data clearly show that both ADRB assays distinguish PMN activation by IgGs that are Pf antigen specific or whole merozoite specific. There were similar kinetics of ROS production in all samples, and therefore, we chose two representative samples, IgG from a single SIP donor (SIP 5) and the SIP-pool, to characterize the neutrophilic ADRB response in depth. We verified the specificity of the luminol-based assays by determining the oxidation of dihydrorhodamine-123 by flow cytometry after 10 min and 30 min stimulation with opsonized merozoites (Supplemental Fig. 4). In these experiments, 30–40% of PMNs were fluorescent after 10 min, and 70–80% were positive after 30 min. In these experiments, using OZ as agonist, 65% and 80% of PMNs were fluorescent after 10 and 30 min, respectively. As we opted to investigate the kinetics of the PMN response, we focused on the luminol-based readout throughout the study.
Table 1. Sample Positivity and Significance of Correlation between ELISAs and ADRB Assays.
| Antigen used in ELISA | Positive samples in ELISA, % | ADRB assay type | Positive samples in ADRB assay, %a | Significance of correlation, P value of Kendall's τ |
|---|---|---|---|---|
| AMA-1 | 94 | sADRB | 100 | 1 × 10−12 |
| mADRB | 97 | 0.001 | ||
| MSP-119 | 81 | sADRB | 74 | <1 × 10−5 |
| mADRB | 97 | 0.0002 | ||
| SZ-lysate | 94 | sADRB | 97 | 0.003 |
| mADRB | 97 | <1 × 10−6 |
Sample positivity was defined as the reactivity of the corresponding NIP control plus two sds.
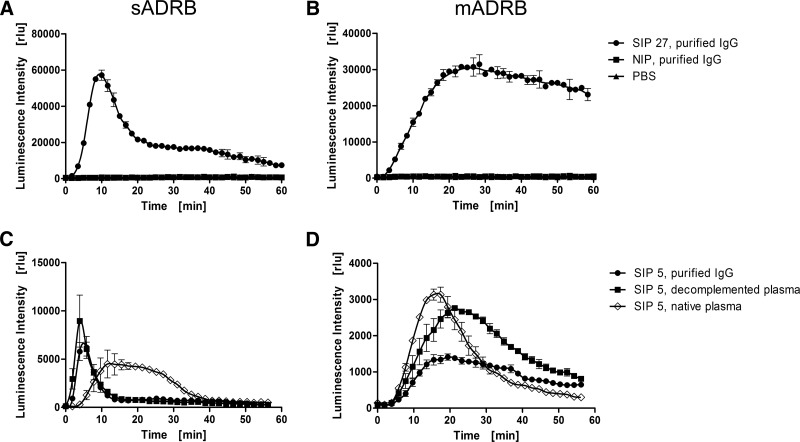
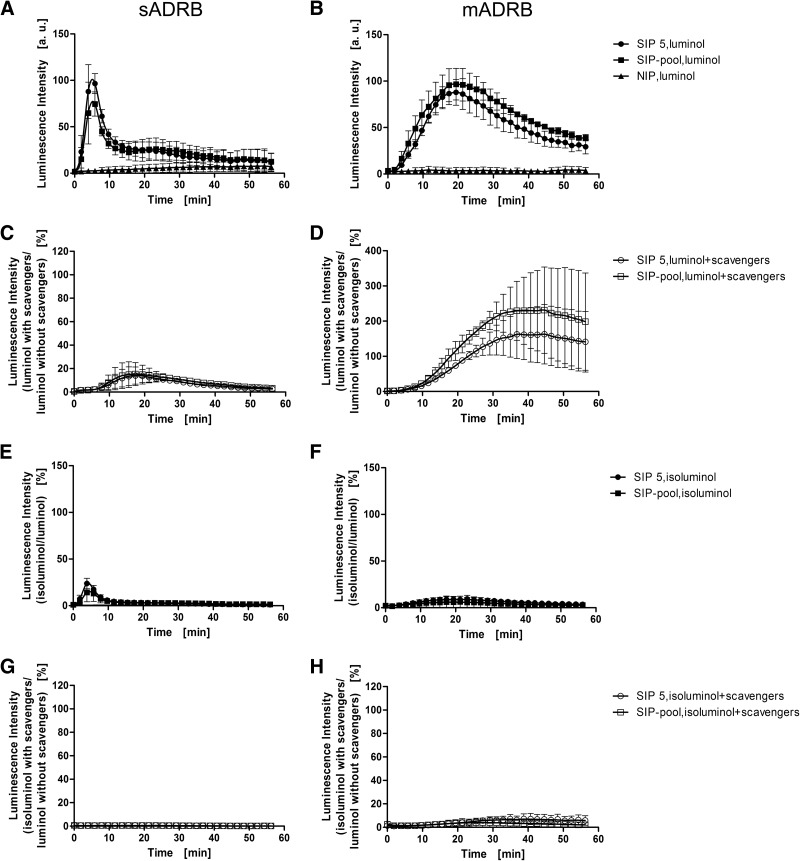
The profile of ROS production during the neutrophilic sADRB clearly differed from the mADRB in terms of overall kinetics and the time of onset (Fig. 1A and B). In the sADRB assay, PMNs underwent single-peak activation after ∼5 min, followed by a reduction in activity, whereas the mADRB assay resulted in a bell-like or plateau-shaped response curve. To avoid the potential loss of IgG fractions during purification, we verified the suitability of decomplemented plasma as a replacement for purified IgG. In both assays, we found that the IgG and decomplemented plasma profiles were similar, although they differed in intensity, whereas the profile generated by native plasma was distinct (Fig. 1C and D). We therefore used decomplemented plasma to investigate IgG-triggered PMN responses. This experimental set-up has the inherited weakness of not completely reflecting the in vivo situation in humans, where PMNs are exposed to complement as well. Nevertheless, we opted for this strategy to characterize the in-depth role of the FcγRs on PMN stimulation without the interfering effects of the interaction between the complement and its receptors.
Figure 1. Characterization of the ADRB response profile.
PMN responses toward purified IgGs obtained from Ghanaian SIP samples were analyzed using the sADRB (A and C) and mADRB (B and D) assays over a 60-min kinetic window. (A and B) One representative sample (SIP 27, black circles) is shown, which elicits a typical sADRB kinetic profile using MSP-119 as the antigen (A) and a typical mADRB kinetic profile (B) using PMNs from one donor. IgG from a European NIP (black squares) and PBS (black triangles) were used as negative controls. The influence of complement in the sADRB (C) and mADRB (D) assays was assessed using native plasma (open diamonds), decomplemented plasma (black squares), and purified IgG (black circles), using mixed PMNs from two donors. The response profiles toward the IgG/plasma from SIP 5 are shown as a representative example. Profiles represent the mean of the detected luminescence intensity in relative luminescence units (rlu) ± range of one technical duplicate.
