The expression of INF-ε in multiple mucosal tissues of rhesus macaques.
Keywords: Simian Immunodeficiency Virus, SIV, type I interferons, IFN-I, mucosal immunity
Abstract
Type I IFNs play an important role in innate and adaptive immunity against viral infections. A novel type I IFN, namely IFN-ε, which can protect against vaginal transmission of HSV2 and Chlamydia muridarum bacterial infection, has been described in mice and humans. Nevertheless, the principle cell type and the expression pattern of IFN-ε in tissues remain uncertain. In addition, the expression of IFN-ε in Indian rhesus macaques (Macaca mulatta) has not been reported. Here, we analyzed IFN-ε expression in multiple mucosal sites of uninfected or SIV-infected Indian rhesus macaques using IHCS. We report for the first time the detection of IFN-ε expression in situ in the lung, foreskin, vaginal, cervical, and small and large intestinal mucosae of rhesus macaques. We found that the expression of IFN-ε was exclusive to the epithelial cells in all of the aforementioned mucosal tissues. Furthermore, the macaque IFN-ε sequence in this study revealed that macaque IFN-ε is highly conserved among human and other nonhuman primates. Lastly, SIV rectal infection did not significantly alter the expression of IFN-ε in rectal mucosae. Together, these findings indicate that IFN-ε may function as the first line of defense against the invasion of mucosal pathogens. Further studies should be conducted to examine IFN-ε protection against gastrointestinal as well as respiratory infections.
Introduction
Type I IFNs are fundamentally important in innate immunity against viral infections, cellular proliferation, regulation, and effector cell activation of the adaptive immune system [1, 2]. Viral infection can induce the expression of type I IFNs by activating TLRs through their pathogen-associated molecular patterns [3–6].
In recent years, a novel type I IFN, IFN-ε, has been described [7]. The IFN-ε gene is located within the type I IFN gene locus and is conserved across many mammalian species [8–12]. Although the IFN-ε protein has only 30% aa homology with IFN-α and -β [7], IFN-ε was found to use the type I IFNR chains IFN-α-R1 and IFN-α-R2 for signaling; therefore, IFN-ε is classified as a type I IFN [11, 13]. However, compared with type I IFN-α and -β, IFN-ε had significantly lower antiviral and NK enhancement activity in vitro [9]. In addition, the expression of IFN-ε cannot be induced by pattern recognition receptors or viral infection [8–12]. In contrast, TNF-α stimulation of HeLa cells and seminal plasma in cervico-vaginal tissues could increase the expression of IFN-ε [14, 15]. Furthermore, the constitutive expression of IFN-ε in mouse lung, brain, small intestine, and male reproductive tissues has been reported [7, 9, 12, 16]. In addition, the expression of IFN-ε in epithelial cells in the genital tracts of women and female mice was found to be regulated by estrogen [11]. Very recently, it was also shown that IFN-ε-deficient mice were more susceptible to vaginal transmission of HSV2 and C. muridarum bacteria, the etiologic agent of chlamydiosis, suggesting that IFN-ε may serve as the first line of defense against sexually transmitted pathogens [11]. Despite the clear evidence of IFN-ε expression in mucosal tissues, the spatial distribution pattern in the mucosae, the cell type responsible for IFN-ε expression and the expression level change in response to SIV infection remain poorly understood.
In this study, we sought to determine the following: 1) whether the mucosae of the Indian rhesus macaque, a commonly used, nonhuman primate model of human infectious diseases, including HIV-1, express IFN-ε; 2) which mucosal sites and what type of cells express IFN-ε; and 3) whether IFN-ε expression is altered in the rectal mucosa after early SIV infection of rhesus macaques. Here, we show for the first time that IFN-ε is expressed in the lung, male and female reproductive tracts, and the gastrointestinal tract of the Indian rhesus macaques. We also show that the distribution of IFN-ε in all of the aforementioned mucosae was exclusive to epithelial cells, and the expression of IFN-ε in the rectal mucosae was unaffected by SIV rectal infection. Finally, we determined the full-length sequence of rhesus macaque IFN-ε mRNA, of which only a partial sequence was available previously. This macaque sequence reveals a high level of conservation across human and other nonhuman primate species. Together, these findings will aid future studies examining the role of IFN-ε in combating mucosal pathogens, not just in the genital tract but also in respiratory and gastrointestinal tract tissues, which have yet to be explored.
