Therapeutic manipulation of macrophage differentiation to enhance the AAM phenotype may be a viable approach for ameliorating RSV-induced disease.
Keywords: rosiglitazone, macrophage differentiation, azithromycin, PPARγ
Abstract
RSV is the most significant cause of serious lower respiratory tract infection in infants and young children worldwide. There is currently no vaccine for the virus, and antiviral therapy (e.g., ribavirin) has shown no efficacy against the disease. We reported that alternatively activated macrophages (AAMs) mediate resolution of RSV-induced pathology. AAM differentiation requires macrophage-derived IL-4 and -13, autocrine/paracrine signaling through the type I IL-4 receptor, and STAT6 activation. Based on these findings, we reasoned that it would be possible to intervene therapeutically in RSV disease by increasing AAM differentiation, thereby decreasing lung pathology. Mice treated with the IL-4/anti-IL-4 immune complexes, shown previously to sustain levels of circulating IL-4, increased the RSV-induced AAM markers arginase-1 and mannose receptor and decreased the lung pathology. Induction of PPARγ, shown to play a role in AAM development, by the PPARγ agonist rosiglitazone or treatment of mice with the macrolide antibiotic AZM, also reported to skew macrophage differentiation to an AAM phenotype, increased the AAM markers and mitigated RSV-induced lung pathology. Collectively, our data suggest that therapeutic manipulation of macrophage differentiation to enhance the AAM phenotype is a viable approach for ameliorating RSV-induced disease.
Introduction
RSV is the most significant cause of serious lower respiratory tract infection in infants and young children worldwide [1]. Global RSV-associated ALRIs in children under age 5, mostly in the developing world, are even more alarming: ∼34 million annually, or 22% of all ALRIs, with a mortality rate of 3–9% [1]. There is currently no vaccine for RSV. The first clinical trial of a formalin-inactivated RSV vaccine in the 1960s resulted in a highly significant increase in hospitalizations of vaccinated infants and children, as well as 2 deaths, during the following RSV season [2]. Since that time, development of RSV subunit vaccines has been challenged by lingering concerns over “vaccine-enhanced disease.”
RSV is relatively stable antigenically, yet most adults are reinfected every few years, and are seropositive, suggesting that immunity from infection is neither sufficient nor long-lasting [3]. In children, severe RSV disease is highly associated with prematurity, bronchopulmonary dysplasia, or congenital heart disease [4], and infection in early infancy often results in allergic and asthmatic symptoms later in life [5]. More recently, RSV has been identified as an increasing cause of morbidity and mortality in the elderly [6, 7] and in transplant and immunodeficient patients [8, 9]. In the elderly, infections can be quite severe and are associated with a debilitated or senescent immune system [6, 7]. Recently, we showed a reciprocal relationship in which cyclooxygenases and lipoxygenases increase or decrease, respectively, the degree of pathology observed in response to RSV infection. Decreased pathology is also associated with increased AAM differentiation [10, 11]. Taken together, these observations suggest that the complex balance of pro- and anti-inflammatory mediators induced by RSV infection center on the differentiation state of macrophages, and that therapeutic interventions can therefore be developed for RSV infection based on increasing AAM differentiation.
MATERIALS AND METHODS
Mice
Six- to 8-wk-old WT BALB/cByJ mice were purchased (Jackson Laboratory, Bar Harbor, ME, USA). Each treatment group per experiment consisted of 4–5 mice. All animal experiments were conducted with institutional approval.
Virus and reagents for in vivo studies
RSV Long strain (group A) was obtained from American Type Culture Collection (Manassas, VA, USA) and propagated as described [10]. AZM was purchased from Teva Pharmaceuticals (North Wales, PA, USA). Rosiglitazone (Avandia) was purchased from GSK (Clifton, NJ,USA). Recombinant murine IL-4 was purchased from R&D Systems (Minneapolis, MN, USA). BVD4-1D11, a rat IgG2b mAb to mouse IL-4 was prepared, as well as the rIL-4/anti-IL-4 immune complexes, as described previously [12]. Briefly, for the IL-4/anti-IL-4 complex experiments, we first prepared the complexes by using rmIL-4 with anti-IL-4 (BVD4-1D11) at a 1:5 ratio and letting the complexes form by gentle mixing at room temperature for 5 min. The complexes were then diluted in 0.9% saline for treatments.