Evaluation of the dependence on FcγR CD16(b) or CD32(a)
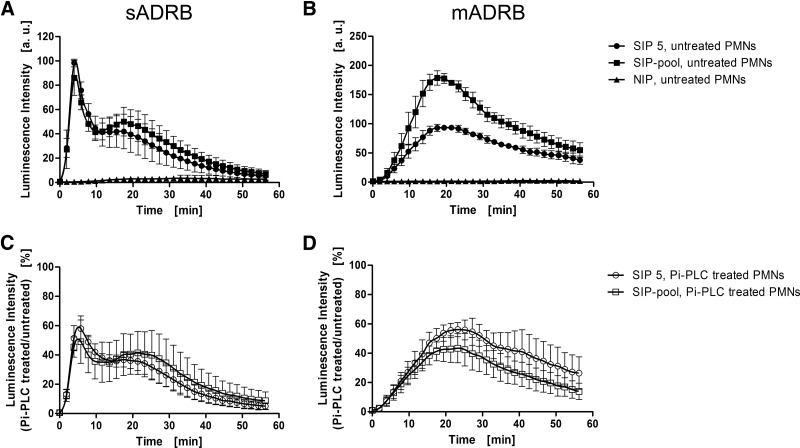
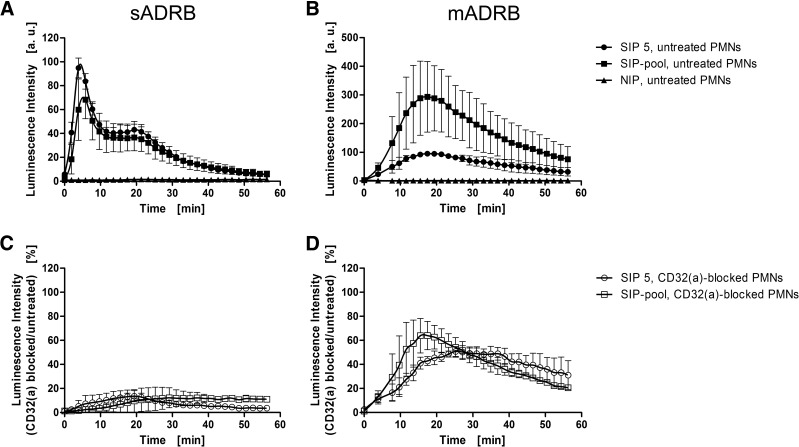
We next characterized the FcγR-specific dependence of sADRB and mADRB responses using unstimulated PMNs that express CD16(b) and CD32(a) but not CD64 (Supplemental Fig. 5A). The binding to CD16(b) was selectively prevented by enzymatic removal of the receptor using Pi-PLC (Fig. 2), whereas the binding to CD32(a) was blocked with an antibody (Fig. 3). Flow cytometry revealed in each case that receptor availability was reduced by ∼75% without markedly affecting the other receptors (Supplemental Fig. 5B). We made sure that the treatments did not induce any background activity using NIP controls, in which no PMN activation occurred after treatment.
Figure 2. Dependence of the neutrophilic ADRB on CD16(b).
The role of CD16(b) in the neutrophilic sADRB (A and C) and mADRB (B and D) was assessed in a 60-min kinetic window using untreated PMNs (black symbols; A and B) and PMNs with enzymatically removed CD16(b) (open symbols; C and D). The reactivities of PMNs were measured against SIP 5 (circles), the SIP-pool (squares), and one NIP (triangles), as representative examples. The data represent the mean ± sd of two experiments, using PMNs from two different donors, each in technical duplicates. (A and B) luminescence intensities were normalized to the reactivity of SIP 5 in each assay and therefore, are presented in arbitrary units (a.u.). (C and D) The residual percentages compared with the respective kinetics without Pi-PLC treatment are depicted.
Figure 3. Dependence of the neutrophilic ADRB on CD32(a).
The importance of CD32(a) in the neutrophilic sADRB (A and C) and mADRB (B and D) was assessed in a 60-min kinetic window using untreated PMNs (black symbols; A and B) and PMNs treated with a CD32(a)-blocking antibody (open symbols; C and D). The reactivities of PMNs were measured against SIP 5 (circles), the SIP-pool (squares), and one NIP (triangles) as representative examples. The data represent the mean ± sd of two experiments using PMNs from two different donors, each in technical duplicates. (A and B) Luminescence intensities were normalized to the reactivity of SIP 5 in each assay and therefore, are presented in arbitrary units. (C and D) The residual percentages compared with the respective kinetics without blocking of CD32(a) are depicted.
In the sADRB assay, the removal of CD16(b) from PMNs resulted in a 50% reduction of signal intensity but without influence on the overall response profile (Fig. 2A and C). The blocking of CD32(a) reduced PMN activity to <20% compared with untreated PMNs. In particular, the first distinct peak was abolished almost completely, resulting in a substantially different response profile (Fig. 3A and C). In the mADRB assay, both the removal of CD16(b) (Fig. 2B and D) and the blocking of CD32(a) (Fig. 3B and D) led to a 50% reduction in PMN activity.