MATERIALS AND METHODS
Rhesus macaques and viral inoculation
The mucosal tissues of Indian rhesus macaques (M. mulatta) came from two studies, in which the macaques were housed and maintained in animal housing facilities at Bioqual (Rockville, MD, USA) and the Caribbean Primate Research Center (Puerto Rico), in accordance with the Guide for the Care and Use of Laboratory Animals. All animals were free of simian retrovirus type D, simian T-lymphotropic virus type 1, and herpes B virus. Animals were sedated with ketamine or telazol for all technical procedures and were fully anesthetized for SIV inoculation with SIVmac251 at the dose of 3.4 × 104 50% tissue culture infective dose intrarectally. Animals were euthanized by exsanguinations under deep (surgical plane) anesthesia using telazol, performed under the direction of the attending veterinarian on a designated date. Tissues collected from a total of 23 adult macaques, of which 11 were uninfected, and 12 were infected with SIVmac251, were examined in this study. Nineteen of the animals were male, and four animals were female. The collected tissues were fixed in 4% paraformaldehyde for 4–6 h and embedded in paraffin.
IHCS
IFN-ε was detected using IHCS [17]. Briefly, 6 μm sections were cut and adhered to silanized slides from 4% paraformaldehyde fixed and paraffin-embedded tissues. The histological sections were pretreated in a 98°C water bath in 10 mM citrate buffer (pH 6.0) for 15 min to unmask the IFN-ε antigen. The sections were blocked with 5% nonfat dry milk in PBS and incubated with an IFN-ε antibody (1:400 dilution, clone number HPA041028; Atlas Antibodies, Stockholm, Sweden) or normal rabbit IgG as an isotype-negative control, overnight at 4°C. The stained color was developed using the Dako Envision System-HRP Rabbit-IgG kit (Dako North America, Carpinteria, CA, USA), using DAB as the substrate. The sections were then counterstained with hematoxylin.
Peptide antigen and antibody competition assays for the confirmation of the specificity of anti-IFN-ε antibody staining
The peptide antigen used to raise the anti-IFN-ε antibody was synthesized in two parts: IFN-ε-FC-25, FQQRQVNQESLKLLNKLQTLSIQQC, and IFN-ε-LE-30, LPHRKNFLLPQKSLSPQQYQKGHTLAILHE (Biomatik, Cambridge, ON, Canada). In addition to the IFN-ε peptide antigen, HTLV ENV-1: LPHSNLDHILEP and ENV-2: VHDSDLEHVLT were used as controls (from Dr. Renu Lal, AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health, Bethesda, MD, USA). The two IFN-ε or HTLV peptides were mixed in an equal molar ratio. The competition assay was conducted by the coincubation of the peptide antigen with the anti-IFN-ε antibody (1:400 or 1:800) at a 5:1- or 10:1-M ratio at room temperature for 2 h. Then, IHCS was performed as described above.
Immunofluorescence staining to colocalize IFN-ε and Mak6
The experiment was conducted as described above for IHCS, except that the tissue sections were coincubated with a rabbit anti-IFN-ε antibody (1:400) and a mouse Mak6 antibody (1:200 dilution; Invitrogen, Carlsbad, CA, USA), a pan-cytokeratin marker for epithelial cells, overnight at 4°C. After washing in PBS, the slides were incubated at room temperature for 2 h with anti-mouse IgG conjugated to AlexaFluor 594 (1:200 dilution; Life Technologies, Carlsbad, CA, USA) and anti-rabbit IgG conjugated to AlexaFluor 488 (1:200 dilution; Life Technologies). After washing, the slides were coverslipped and examined using an inverted confocal microscope (Olympus IX 81; Olympus, Center Valley, PA, USA).