To test the IL-4/anti-IL-4 immune complexes, WT BALBc/ByJ mice were injected intraperitoneally with the complex or an isotype-matched control Ab starting 2 days before infection (5 μg recombinant mIL-4/25 μg anti-IL-4). On day 0, the mice were mock infected with PBS or infected with RSV (2×106 PFU/mouse intranasally). The complexes were then injected 2 more times (days 2 and 5 p.i.), and the lungs were harvested on day 6 p.i. To assess the effect of rosiglitazone on RSV-induced lung pathology, WT BALB/cByJ mice were infected with RSV on day 0 and treated with rosiglitazone (25 mg/kg i.p.), on days 1–5 p.i. The lungs were harvested on day 6 p.i. for determining mRNA gene expression and for lung histopathology. To assess the effect of AZM, WT BALB/cByJ mice were infected with RSV. On days 2–5 p.i., the mice were treated once daily with AZM (2 mg/mouse; i.p.). On day 6 p.i., the lungs were harvested and analyzed as described above.
qRT-PCR
Total RNA isolation and real-time PCR were performed as previously described [11, 13, 14]. Levels of mRNA for specific genes are reported as relative gene expression normalized to mock-infected samples.
Histopathology
Fixed sections (10 μm) of paraffin-embedded lungs were stained with H&E. Four inflammatory parameters were scored independently from 0 to 4 for each section [15]: peribronchiolitis (inflammatory cells, primarily lymphocytes, surrounding a bronchiole), perivasculitis (inflammatory cells, primarily lymphocytes, surrounding a blood vessel), alveolitis (inflammatory cells within alveolar spaces), and interstitial pneumonitis (increased thickness of alveolar walls associated with inflammatory cells). Slides were randomized, read blindly, and scored for each. The data shown in the figures were combined from 2 independent experiments, and where no error bars are visible, all the mice from that group had identical histology scores.
Statistics
Significant differences between 2 groups were determined with an unpaired, 2-tailed Student's t test with significance set at P < 0.05. For comparisons between 3 or more groups, analysis was performed by 1-way ANOVA, followed by Tukey's multiple comparison test with significance determined at P < 0.05.
RESULTS AND DISCUSSION
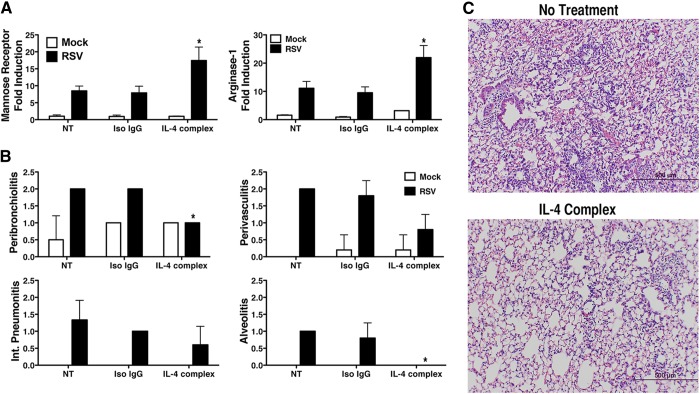
Our previous findings showed that RSV-induced AAM is TLR4 and IFN-β dependent and requires production of macrophage-derived IL-4 and -13. Signaling via the IL-4Rα chain activates STAT6 that drives differentiation of macrophages to AAMs. AAMs, in turn, mediate resolution of RSV-induced lung pathology [14]. We reasoned that administration of exogenous IL-4 could facilitate resolution of RSV-induced tissue damage; however, it is well documented that IL-4, like most cytokines, is cleared very rapidly in vivo [12, 16, 17]. Finkelman and colleagues [12] developed an approach using the IL-4/anti-IL-4 complexes, which substantially extends the therapeutic efficacy of IL-4. When the rIL-4/anti-IL-4 immune complexes were administered to RSV-infected BALB/cByJ mice 2 days before infection and then on days 2 and 5 p.i., and the lungs were harvested 6 days p.i., we observed a significant increase in the expression of the murine AAM markers mannose receptor and arginase-1 mRNA above that recorded in the control-treated mice (Fig. 1A). Although a minimal level of inflammation was observed in control mice, as indicated by the accumulation of a small number of cells around the bronchioles (peribronchiolitis), this is normal in rodents [18]. Notably, there was a significant reduction in the level of RSV-induced peribronchiolitis and alveolitis in the mice treated with the IL-4/anti-IL-4 immune complexes (Fig. 1B, C), and a similar, but nonsignificant, trend was observed in perivasculitis and interstitial pneumonitis in the treated mice (Fig. 1B, C). These data provide proof of principle that exogenous enhancement of AAMs by IL-4 results in an improved outcome in RSV. Based on these findings, we sought to extend our evidence that therapeutic manipulation of AAM development would result in amelioration of RSV-induced lung damage.