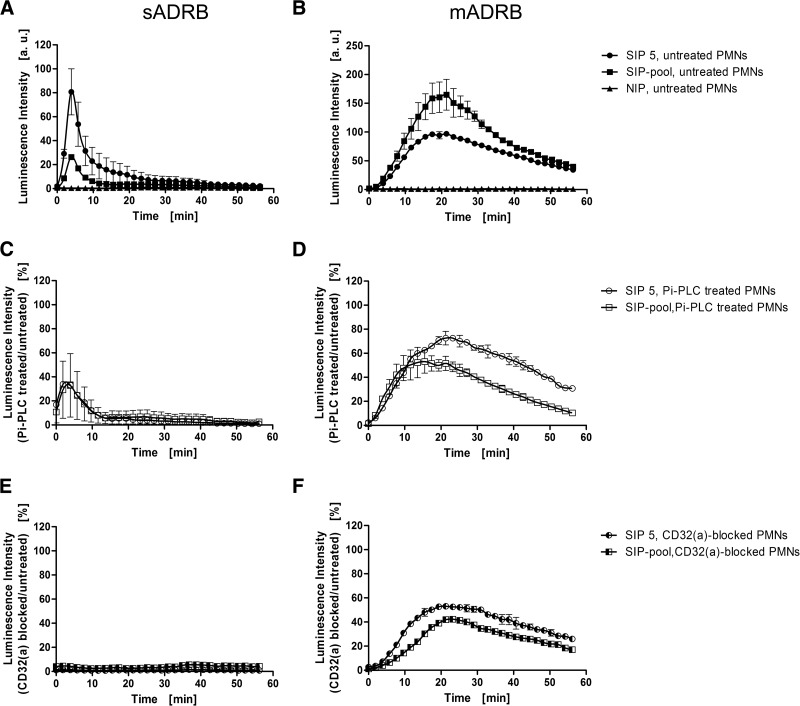
As the former results were not clear, control experiments were performed using CD16(b)-deficient PMNs, whose donor was recruited during the study. The lack of CD16(b) expression was confirmed by flow cytometry (Supplemental Fig. 4A). The comparable behavior of these PMNs during the ADRB assays was ascertained by verifying the same response profiles in both ADRB assays (Fig. 4A and B). After treatment with Pi-PLC, these PMNs also showed a reduction in signal intensity to 40% (Fig. 4C), similar to the reduction observed with nondeficient, Pi-PLC-treated PMNs (Fig. 2C). In contrast, the blocking of CD32(a) resulted in a complete failure of plasma IgG to activate CD16(b)-deficient PMNs in the sADRB assay (Fig. 4E). PMN responses in the mADRB assay were also reduced after Pi-PLC treatment, to 60–80% (Fig. 4D), but the blocking of CD32(a) resulted in an even lower signal of 40–60% (Fig. 4F).
Figure 4. FcγR dependence of the neutrophilic ADRB using CD16(b)-deficient PMNs.
The predominant role of CD32(a) was confirmed by measuring the sADRB (A, C, and E) and mADRB (B, D, and F) in a 60-min kinetic window using CD16(b)-deficient PMNs, which were added untreated (filled symbols; A and B) or treated with Pi-PLC (open symbols; C and D) or with the CD32(a)-blocking antibody (half-filled symbols; E and F). The reactivities of PMNs were measured against SIP 5 (circles), the SIP-pool (squares), and one NIP (triangles) as representative examples. The data represent the mean ± range of one experiment using CD16-deficient PMNs from one donor in technical duplicates. (A and B) Luminescence intensities were normalized to the reactivity of SIP 5 in each assay and therefore, are presented in arbitrary units. (C and D) The residual percentages compared with the respective kinetics without Pi-PLC treatment are shown. (E and F) The residual percentages compared with the respective kinetics without blocking of CD32(a) are depicted.
Definition of the cellular site of ROS production
The different response profiles and extent of dependence on CD32(a) correspond to the different location of ROS during the ADRB response (Fig. 5). In the sADRB assay, the initial peak of ROS activity was abolished completely by adding the extracellular ROS scavengers SOD and CAT (Fig. 5A and C). SOD converts O2− to H2O2, and CAT catalyzes the decomposition of H2O2 to H2O and O2. The amplifier luminol is cell permeable, whereas SOD and CAT are not, resulting in the selective depletion of extracellular ROS. After 15–20 min, the sADRB response matched the corresponding response curve without ROS scavengers. In contrast, ROS detection, using the less lipophilic and therefore, less membrane-permeable derivative isoluminol, only showed the first extracellular peak, which was abolished completely by extracellular scavengers (Fig. 5E and G). The peak intensity was 75% lower for isoluminol, which can be explained by the fact that it has a quantum yield approximately tenfold lower than luminol [46]. The increase of the concentration to 600 μM did not enhance the peak height, indicating that the initial 83.3 μM was sufficient to cause saturation (data not shown). In the mADRB assay, the luminol-enhanced ROS signal was not reduced but slightly delayed when scavengers were present (Fig. 5B and D). When isoluminol was used as the chemiluminescence amplifier, a basal level of ROS was detected (Fig. 5F). The addition of extracellular scavengers did not reduce the luminescence, confirming that ROS produced in the mADRB are mainly intracellular (Fig. 5H).
Figure 5. The cellular sites of ROS production during the sADRB and mADRB.
The cellular sites of ROS production during the sADRB (A, C, E, and G) and mADRB (B, D, F, and H) responses were determined by measuring the total chemiluminescence (standard sADRB and mADRB, filled symbols, A and B) compared with the intracellular chemiluminescence (C and D) and extracellular chemiluminescence (E and F). Intracellular ROS were quantified by depleting the extracellular ROS for luminol-enhanced chemiluminescence with SOD and CAT (C and D, open symbols). Extracellular ROS were quantified using the less membrane-permeable chemiluminescence amplifier isoluminol, also with (open symbols, G and H) and without (filled symbols, E and F) the addition of SOD and CAT. SIP 5 (squares), the SIP-pool (circles), and one NIP (triangles) are shown as examples. The data represent the mean ± sd of two experiments using PMNs from two different donors, each in technical duplicates. (A and B) Luminescence intensities were normalized to the reactivity of SIP 5 in each assay and therefore, are presented in arbitrary units. (C–H) The residual percentages compared with the respective kinetics using luminol without extracellular ROS scavengers are shown.
Analysis of phagocytosis as a possible antimerozoite mode of action of PMNs
Next, we confirmed that the intracellular production of ROS represents the phagocytosis of Pf merozoites at the single-cell level by using a parasite strain expressing GFP in the cytosol [Pf D10 ACP(transit)-GFP]. PMNs were exposed to GFP-producing, opsonized merozoites and showed varying degrees of fluorescence, according to the source of the opsonization, i.e., decomplemented SIPs or NIPs (Fig. 6A; showing SIP 5, the SIP-pool, and one NIP as an example). We found that 97% of the SIPs were able to induce phagocytosis, but no NIPs (Fig. 6B). Median phagocytosis rates were 8.1% and 1.1% for the SIPs and NIPs, respectively, representing a 7.4-fold enhancement, as determined by the Mann-Whitney test (P=0.0001). The intracellular location of the merozoites in fluorescent PMNs was confirmed by confocal laser-scanning microscopy (Fig. 6C). Merozoite GFP fluorescence and merozoite nuclear staining were colocalized, as indicated by the red arrows. Furthermore, merozoite nuclei and PMN nuclei were present in the same focal plane of the image, thus excluding the possibility that extracellular merozoites were located above or below the PMN.
Figure 6. Confirmation of phagocytosis by flow cytometry and confocal laser-scanning microscopy.