Detection of SIV RNA in rectal tissue using in situ hybridization
SIV RNA in rectal tissues was detected using in situ hybridization as described previously [18]. Briefly, 6 μm sections were cut and adhered to slides. After deparaffinization in xylene; rehydration in PBS; and permeabilization with HCl, digitonin, and proteinase K, the sections were acetylated and hybridized to 35S-labeled, SIV-specific antisense riboprobes or sense riboprobes as a negative control. After washing and digesting with RNases, the sections were coated with a nuclear track emulsion and exposed, developed, and counterstained with H&E.
IFN-ε quantification in the mucosal tissues of uninfected and infected macaques
Tissue sections stained using IHCS were digitized using Scanscope, and the IFN-ε signal was quantified using the Spectrum Plus analysis program (Version 9.1; Aperio ePathology Solutions, Vista, CA, USA). Briefly, a scanned digital slide was opened in ImageScope, and areas morphologically representative of each tissue were selected for analysis using the ImageScope drawing tools; the signal was quantified using a positive pixel count algorithm in the Spectrum Plus analysis program. The algorithm parameters were manually tuned to match the positive markup image accurately over the DAB stain. Once the parameters were set, the algorithm was applied automatically to all of the digital images to measure the IFN-ε expression in the tissues.
Statistics
A statistical analysis of rectal IFN-ε image signal quantification and qPCR data was conducted using an unpaired Student's t-test, as well as a Wilcoxon rank sum test with R statistical software (http://www.r-project.org). P < 0.05 was considered significant.
Total RNA extraction and PCR amplification of IFN-ε mRNA
Total RNA was extracted from rhesus rectal tissues using a previously published protocol [19]. Briefly, rectal tissues were homogenized with a power homogenizer in TRIzol solution (Life Technologies), followed by purification with an RNeasy Mini Kit (Qiagen, Hilden, Germany). Five micrograms of total RNA was used for RT-PCR with Superscript III RT (Life Technologies) and the IFN-ε-R1 primer 5′-TCATGTCGTTCAAGGGTCTTC-3′. The resulting cDNA was amplified via nested PCR using High Fidelity Platinum Taq Polymerase (Life Technologies), the first-round IFN-ε-R1 antisense primer, and the IFN-ε-FORWARD sense primer 5′-ATG ATT ATC AAG CAC TTC TTT GAA-3′. Second-round nested PCR was performed using the IFN-ε-F2 sense primer 5′-ACT CTT GAA TAA GTT GCA AAC C-3′ and the IFN-ε-R2 antisense 5′-TCTGTGAGACTGAACACAAAG-3′ primer. The amplicons were sequenced, and the resulting sequence was used to design primers for amplifying the 5′ and 3′ UTR to characterize the IFN-ε mRNA regulatory elements. The amplification of the IFN-ε mRNA 3′ UTR was performed using the IFN-ε-RACE1 sense primer 5′-CCTGGGCCATTGTCCAAGTA-3′ and the antisense primer 5′-TTTGAAGAATCAACCATATTAATG-3′. For the amplification of the IFN-ε mRNA, the 5′ UTR AD02413B sense primer 5′-CTTAGATATTAAACTGATAGGATA-3′ and the antisense IFN-ε-5RACE2 primer 5′-GCCAGCAGCACCAACATAATT-3′ were used. The final PCR products were run on a 1% agarose gel, purified using a QIAquick Gel Extraction Kit (Qiagen), and sequenced directly.