Figure 1. Administration of the IL-4/anti-IL-4 complexes during RSV infection.
WT BALB/cByJ mice (4 mice/group) were injected (i.p.) with either isotype control IgG or the IL-4/anti-IL-4 (1:5) complex 2 days before infection with RSV. The mice were again treated on days 2 and 5 p.i., with either control IgG or the IL-4/anti-IL-4 complexes. The lungs were harvested on day 6 p.i. for (A) mRNA gene expression and (B and C) histopathology. *P < 0.05, based on analysis of results of 2 independent experiments.
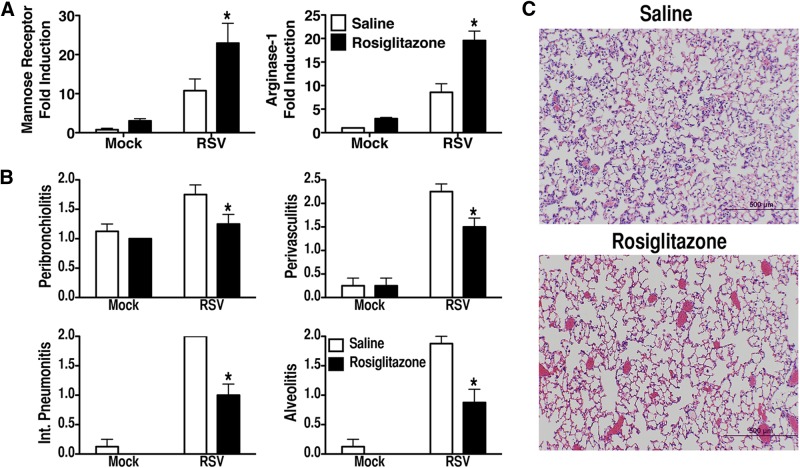
PPARγ is a critical transcription factor for induction of AAMs [19, 20]. We previously reported that RSV-induced PPARγ mRNA is TLR4 dependent [14]. Rosiglitazone, a PPARγ agonist, has also been shown to alter macrophage differentiation to an M2 phenotype [21]. In addition to having a direct effect on induction of AAMs, PPARγ also decreases the expression of signaling receptors that contribute to COX-2 induction in vivo [22–25]. Therefore, if PPARγ activation reduces the pathology induced by RSV by decreasing levels of COX-2 during infection, it might be predicted that treating RSV-infected animals with a PPARγ agonist would decrease RSV-induced lung injury. To test this hypothesis, BALB/cByJ mice were infected with RSV and treated therapeutically (on days 1–5 p.i.) with rosiglitazone. Lungs harvested on day 6 from mice infected with RSV and treated therapeutically with rosiglitazone exhibited a statistically significant increase in the expression of mannose receptor and arginase-1 mRNA (Fig. 2A), accompanied by a significant decrease in lung pathology (Fig. 2B, C). The recent observation that rosiglitazone reduces brain inflammation by activating both the PPARγ and 5-LO pathways [26] is supportive of our recently published findings showing that RSV-induced induction of AAMs also requires the 5-LO signaling pathway [11]. Although both rosiglitazone and the more potent PPARγ receptor agonist pioglitazone have been taken off the market as treatments for type II diabetes because of cardiac side effects, we anticipate that an acute treatment regimen with these drugs for RSV would be considerably less prolonged and would not result in significant cardiotoxicity.
Figure 2. Administration of rosiglitazone during RSV infection.
WT BALB/cByJ mice (4–5 mice/group) were infected with RSV. Starting on day 1 p.i., the mice were treated with either saline or rosiglitazone (25 mg/kg/mouse i.p.) once daily for 5 consecutive days (days 1–5 p.i). The lungs were harvested for (A) mRNA gene expression and (B and C) histopathology on day 6 p.i. *P < 0.05, based on analysis of results of 2 independent experiments.