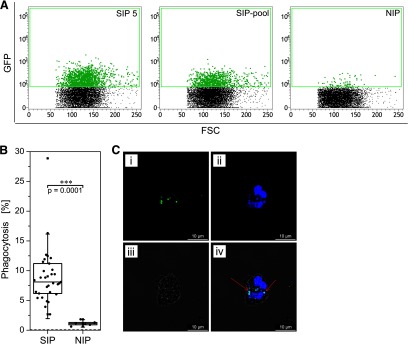
PMNs were isolated and exposed to Pf D10 ACP(transit)-GFP merozoites (opsonized with decomplemented plasma) for 10 min at 37°C and analyzed immediately by flow cytometry. GFP-positive PMNs (phagocytosis signal) were gated and are highlighted in green (A). Differences in the rates of phagocytosis when comparing merozoites opsonized by 31 SIPs or six NIPs were estimated using the Mann-Whitney test (B). The dashed line at the bottom of the panel shows the background phagocytosis of merozoites incubated in HBSS instead of plasma. (C) The intracellular location of the merozoites was verified by confocal laser-scanning microscopy. (i) GFP-fluorescent merozoites are shown. (ii) The nuclei of PMNs and merozoites were counterstained with Hoechst 33342. (iv) The overlay image includes (iii) the bright field image. (iv) Red arrows show representative merozoites in which the GFP and Hoechst 33342 fluorescence signals are colocalized. Original scale bars, 10 μm.
DISCUSSION
Several assays have been developed to characterize Pf-reactive IgGs, originating from semi-immune individuals or vaccine-trial volunteers, or produced as recombinant antibodies to study effector cell stimulation and antimalarial activity. These include growth-inhibition assays to determine the ability of IgGs to inhibit invasion [10, 36]; the ADCI assay, which determines the cooperative effect of IgGs and blood monocytes [12]; and the ADRB assay, which measures antibody-mediated PMN activation [21, 22]. Recently, the demand for precise assays to evaluate the protective immune response in malaria has increased, and assays involving PMNs as key immune effector cells have been recommended [6].
We investigated the PMN response in two different ADRB assay set-ups, i.e., the sADRB and mADRB assays. We developed two optimized protocols that make these assays stable and reproducible, as demonstrated by comparable sample positivity and significant correlations between ELISA and sADRB assay reactivities for 31 Ghanaian SIPs. We also characterized both assays to an unprecedented level of detail by determining the reaction kinetics, defining their selective dependence on FcγRs, localizing ROS, and identifying the neutrophilic, antiparasitic mode of action. The ADRB characterization experiments were important, as PMNs must react quickly to kill merozoites. This extracellular stage of the parasite only lasts a few minutes [35]. Therefore, the PMNs must either kill the parasite before invasion by mounting a strong ROS response or by removing the merozoite from the bloodstream by phagocytosis, thus allowing the parasite to be digested more slowly in the intracellular phagosome.
We demonstrate that the sADRB assay is a valuable tool that can be used to screen polyclonal samples for single antigen reactivity and concurrently, PMN stimulation. However, the stimulation is only qualitative and differs from the natural process in the bloodstream, as reflected by the mADRB assay. PMN-activation mechanisms differ between the two ADRB assay formats, as confirmed by the alternative ROS response profiles. The mADRB assay, however, represents the natural-occurring type of immune complex in the bloodstream and additionally, covers a broader variety of antigen specificity. This was demonstrated by the significant correlation between mADRB reactivity and SZ-lysate reactivity in the ELISA. Although our data are no measure for clinical protection or rapid in vivo parasite clearance, as shown in two previous studies [21, 47], they demonstrate that semi-immune people bear antibodies of widespread specificity, which are able to induce a neutrophilic ADRB response and moreover, are able to induce phagocytosis in vitro. Surprisingly, our resulting kinetic profiles of ROS production differed from those reported earlier [21]. This phenomenon is described in detail below.
CD16(b) is a glycophosphatidylinositol-anchored FcγR that lacks a cytoplasmic tail, whereas the cytoplasmic domain of CD32(a) contains immunoreceptor tyrosine-based activation motifs that allow the direct induction of intracellular phosphorylation cascades and phagocytosis [48]. The importance of different FcγRs in PMN activation is a matter of debate. In some studies, soluble immune complexes were shown to activate PMNs via CD16(b) and CD32(a) [49], whereas in others, one of the receptors was found to play a predominant role, either CD16(b) [27, 50] or CD32(a) [51, 52].
In our sADRB experiments, CD32(a) was clearly identified as the main FcγR, as no sADRB response was induced in CD16(b)-deficient PMNs when CD32(a) was blocked. Residual ROS production in nondeficient PMNs with blocked CD32(a) may rely on antibody binding by CD16(b), followed by the displacement of the CD32(a)-blocking antibody [52] and induction of a weaker and altered respiratory burst via CD32(a).
Similar results were obtained in the mADRB assay, although not to the same clear extent. The blocking of CD32(a) resulted in a lower but still comparably strong signal, even on CD16(b)-deficient PMNs. A potential explanation is that the remaining 25% of accessible CD32(a) surface receptors are sufficient for initial immune complex binding, the induction of phagocytosis, and the production of low amounts of ROS in the mADRB assay set-up, suggesting that there are significant differences in the signaling pathways and response mechanisms toward these different kinds of immune complexes. This agrees with earlier reports, showing that variable PMN responses to soluble and insoluble immune complexes were differentially sensitive to treatment with pertussis toxin or PGE1 [49]. Furthermore, exposure to (exogenous) ROS can enhance the transport of CD32(a) to the plasma membrane [53]; thus, the minimal activation represented by the 25% of residual CD32(a) could facilitate the transport of more CD32(a) molecules to the PMN surface. CD32(a) is also described as a highly efficient receptor for phagocytosis, so low amounts of CD32(a) could be sufficient to support partial activity [48].
Four possible models for CD16(b) and CD32(a), cooperation and signaling have been discussed [54]: 1) both receptors bind their ligands and induce separate signaling cascades; 2) CD16(b) serves as the initial ligand-binding and ligand-presenting receptor, as a result of its higher expression level, but signaling occurs only through CD32(a); 3) initial engagement of CD16(b) by ligands enhances the functional response of CD32(a); and 4) both receptors act synergistically to induce one response. Our results support the model that CD16(b) serves as an additional ligand-presenting receptor, as a result of its higher expression level on the surface [52]. In the context of malaria, it should be noted that CD32(a) is polymorphic, and an arginine to histidine substitution at position 131 reduces the affinity of the receptor toward IgG2 and IgG3. The importance of this polymorphism is a matter of debate. Some authors have associated the homozygous arginine genotype with higher susceptibility to severe malaria and anemia [55, 56], whereas others found a correlation between this genotype and a lower incidence of high-density parasitemia [57, 58]. The influence of this polymorphism, therefore, requires further investigation, as it appears to play a major role in the predominance of CD32(a) in the antimalarial response of PMNs.