The quantification of IFN-ε expression in rectal tissues using qRT-PCR
qRT-PCR was conducted in a final volume of 20 μl with 800 ng cDNA, 0.2 μM of each primer, and Platinum Taq High Fidelity Polymerase (Invitrogen) using the CFX96 Real-Time detection system (Bio-Rad Laboratories, Hercules, CA, USA), using a hot start (95°C for 3 min) and 40 amplification cycles (95°C for 15 s, 57°C for 30 s). The cDNA was synthesized using an Oligo (DT) primer and Superscript III RT (Life Technologies). The following primers and probes were used for amplification and detection: Rh-IFN-ε forward CTC TTG AAT AAG TTG CAA ACC TCA and Rh-IFN-ε reverse 5′-TCT GCT GAA GCA TCT CAT GG-3′; GAPDH forward 5′-ACA TCA TCC CTG CCT CTA CT-3′, Rh-IFN-ε probe 5′-/56-FAM/AGA AGT CTT /ZEN/TGA GTC CTC AGC AGT ACC A/3IABkFQ/-3′; GAPDH probe 5′-/56-FAM/CAA GGT CAT/ZEN/CCC TGA GCT GAA CGG/3IABkFQ/-3′.
Multiple sequence alignment and phylogenetic analysis of IFN-ε mRNA
The full-length Indian rhesus macaque IFN-ε mRNA sequence derived from this study was aligned with other mammalian IFN-ε mRNA sequences obtained from NCBI using the MUSCLE multiple alignment tool [20] with default settings and a maximum iteration of 16 times. The resulting multiple alignments were verified and edited manually in BioEdit. A phylogenetic analysis was conducted using the maximum likelihood method, and the tree was generated using the PhyML program [21]. The HKY85 nucleotide substitution model and the nearest neighbor interchange algorithm were used for the tree topological search. A BioNJ tree was built for the starting tree. The branch lengths and substitution model parameters were optimized for the best tree output. The phylogenetic accuracy and reliability were tested by bootstrapping with 1000 repeat calculations. The tree was viewed and edited using FigTree (by Andre Rambaut; http://tree.bio.ed.ac.uk/software/figtree/).
Nucleotide sequence accession numbers
The full-length Indian rhesus macaque IFN-ε mRNA sequence derived from this study was used for MUSCLE multiple alignment and phylogenetic analysis against the following sequences: chimpanzee (Pan troglodytes), accession #GABE01011555; northern white-cheeked gibbon (Nomascus leucogenys), accession #XM_004092857.1; gorilla (Gorilla gorilla), accession #XM_004047874; and human (Homo sapiens), accession #NM_176891, and the full length was determined experimentally from this study from the rhesus macaque (M. mulatta), accession #KF955535.
RESULTS
IFN-ε is expressed in the cervix, vagina, and foreskin of the Indian rhesus macaque
Previous studies have demonstrated that IFN-ε could be detected in epithelial cells in the female reproductive tracts of mice and humans [11, 12]. In addition, mouse testes were found to express IFN-ε, demonstrating that both the male and female reproductive organs can express IFN-ε [12]. However, whether IFN-ε is expressed in nonhuman primates is unknown. We examined IFN-ε expression in the vagina and cervix tissues of four SIV-uninfected female Indian rhesus macaques and the foreskin tissue of seven SIV-uninfected male Indian rhesus macaques using IHCS. In the vagina and ectocervix tissues, IFN-ε was expressed exclusively in epithelial cells (Fig. 1A and B and Supplemental Fig. 1) but not in the LP, follicular aggregates, or any other cells in these tissues. This result was confirmed by the colocalization of IFN-ε and Mak6, a marker for pan-cytokeratin (epithelial-specific proteins), using immunofluorescence staining (Supplemental Fig. 1). Of note, the highest IFN-ε-expressing cells in the vagina and ectocervix were the basal epithelial cells. Similar to the ectocervix and vagina, in the endocervix, IFN-ε expression was localized solely in the single layer of columnar epithelial cells (Fig. 1C and Supplemental Fig. 1).
Figure 1. IFN-ε expression in the reproductive tracts of male and female rhesus macaques.