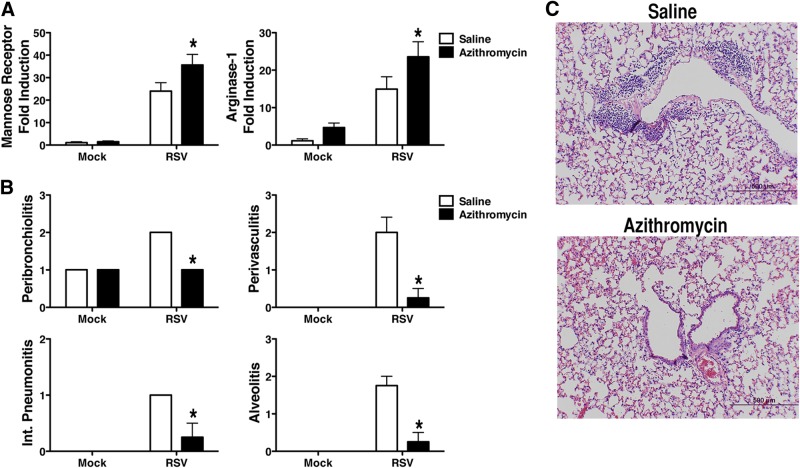
Several reports show that the macrolide antibiotic AZM reduces the production of proinflammatory cytokines and increases arginase-1 activity in vitro and in vivo by down-regulating both NF-κB and STAT1 transcription factors [27–29]. AZM has also been found to be clinically useful in patients with cystic fibrosis because of its anti-inflammatory effects [30, 31]. Murphy et al. [27] reported that AZM alters macrophages to an AAM phenotype in vitro. Therefore, we hypothesized that a short course of AZM in RSV-infected mice would facilitate resolution of tissue damage by enhancing AAM differentiation. To test this hypothesis, BALB/cByJ mice were infected with RSV. On days 2–5 p.i., the mice were treated once daily with AZM (2 mg/mouse i.p.). This dose of AZM has been shown to result in increased AAM expression in mice infected with Pseudomonas aeruginosa [28]. In the RSV-infected mice treated therapeutically with AZM, we observed a significant increase in mannose receptor and arginase-1 mRNA expression in the lung (Fig. 3A) accompanied by a significant decrease in pathology on day 6 p.i (Fig. 3B, C). Arginase-1 mRNA was also increased in AZM-treated, RSV-infected cotton rats (data not shown). Thus, our data support the contention that repurposing drugs such as rosiglitazone and AZM would provide a novel and safe approach to the amelioration of severe RSV-induced pathology.
Figure 3. Administration of AZM during RSV infection.
WT BALB/cByJ mice (4–5 mice/group) were infected with RSV. Starting on day 2 p.i., the mice were treated with either saline or AZM (2 mg/mouse i.p.) once daily for 5 consecutive days (days 2–6 p.i.). The lungs were harvested for (A) mRNA gene expression and (B and C) histopathology on day 6 p.i. * P < 0.05, based on analysis of results of 2 independent experiments.
RSV remains a leading global cause of respiratory illness in infants, for which no effective vaccine or therapy exists. The ability to intervene therapeutically with drugs developed for other diseases that have the ability to promote induction of AAMs and mitigate RSV-induced pathology provides a new approach that could be used to manage RSV infection in individuals at risk for severe disease. Keegan and colleagues [32] and Saenz et al. [33] have shown that AAMs have the ability to initiate airway hyper-responsiveness to subsequent exposure to Ag. Since many young children who have had RSV as infants develop asthma and atopy [5, 34], the possible use of AAM-inducing therapeutics will have to be assessed for their long-term effects on the development of airway hyper-responsiveness.
ACKNOWLEDGMENTS
J.C.B. was supported by U.S. National Institutes of Health (NIH) grant AI-057575, S.N.V. by NIH AI-18797, and D.J.F. by NIH AI-095307; F.D.F. was supported by NIH AI-070300 and is the recipient of a Veterans Affairs Merit award.
SEE CORRESPONDING EDITORIAL ON PAGE 945
- 5-LO
- 5-lipoxygenase
- AAM
- alternatively activated macrophage
- ALRI
- acute lower respiratory infection
- AZM
- azithromycin
- COX-2
- cyclooxygenase-2
- p.i.