The readout system for the respiratory burst relies on the visualization of ROS that are produced by a membrane-associated NADPH oxidase. This enzyme may be activated within intracellular phagosomes or on the outer cell membrane [43]. In agreement with previous studies [25, 59], the assay set-up and therefore, the solubility of the immune complex played a major role in the overall ROS response, not only (as already discussed) in the context of the FcγR but also concerning the cellular origin. Whereas the sADRB (with solid-phase immobilized “soluble” antigens) is characterized by a predominantly extracellular ROS response, the mADRB (with “insoluble” opsonized merozoites) is mainly intracellular and represents phagocytosis events (Figs. 5 and 6). The phagocytosis of merozoites is generally accepted as an antimalarial mode of action used by PMNs, although mediated by complement factors to a greater extent than solely by IgGs [60–62]. The addition of ROS scavengers to the mADRB assay destroyed the early extracellular ROS, resulting in a slight shift in the onset of the detected response. The presence of early extracellular ROS in the mADRB assay without scavengers may thus reflect secretory granule release before phagocytosis, meaning that opsonized merozoites do not specifically activate the outer membrane-associated NADPH oxidase, but intracellular ROS from specific granules may be transported to the extracellular space [63]. Therefore, it is not surprising that our response curves differ from those reported previously based on the use of isoluminol [21], as opsonized merozoites induce a ROS response at an intracellular level, but isoluminol predominantly detects extracellular ROS. These observations clearly show that phagocytosis rather than ROS secretion is the main antiparasitic mode of action of PMNs against Pf merozoites in vitro. In vivo, if the same mode of response is induced, this would be advantageous, as only small amounts of ROS are targeted to the extracellular space, thus minimizing damage to the surrounding space.
Experimentally, it is therefore beneficial to determine the luminol-enhanced neutrophilic mADRB for the assessment of antimalarial antibody samples in the context of PMN stimulation. The ROS response appears concomitant with phagocytosis and may therefore be used as a substitute for the labor-intensive characterization of phagocytosis when studying PMN stimulation by antibodies. The sADRB assay may be useful for initial antigen binding and PMN stimulation studies, but the underlying activating effect is only represented in the mADRB and phagocytosis assays.
The role of neutrophils for protection in vivo has not been confirmed thus far. Recently, digestive vacuoles released following schizont rupture were shown to impair PMN phagocytosis events and may therefore inhibit their proposed antimalarial activities [64]. Moreover, the neutrophil-activating potential of certain IgG subclasses specific for MSP-119 was not shown to correlate with increased protection from malaria in mice [65], and although showing increased (sADRB-based) PMN stimulation, IgA1 was also not shown to be protective in human FcαR-transgenic mice [66]. More recently, a study investigating the role of PMNs in a murine malaria infection model showed no correlation between the ex vivo activity of the neutrophils from wild-type mice and FcγR knockouts (measured using assays similar to those presented here) and in vivo protection [67]. Despite a two- to threefold increase in ADRB, the mice were not protected after vaccination with MSP-142 or a sublethal dose of Plasmodium yoelii. The authors suggested that the choice of vaccine antigen is critical, as the PMN response depends not only on the antibody concentration but also on the ability of the antibody to access the PMN. This agrees with the observation that antibodies against MSP-3 do not perform well in growth-inhibition assays but induce a strong response in ADCI assays [11]. Also, as murine PMNs express CD32(b) but not CD32(a), this model might not be completely transferrable to human malaria infections, and the experiments should be repeated using human PMNs.
To conclude, PMNs may act as the first line of defense to control parasite density until more effective mechanisms are activated [16]. This defense was shown to be based on phagocytosis rather than extracellular ROS-dependent mechanisms, which agrees with early, previous reports [17, 68]. Here, also, extracellular scavenging of ROS did not reduce in vitro multiplication of Pf, and also, PMNs defective in oxygen metabolism were able to inhibit parasite growth in vitro. This was emphasized by our results confirming the intracellular localization of ROS during the mADRB assay, which corresponds to phagocytosis at a single-cell level. We have validated two related assays that characterize PMN-mediated, antiparasitic responses against Pf malaria, providing insight into the activation mechanisms and corresponding responses. The mADRB assay results offer a valuable prediction of potential in vivo vaccine efficacy (induction of protective antibodies), and recombinant antibody subtypes could be analyzed more efficiently for their potential use as passive vaccines or therapeutic antibodies in vivo. The field requires further investigations based on robust and stable methods to allow the prediction of vaccine efficacy and antibody functionality. Our results support this approach by providing stable and well-characterized methods to fulfill these objectives.
Supplementary Material
ACKNOWLEDGMENTS
This study was financed by the “Fraunhofer Future Foundation” (125-300004). S.K. was supported by a Richtlinien zur Förderung des wissenschaftlichen Nachwuchses (RFwN) Ph.D. grant from Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen University. The following reagents were obtained through the Malaria Research and Reference Reagent Resource Center (MR4) as part of the Biodefense and Emerging Infections (BEI) Resources Repository, National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health: Pf 3D7A, MRA-151 (deposited by D. Walliker), and Pf D10 ACP(transit)-GFP, MRA-569 (deposited by A. F. Cowman).