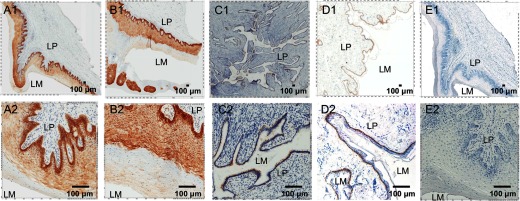
Representative micrographs of IFN-ε expression (stained as brown) in the cervixes, vaginas, and foreskins from at least four SIV-uninfected Indian rhesus macaques detected using IHCS. Five histological sections for each tissue type from each animal were stained. (Upper and lower) The same field at low and high magnifications. IFN-ε expression in the epithelial cells lining the vagina (A1 and A2), ectocervix (B1 and B2), endocervix (C1 and C2), and foreskin (D1 and D2). E1 and E2 show staining of the vaginal tissue with a rabbit IgG isotype as an antibody control. LM, Lumen. Original scale bars: 100 μ.
Similar to the female reproductive tract tissues, IFN-ε expression in the foreskin (Fig. 1D) was localized in unkeratinized epithelial cells. Again, the highest IFN-ε expression was in the basal epithelial cells. However, the signal intensity in the foreskin relative to the female reproductive tissues was significantly lower. Together, these findings demonstrate for the first time that the epithelial cells lining the cervix, vagina, and foreskin of rhesus macaques constitutively express IFN-ε.
The antibody used in this study is against human IFN-ε and its specificity for rhesus macaques IFN-ε was unknown. To confirm the specificity of the antibody, we conducted IHCS using an isotype control antibody, in which no staining was observed (Fig. 1E and Supplemental Fig. 2). As the human IFN-ε antigen amino acid sequence contains five mismatches with the same region of the rhesus macaque sequence, to confirm further the specificity of the antibody and ensure that the human IFN-ε antibody was recognizing rhesus macaque IFN-ε, we performed a peptide antigen and antibody competition assay. The preincubation of the peptide antigen with the IFN-ε antibody at a 5:1-M ratio resulted in reduced staining compared with the nonpeptide control (Supplemental Fig. 3), and a 10:1 ratio resulted in complete elimination of IFN-ε staining of the tissue. This reduction in staining could be completely rescued when a nonspecific peptide (HTLV) of similar length was used. Together, these data demonstrate the specificity of this antibody in recognizing rhesus macaque IFN-ε protein (Supplemental Fig. 3).
IFN-ε is expressed in lung mucosal tissue
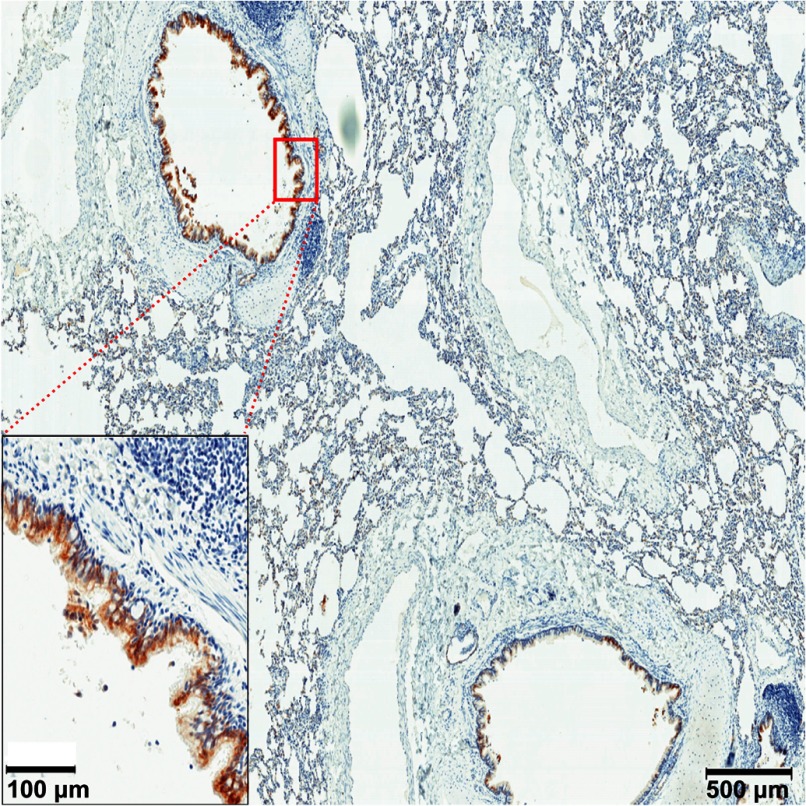