- postinfection
- PPARγ
- peroxisome proliferator-activated receptor γ
- RSV
- respiratory syncytial virus
- WT
- wild-type
AUTHORSHIP
K.A.S. and S.N.V. carried out the study design, with advice from J.C.B., F.F., and D.F.; K.A.S., W.L., J.C.B., and L.M.P. performed experiments; F.F. and D.F. provided crucial reagents for study; K.A.S. and S.N.V. prepared the manuscript, with the input and approval of all other coauthors.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Nair H., Nokes D. J., Gessner B. D., Dherani M., Madhi S. A., Singleton R. J., O'Brien K. L., Roca A., Wright P. F., Bruce N., Chadran A., Theodoratou E., Sutanto A., Sedyaningsih E. R., Ngama M., Munywoki P. K., Kartasasmita C., Simoes E. A. F., Rudan I., Weber M. W., Campbell H. (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375, 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blanco J. C. G., Boukhvalova M. S., Shirey K., Prince G. A., Vogel S. N. (2010) New insights for development of a safe and protective RSV vaccine. Hum. Vaccin. 6, 482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The PREVENT Study Group. (1997) Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 99, 93–99. [DOI] [PubMed] [Google Scholar]
- 4. Chin J., Magoffin R. L., Shearer L. A., Schieble J. H., Lennette E. H. (1969) Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 89, 449–446. [DOI] [PubMed] [Google Scholar]
- 5. The IMpact-RSV Study Group. (1998) Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102, 531–537. [PubMed] [Google Scholar]
- 6. Falsey A. R. (2007) Respiratory syncytial virus infection in adults. Semin. Respir. Crit. Care Med. 28, 171–181. [DOI] [PubMed] [Google Scholar]
- 7. Lee N., Lui G. C. Y., Wong K. T., Li T. C. M., Tse E. C. M., Chan J. Y. C., Yu J., Wong S. S. M., Choi K. W., Wong R. Y. K., Ngai K. L. K., Hui D. S. C., Chan P. K. S. (2013) High morbidity and mortality in adults hospitalized for respiratory syncytial virus infection. Clin. Infect. Dis. 57, 1069–1077. [DOI] [PubMed] [Google Scholar]
- 8. Harrington R. D., Hooton T. M., Hackman R. C., Storch G. A., Osborne B., Gleaves C. A., Benson A., Meyers J. D. (1992) An outbreak of respiratory syncytial virus in a bone marrow transplant center. J. Infect. Dis. 165, 987–993. [DOI] [PubMed] [Google Scholar]
- 9. Bowden R. A. (1997) Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am. J. Med. 102, 27–30, discussion 42-3. [DOI] [PubMed] [Google Scholar]
- 10. Richardson J. Y., Ottolini M. G., Pletneva L., Boukhvalova M., Zhang S., Vogel S. N., Prince G. A., Blanco J. C. (2005) Respiratory syncytial virus (RSV) infection induces cyclooxygenase 2: a potential target for RSV therapy. J. Immunol. 174, 4356–4364. [DOI] [PubMed] [Google Scholar]
- 11. Shirey K., Lai W., Pletneva L.M., Karp C.L., Divanovic S., Blanco J. C. G., Vogel S. N. (2014) Role of the lipoxygenase pathway in RSV-induced alternatively activated macrophages leading to resolution of lung pathology. Mucosal Immunol. 7, 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finkelman F. D., Madden K. B., Morris S. C., Holmes J. M., Boiani N., Katona I. M., Maliszewski C. R. (1993) Anti-cytokine antibodies as carrier proteins: prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol. 151, 1235–1244. [PubMed] [Google Scholar]
- 13. Shirey K., Cole L. E., Keegan A. D., Vogel S. N. (2008) Francisella tularensis LVS induces macrophage alternative activation as a survival mechanism. J. Immunol. 181, 4159–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shirey K., Pletneva L. M., Puche A. C., Keegan A. D., Prince G. A., Blanco J. C., Vogel S. N. (2010) Control of RSV-induced lung injury by alternatively activated macrophages is IL-4Rα-, TLR4-, and IFN-β-dependent. Mucosal Immunol. 3, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prince G. A., Prieels J. P., Slaoui M., Porter D. D. (1999) Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus). Lab. Invest. 79, 1385–1392. [PubMed] [Google Scholar]
- 16. Lotze M. T., Matory Y. L., Ettinghausen S. E., Rayner A. A., Sharrow S. O., Seipp C. A. Y., Custer M. C., Rosenberg S. A. (1985) In vivo administration of purified human interleukin 2: II, half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL2. J. Immunol. 135, 2865–2875. [PubMed] [Google Scholar]