We acknowledge the Regional Blood Bank of the University Hospital Aachen for the regular supply of blood samples. We thank all voluntary PMN donors, without whom the study could not have been performed. We gratefully acknowledge Susanne Bethke for assistance during the experiments; Otchere Addai-Mensah and Margaret T. Frempong of the Kwame Nkrumah University of Science and Technology for the collection of blood samples in Kumasi; Dominika Maskus for providing us with rMSP-119; and Bart Faber (Biomedical Primate Research Centre, Rijswijk, The Netherlands) for the AMA-1 DiCo1–3 protein. We thank Richard M. Twyman, who critically reviewed the manuscript.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ACP
- acyl carrier protein
- ADCI
- antibody-mediated cellular inhibition
- ADRB
- antibody-dependent respiratory burst
- AMA-1
- apical membrane antigen 1
- CAT
- catalase
- DiCo
- diversity-covering variant
- FSC
- forward-scatter
- mADRB
- antibody-dependent respiratory burst in response to IgG-opsonized merozoites
- MSP
- merozoite surface protein
- NIP
- nonimmune plasma
- OZ
- serum-opsonized zymosan particles
- Pf
- Plasmodium falciparum
- Pi-PLC
- phosphatidylinositol-specific phospholipase C
- PMN
- polymorphonuclear neutrophil granulocyte
- ROS
- reactive oxygen species
- sADRB
- antibody-dependent respiratory burst in response to IgG bound to solid-phase immobilized antigens
- SIP
- semi-immune plasma
- SIP 5
- highly reactive semi-immune Ghanaian plasma sample from Donor 5
- SIP-pool
- pool of four highly reactive semi-immune Ghanaian plasma samples
- SOD
- superoxide dismutase
- SSC
- side-scatter
- SZ-lysate
- Plasmodium falciparum 3D7A schizont lysate
AUTHORSHIP
S.K. and R. Fendel conceived of, designed, and performed the experiments; analyzed the data; and wrote the manuscript. R. Fischer, S.B., T.K., and R. Fendel conceived of the overall project strategy. All authors read and approved the manuscript.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1. Langhorne J., Ndungu F. M., Sponaas A. M., Marsh K. (2008) Immunity to malaria: more questions than answers. Nat. Immunol. 9, 725–732. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz L., Brown G. V., Genton B., Moorthy V. S. (2012) A review of malaria vaccine clinical projects based on the WHO rainbow table. Malaria J. 11, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crompton P. D., Pierce S. K., Miller L. H. (2010) Advances and challenges in malaria vaccine development. J. Clin. Invest. 120, 4168–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dondorp A. M., Nosten F., Yi P., Das D., Phyo A. P., Tarning J., Lwin K. M., Ariey F., Hanpithakpong W., Lee S. J., Ringwald P., Silamut K., Imwong M., Chotivanich K., Lim P., Herdman T., An S. S., Yeung S., Singhasivanon P., Day N. P., Lindegardh N., Socheat D., White N. J. (2009) Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361, 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization (2013) World Malaria Report 2013. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 6. Sheehy S. H., Douglas A. D., Draper S. J. (2013) Challenges of assessing the clinical efficacy of asexual blood-stage Plasmodium falciparum malaria vaccines. Hum. Vaccin. Immunother. 9, 1831–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Duncan C. J., Hill A. V., Ellis R. D. (2012) Can growth inhibition assays (GIA) predict blood-stage malaria vaccine efficacy? Hum. Vaccin. Immunother. 8, 706–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen S., Mc G. I., Carrington S. (1961) gamma-Globulin and acquired immunity to human malaria. Nature 192, 733–737. [DOI] [PubMed] [Google Scholar]
- 9. Sabchareon A., Burnouf T., Ouattara D., Attanath P., Bouharoun-Tayoun H., Chantavanich P., Foucault C., Chongsuphajaisiddhi T., Druilhe P. (1991) Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hygiene 45, 297–308. [DOI] [PubMed] [Google Scholar]
- 10. Treeck M., Tamborrini M., Daubenberger C. A., Gilberger T. W., Voss T. S. (2009) Caught in action: mechanistic insights into antibody-mediated inhibition of Plasmodium merozoite invasion. Trends Parasitol. 25, 494–497. [DOI] [PubMed] [Google Scholar]
- 11. Oeuvray C., Bouharoun-Tayoun H., Gras-Masse H., Bottius E., Kaidoh T., Aikawa M., Filgueira M. C., Tartar A., Druilhe P. (1994) Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84, 1594–1602. [PubMed] [Google Scholar]
- 12. Druilhe P., Perignon J. L. (1994) Mechanisms of defense against P. falciparum asexual blood stages in humans. Immunol. Lett. 41, 115–120. [DOI] [PubMed] [Google Scholar]
- 13. Bouharoun-Tayoun H., Oeuvray C., Lunel F., Druilhe P. (1995) Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J. Exp. Med. 182, 409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fendel R., Brandts C., Rudat A., Kreidenweiss A., Steur C., Appelmann I., Ruehe B., Schroder P., Berdel W. E., Kremsner P. G., Mordmuller B. (2010) Hemolysis is associated with low reticulocyte production index and predicts blood transfusion in severe malarial anemia. PloS One 5, e10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Casals-Pascual C., Kai O., Cheung J. O., Williams S., Lowe B., Nyanoti M., Williams T. N., Maitland K., Molyneux M., Newton C. R., Peshu N., Watt S. M., Roberts D. J. (2006) Suppression of erythropoiesis in malarial anemia is associated with hemozoin in vitro and in vivo. Blood 108, 2569–2577. [DOI] [PubMed] [Google Scholar]
- 16. Brown J., Smalley M. E. (1981) Inhibition of the in vitro growth of Plasmodium falciparum by human polymorphonuclear neutrophil leucocytes. Clin. Exp. Immunol. 46, 106–109. [PMC free article] [PubMed] [Google Scholar]
- 17. Kharazmi A., Jepsen S. (1984) Enhanced inhibition of in vitro multiplication of Plasmodium falciparum by stimulated human polymorphonuclear leucocytes. Clin. Exp. Immunol. 57, 287–292. [PMC free article] [PubMed] [Google Scholar]
- 18. Metzger W. G., Mordmuller B. G., Kremsner P. G. (1995) Malaria pigment in leucocytes. Transact. Royal Soc. Trop. Med. Hygiene 89, 637–638. [DOI] [PubMed] [Google Scholar]
- 19. Clark I. A., Hunt N. H. (1983) Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect. Immun. 39, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. (2000) Neutrophils: molecules, functions and pathophysiological aspects. Lab. Invest. 80, 617–653. [DOI] [PubMed] [Google Scholar]