IFN-ε mRNA has been detected previously in mouse lung tissue through qPCR [7]. However, it had not been reported in any other animals. Here, we sought to determine if IFN-ε is expressed in the lungs of Indian rhesus macaques. We identified IFN-ε expression in the lungs of all three animals examined. IFN-ε was expressed in the epithelial cells lining the bronchioles (Fig. 2 and Supplemental Fig. 1) but not in other cells, including epithelial cells of the alveoli. This finding revealed that the respiratory mucosae of Indian rhesus macaques express IFN-ε, with expression exclusive to bronchial epithelial cells, supporting the notion that IFN-ε expression is limited to epithelial cells in the mucosae.
Figure 2. IFN-ε expression in rhesus respiratory mucosae.
Representative micrographs of IFN-ε expression (stained as brown) in the epithelial cells lining the bronchioles of at least four SIV-uninfected Indian rhesus macaques detected using IHCS. Three tissue sections for each animal were stained.
IFN-ε is expressed in the mucosae of small and large intestines
Next, we sought to determine whether IFN-ε expression could be detected in intestinal mucosae, especially the rectum, which is an important portal of entry for many pathogens, including HIV-1 [22]. In the jejunum (Fig. 3A and B) and the rectum (Fig. 3D–G, top, and Supplemental Fig. 1), robust expression of IFN-ε was detected in the epithelial cells but not in the LP or any other cells using IHCS. These data demonstrate that epithelial cells of the small and large intestinal mucosae of the rhesus macaque express IFN-ε.
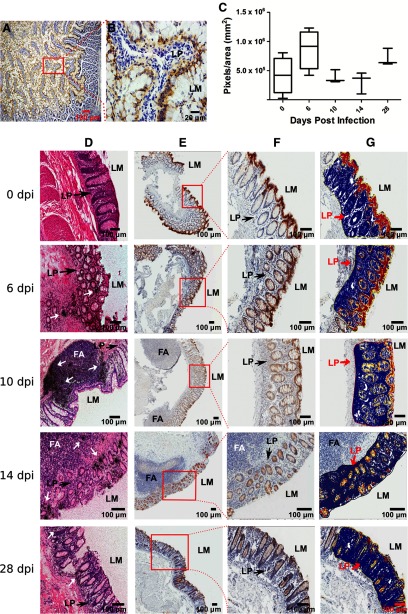
Figure 3. IFN-ε expression in the gut mucosae of uninfected and SIV-infected macaques.
Representative micrographs of IFN-ε expression (stained as brown) in the epithelial cells lining the jejunum (A and B) and rectums (E–G, top) of seven SIV-uninfected Indian rhesus macaques, detected using IHCS. (C) Quantitative analysis of IFN-ε expression in the rectums of uninfected or 12 SIV-infected macaques; the box plot shows the ratio of IFN-ε-positive pixels versus the epithelial area in pixels. The differences were not significant (horizontal black line denotes the median, boxes denote 25 and 75 percentiles, and whiskers denote the sd). (D) SIV vRNA+ cells in rectums detected using in situ hybridization with an S35-labeled riboprobe. After radioautographic development, the clusters of discrete black silver grains (white arrows) overlay the vRNA+ cells at 6, 10, 14, and 28 days postinoculation (dpi). (E) Representative images of IFN-ε staining in infected rectal tissues from three male animals at each time-point postinfection. (F) The images at high magnification from the boxes in image E. (G) Markup images, in which red, pink, and yellow represent strong, medium, and weak positive signals, respectively, used for quantification of IFN-ε in SIV-uninfected and infected rectums at different time-points postinfection. FA, Follicular aggregate.