- 17. Sherman M.L., Spriggs D.R., Arthur K.A., Imamura K., Frei E., III, Kufe D.W. (1988) Recombinant human tumor necrosis factor administered as a five-day continuous infusion in cancer patients: phase I toxicity and effects on lipid metabolism. J. Clin. Oncol. 6:344–350. [DOI] [PubMed] [Google Scholar]
- 18. Prince G. A., Mathews A., Curtis S. J., Porter D. D. (2000) Treatment of Respiratory syncytial virus bronchiolitis and pneumonia in a cotton rat model with systemically administered monoclonal antibody (Palivizumab) and glucocorticosteroid. J. Infect. Dis. 182, 1326–1330. [Google Scholar]
- 19. Malur A., McCoy A. J., Arce S., Barna B. P., Kavuru M. S., Malur A. G., Thomassen M. J. (2009) Deletion of PPAR gamma in alveolar macrophages is associated with a Th-1 pulmonary inflammatory response. J. Immunol. 182, 5816–5822. [DOI] [PubMed] [Google Scholar]
- 20. Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A. W., Chawla A. (2007) Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447, 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hasegawa-Mariyama M., Kurimoto T., Nakama M., Godai K., Kojima M., Kuwaki T., Kanmura Y. (2013) Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuates inflammatory pain through the induction of heme oxygenase-1 in macrophages. Pain 154, 1402–1412. [DOI] [PubMed] [Google Scholar]
- 22. Springer T., Galfre G., Secher D., Milstein C. (1979) Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur. J. Immunol. 9, 301–306. [DOI] [PubMed] [Google Scholar]
- 23. Ho M. K., Springer T. A. (1982) Mac-1 antigen: quantitation and expression in macrophage populations and tissues, and immunofluorescent localization in spleen. J. Immunol. 128, 2281–2286. [PubMed] [Google Scholar]
- 24. Lim J., Hotchkin N. A. (2012) Signaling mechanisms of the leukocyte integrin αMβ2: current and future perspectives. Biol. Cell 104, 631–640. [DOI] [PubMed] [Google Scholar]
- 25. Perera P. Y., Mayadas T. N., Takeuchi O., Akira S., Zaks-Zilberman M., Goyert S. M., Vogel S. N. (2001) CD11b/CD18 acts in concert with Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol. 166, 574–581. [DOI] [PubMed] [Google Scholar]
- 26. Ballesteros I., Cuartero M. I., Pradillo J. M., de la Parra J., Perez-Ruiz A., Corni A., Ricote M., Hamilton J. A., Sobrado M., Vivancos J., Nombela F., Lizasoain I., Moro M. A. (2014) Rosiglitazone-induced CD36 up-regulation resolves inflammation by PPARγ and 5-LO-dependent pathways. J. Leukoc. Biol. 95, 587–598. [DOI] [PubMed] [Google Scholar]
- 27. Murphy B. S., Sundareshan V., Cory T. J., Hayes D., Jr., Anstead M. I., Feola D. J. (2008) Azithromycin alters macrophage phenotype. J. Antimicrob. Chemother. 61, 554–560. [DOI] [PubMed] [Google Scholar]
- 28. Feola D. J., Garvy B. A., Cory T. J., Birket S. E., Hoy H., Hayes D., Jr., Murphy B. S. (2010) Azithromycin alters macrophage phenotype and pulmonary compartmentalization during lung infection with Pseudomonas. Antimicrob. Agents Chemother. 54, 2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vrancic M., Banjanac M., Nujic K., Bosnar M., Murati T., Munic V., Stupin Polancec D., Belamaric D., Parnham M. J., Erakovic Haber V. (2012) Azithromycin distinctly modulates classical activation of human monocytes in vitro. Br. J. Pharmacol. 165, 1348–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Equi A., Balfour-Lynn I. M., Bush A., Rosenthal M. (2002) Long term azithromycin in children with cystic fibrosis: a randomized, placebo-controlled crossover trial. Lancet 360, 978–984. [DOI] [PubMed] [Google Scholar]
- 31. Florescu D. F., Murphy P. J., Kalil A. C. (2009) Effects of prolonged use of azithromycin in patients with cystic fibrosis: a meta-analysis. Pulmonary Pharmacol. Therapeut. 22, 467–472. [DOI] [PubMed] [Google Scholar]
- 32. Ford A.Q., Dasgupta P., Mikhailenko I., Smith E.M., Noben-Trauth N., Keegan A. D. (2012) Adoptive transfer of IL-4Ralpha+ macrophages is sufficient to enhance eosinophilic inflammation in a mouse model of allergic lung inflammation. BMC Immunol. 13, 6 doi: 10.1186/1471–2172-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saenz S. A., Noti M., Artis D. (2010) Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 31, 407–413. [DOI] [PubMed] [Google Scholar]
- 34. Sigurs N., Bjarnason R., Sigurbergsson F., Kjellman B., Bjorksten B. (1995) Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics 95, 500–505. [PubMed] [Google Scholar]