- 21. Joos C., Marrama L., Polson H. E., Corre S., Diatta A. M., Diouf B., Trape J. F., Tall A., Longacre S., Perraut R. (2010) Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PloS One 5, e9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pleass R. J., Dunlop J. I., Anderson C. M., Woof J. M. (1999) Identification of residues in the CH2/CH3 domain interface of IgA essential for interaction with the human Fcalpha receptor (FcalphaR) CD89. J. Biol. Chem. 274, 23508–23514. [DOI] [PubMed] [Google Scholar]
- 23. Norton L. J., Zhang Q., Saqib K. M., Schrewe H., Macura K., Anderson K. E., Lindsley C. W., Brown H. A., Rudge S. A., Wakelam M. J. (2011) PLD1 rather than PLD2 regulates phorbol-ester-, adhesion-dependent and Fc{gamma}-receptor-stimulated ROS production in neutrophils. J. Cell. Sci. 124, 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee C. Y., Herant M., Heinrich V. (2011) Target-specific mechanics of phagocytosis: protrusive neutrophil response to zymosan differs from the uptake of antibody-tagged pathogens. J. Cell. Sci. 124, 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fossati G., Moots R. J., Bucknall R. C., Edwards S. W. (2002) Differential role of neutrophil Fcgamma receptor IIIB (CD16) in phagocytosis, bacterial killing, and responses to immune complexes. Arthritis Rheum. 46, 1351–1361. [DOI] [PubMed] [Google Scholar]
- 26. Shi J., McIntosh R. S., Pleass R. J. (2006) Antibody- and Fc-receptor-based therapeutics for malaria. Clin. Sci. 110, 11–19. [DOI] [PubMed] [Google Scholar]
- 27. Nagarajan S., Venkiteswaran K., Anderson M., Sayed U., Zhu C., Selvaraj P. (2000) Cell-specific, activation-dependent regulation of neutrophil CD32A ligand-binding function. Blood 95, 1069–1077. [PubMed] [Google Scholar]
- 28. Yoneto T., Waki S., Takai T., Tagawa Y., Iwakura Y., Mizuguchi J., Nariuchi H., Yoshimoto T. (2001) A critical role of Fc receptor-mediated antibody-dependent phagocytosis in the host resistance to blood-stage Plasmodium berghei XAT infection. J. Immunol. 166, 6236–6241. [DOI] [PubMed] [Google Scholar]
- 29. Clatworthy M. R., Willcocks L., Urban B., Langhorne J., Williams T. N., Peshu N., Watkins N. A., Floto R. A., Smith K. G. (2007) Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc. Natl. Acad. Sci. USA 104, 7169–7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waller R. F., Reed M. B., Cowman A. F., McFadden G. I. (2000) Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 19, 1794–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trager W., Jensen J. B. (1976) Human malaria parasites in continuous culture. Science 193, 673–675. [DOI] [PubMed] [Google Scholar]
- 32. Lambros C., Vanderberg J. P. (1979) Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65, 418–420. [PubMed] [Google Scholar]
- 33. Mordmuller B., Fendel R., Kreidenweiss A., Gille C., Hurwitz R., Metzger W. G., Kun J. F., Lamkemeyer T., Nordheim A., Kremsner P. G. (2006) Plasmodia express two threonine-peptidase complexes during asexual development. Mol. Biochem. Parasitol. 148, 79–85. [DOI] [PubMed] [Google Scholar]
- 34. Radfar A., Mendez D., Moneriz C., Linares M., Marin-Garcia P., Puyet A., Diez A., Bautista J. M. (2009) Synchronous culture of Plasmodium falciparum at high parasitemia levels. Nat. Protoc. 4, 1899–1915. [DOI] [PubMed] [Google Scholar]
- 35. Boyle M. J., Wilson D. W., Richards J. S., Riglar D. T., Tetteh K. K., Conway D. J., Ralph S. A., Baum J., Beeson J. G. (2010) Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc. Natl. Acad. Sci. USA 107, 14378–14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Remarque E. J., Faber B. W., Kocken C. H., Thomas A. W. (2008) A diversity-covering approach to immunization with Plasmodium falciparum apical membrane antigen 1 induces broader allelic recognition and growth inhibition responses in rabbits. Infect. Immun. 76, 2660–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Belard S., Issifou S., Hounkpatin A. B., Schaumburg F., Ngoa U. A., Esen M., Fendel R., de Salazar P. M., Murbeth R. E., Milligan P., Imbault N., Imoukhuede E. B., Theisen M., Jepsen S., Noor R. A., Okech B., Kremsner P. G., Mordmuller B. (2011) A randomized controlled phase Ib trial of the malaria vaccine candidate GMZ2 in African children. PloS One 6, e22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. R Core Team (2013) R: A Language and Environment for Statistical Computing, Vienna, Austria. European Environment Agency, Copenhagen, Denmark. [Google Scholar]
- 39. Nauseef W. M. (2007) Isolation of human neutrophils from venous blood. Methods Mol. Biol. 412, 15–20. [DOI] [PubMed] [Google Scholar]
- 40. Vely F., Gruel N., Moncuit J., Cochet O., Rouard H., Dare S., Galon J., Sautes C., Fridman W. H., Teillaud J. L. (1997) A new set of monoclonal antibodies against human Fc gamma RII (CD32) and Fc gamma RIII (CD16): characterization and use in various assays. Hybridoma 16, 519–528. [DOI] [PubMed] [Google Scholar]
- 41. Lazarou M., Guevara Patino J. A., Jennings R. M., McIntosh R. S., Shi J., Howell S., Cullen E., Jones T., Adame-Gallegos J. R., Chappel J. A., McBride J. S., Blackman M. J., Holder A. A., Pleass R. J. (2009) Inhibition of erythrocyte invasion and Plasmodium falciparum merozoite surface protein 1 processing by human immunoglobulin G1 (IgG1) and IgG3 antibodies. Infect. Immun. 77, 5659–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Freitas M., Porto G., Lima J. L., Fernandes E. (2009) Optimization of experimental settings for the analysis of human neutrophils oxidative burst in vitro. Talanta 78, 1476–1483. [DOI] [PubMed] [Google Scholar]
- 43. Dahlgren C., Karlsson A. (1999) Respiratory burst in human neutrophils. J. Immunol. Methods 232, 3–14. [DOI] [PubMed] [Google Scholar]
- 44. Lundqvist H., Dahlgren C. (1996) Isoluminol-enhanced chemiluminescence: a sensitive method to study the release of superoxide anion from human neutrophils. Free Radic. Biol. Med. 20, 785–792. [DOI] [PubMed] [Google Scholar]
- 45. Briheim G., Stendahl O., Dahlgren C. (1984) Intra- and extracellular events in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect. Immun. 45, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Whitehead T. P., Kricka L. J., Carter T. J., Thorpe G. H. (1979) Analytical luminescence—its potential in the clinical laboratory. Clin. Chem. 25, 1531–1546. [PubMed] [Google Scholar]
- 47. Greve B., Lehman L. G., Lell B., Luckner D., Schmidt-Ott R., Kremsner P. G. (1999) High oxygen radical production is associated with fast parasite clearance in children with Plasmodium falciparum malaria. J. Infect. Dis. 179, 1584–1586. [DOI] [PubMed] [Google Scholar]
- 48. Nordenfelt P., Tapper H. (2011) Phagosome dynamics during phagocytosis by neutrophils. J. Leukoc. Biol. 90, 271–284. [DOI] [PubMed] [Google Scholar]
- 49. Crockett-Torabi E., Fantone J. C. (1990) Soluble and insoluble immune complexes activate human neutrophil NADPH oxidase by distinct Fc gamma receptor-specific mechanisms. J. Immunol. 145, 3026–3032. [PubMed] [Google Scholar]
- 50. Hundt M., Schmidt R. E. (1992) The glycosylphosphatidylinositol-linked Fc gamma receptor III represents the dominant receptor structure for immune complex activation of neutrophils. Eur. J. Immunol. 22, 811–816. [DOI] [PubMed] [Google Scholar]
- 51. Rivas-Fuentes S., Garcia-Garcia E., Nieto-Castaneda G., Rosales C. (2010) Fcgamma receptors exhibit different phagocytosis potential in human neutrophils. Cell. Immunol. 263, 114–121. [DOI] [PubMed] [Google Scholar]
- 52. Huizinga T. W., van Kemenade F., Koenderman L., Dolman K. M., von dem Borne A. E., Tetteroo P. A., Roos D. (1989) The 40-kDa Fc gamma receptor (FcRII) on human neutrophils is essential for the IgG-induced respiratory burst and IgG-induced phagocytosis. J. Immunol. 142, 2365–2369. [PubMed] [Google Scholar]
- 53. Simms H. H., D'Amico R. (1995) Subcellular location of neutrophil opsonic receptors is altered by exogenous reactive oxygen species. Cell. Immunol. 166, 71–82. [DOI] [PubMed] [Google Scholar]
- 54. Edberg J. C., Salmon J. E., Kimberly R. P. (1992) Functional capacity of Fc gamma receptor III (CD16) on human neutrophils. Immunol. Res. 11, 239–251. [DOI] [PubMed] [Google Scholar]
- 55. Schuldt K., Esser C., Evans J., May J., Timmann C., Ehmen C., Loag W., Ansong D., Ziegler A., Agbenyega T., Meyer C. G., Horstmann R. D. (2010) FCGR2A functional genetic variant associated with susceptibility to severe malarial anaemia in Ghanaian children. J. Med. Genet. 47, 471–475. [DOI] [PubMed] [Google Scholar]
- 56. Nasr A., Iriemenam N. C., Troye-Blomberg M., Giha H. A., Balogun H. A., Osman O. F., Montgomery S. M., ElGhazali G., Berzins K. (2007) Fc gamma receptor IIa (CD32) polymorphism and antibody responses to asexual blood-stage antigens of Plasmodium falciparum malaria in Sudanese patients. Scand. J. Immunol. 66, 87–96. [DOI] [PubMed] [Google Scholar]
- 57. Ouma C., Keller C. C., Opondo D. A., Were T., Otieno R. O., Otieno M. F., Orago A. S., Ong'Echa J. M., Vulule J. M., Ferrell R. E., Perkins D. J. (2006) Association of FCgamma receptor IIA (CD32) polymorphism with malarial anemia and high-density parasitemia in infants and young children. Am. J. Trop. Med. Hyg. 74, 573–577. [PubMed] [Google Scholar]
- 58. Shi Y. P., Nahlen B. L., Kariuki S., Urdahl K. B., McElroy P. D., Roberts J. M., Lal A. A. (2001) Fcgamma receptor IIa (CD32) polymorphism is associated with protection of infants against high-density Plasmodium falciparum infection. VII. Asembo Bay Cohort Project. J. Infect. Dis. 184, 107–111. [DOI] [PubMed] [Google Scholar]
- 59. Zhang W., Voice J., Lachmann P. J. (1995) A systematic study of neutrophil degranulation and respiratory burst in vitro by defined immune complexes. Clin. Exp. Immunol. 101, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kumaratilake L. M., Ferrante A. (2000) Opsonization and phagocytosis of Plasmodium falciparum merozoites measured by flow cytometry. Clin. Diagn. Lab. Immunol. 7, 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kumaratilake L. M., Ferrante A., Jaeger T., Rzepczyk C. M. (1992) Effects of cytokines, complement, and antibody on the neutrophil respiratory burst and phagocytic response to Plasmodium falciparum merozoites. Infect. Immun. 60, 3731–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brown I. N. (1969) Immunological aspects of malaria infection. Adv. Immunol. 11, 267–349. [DOI] [PubMed] [Google Scholar]
- 63. Tapper H., Grinstein S. (1997) Fc receptor-triggered insertion of secretory granules into the plasma membrane of human neutrophils: selective retrieval during phagocytosis. J. Immunol. 159, 409–418. [PubMed] [Google Scholar]
- 64. Dasari P., Heber S. D., Beisele M., Torzewski M., Reifenberg K., Orning C., Fries A., Zapf A. L., Baumeister S., Lingelbach K., Udomsangpetch R., Bhakdi S. C., Reiss K., Bhakdi S. (2012) Digestive vacuole of Plasmodium falciparum released during erythrocyte rupture dually activates complement and coagulation. Blood 119, 4301–4310. [DOI] [PubMed] [Google Scholar]
- 65. Adame-Gallegos J. R., Shi J., McIntosh R. S., Pleass R. J. (2012) The generation and evaluation of two panels of epitope-matched mouse IgG1, IgG2a, IgG2b and IgG3 antibodies specific for Plasmodium falciparum and Plasmodium yoelii merozoite surface protein 1–19 (MSP1(19)). Exp. Parasitol. 130, 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shi J., McIntosh R. S., Adame-Gallegos J., Dehal P. K., van Egmond M., van de Winkel J., Draper S. J., Forbes E. K., Corran P. H., Holder A. A., Woof J. M., Pleass R. J. (2011) The generation and evaluation of recombinant human IgA specific for Plasmodium falciparum merozoite surface protein 1–19 (PfMSP1 19). BMC Biotechnol. 11, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Llewellyn D., de Cassan S. C., Williams A. R., Douglas A. D., Forbes E. K., Adame-Gallegos J. R., Shi J., Pleass R. J., Draper S. J. (2015) Assessment of antibody-dependent respiratory burst activity from mouse neutrophils on Plasmodium yoelii malaria challenge outcome. J. Leukoc. Biol. 95, 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kharazmi A., Jepsen S., Valerius N. H. (1984) Polymorphonuclear leucocytes defective in oxidative metabolism inhibit in vitro growth of Plasmodium falciparum. Evidence against an oxygen-dependent mechanism. Scand. J. Immunol. 20, 93–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.