IFN-ε expression in rectal mucosa was not altered in the early rectal transmission of SIV
Receptive rectal intercourse is a common mode of HIV-1 transmission in humans. As we found that IFN-ε is constitutively expressed in the rectal epithelial cells of SIV-uninfected macaques, we next wanted to determine whether IFN-ε expression in the rectal mucosae would be altered during early rectal transmission of SIV. Rectal tissues collected at 10, 14, or 28 days post-SIVmac251 inoculation, confirmed to be SIV vRNA positive through in situ hybridization (Fig. 3D), and qRT-PCR (data not shown) were used for this purpose. Quantitative image analysis of rectal tissues stained using IHCS (Fig. 3E and G) showed no significant difference in IFN-ε between uninfected and acutely infected rectal tissues or between the different time-points postinfection, and qRT-PCR confirmed that IFN-ε mRNA expression was not different between the uninfected and infected animals (Supplemental Fig. 4). Furthermore, the spatial distribution of IFN-ε (Fig. 3E and F) within these tissues was similar. These data indicate that SIV infection of the rectum did not alter the expression of IFN-ε, at least in acute infection, which supports the previous notion that IFN-ε expression is not induced directly by viral infection. However, based on the variability in IFN-ε mRNA and protein expression levels between animals, more animals may be needed to conclude definitely that IFN-ε expression is not altered in SIV infection. Therefore, the role of IFN-ε in protecting against rectal transmission of SIV remains unknown and is beyond the scope of this study.
IFN-ε mRNA is highly conserved among nonhuman primates
Mouse and human IFN-ε have been sequenced previously; however, for rhesus macaques, only a putative sequence was available before this study. To confirm the specificity of our IFN-ε IHCS and to obtain the full-length IFN-ε sequence of the Indian rhesus macaque, IFN-ε mRNA, isolated from rectal tissues, was amplified using RT-PCR and sequenced. The macaque full-length coding sequence of IFN-ε (positions 622–1203 in the full-length sequence), obtained from this study, was aligned and compared with the available IFN-ε sequences derived from the mRNA of human and other nonhuman primates in the NCBI database using MUSCLE multiple sequence alignment [20, 23]. Overall, there was a 94% nucleotide identity in the IFN-ε coding sequences of rhesus macaques, humans, and other nonhuman primate species. The 5′ UTR region of the rhesus macaque had not been predicted before this study. The alignment of the 5′ UTR region derived from this study (positions 1–621 in the full-length sequence) revealed that the rhesus macaque IFN-ε 5′ UTR region has 96% identity to the human and other nonhuman primate 5′ UTR sequences, including several known transcription factor-binding sites (Supplemental Fig. 5). The most notable difference in the 5′ UTR region was at positions 570–574, in which the rhesus macaque and olive baboon (Papio anubis) have a 4-nt insertion/disruption of the HNF-1 transcription factor-binding site (Supplemental Fig. 5). This observation suggests that the function of the IFN-ε protein and the expression regulation of the IFN-ε gene are likely to be highly similar among other nonhuman primates and humans.
Maximum likelihood phylogenetic analysis [21] (Fig. 4) resulted in two distinct clusters or groups. The full-length rhesus macaque IFN-ε mRNA clustered with olive baboon in one group, while human, chimpanzee, and other nonhuman primates clustered in the other group. Overall, the sequence conservation observed in the IFN-ε mRNA Indian rhesus macaque, relative to the other IFN-ε mRNA sequences, demonstrates that IFN-ε is highly conserved among human and nonhuman primates.
Figure 4. Phylogenetic analysis of IFN-ε from the rhesus macaque, other nonhuman primates, mice, and humans.
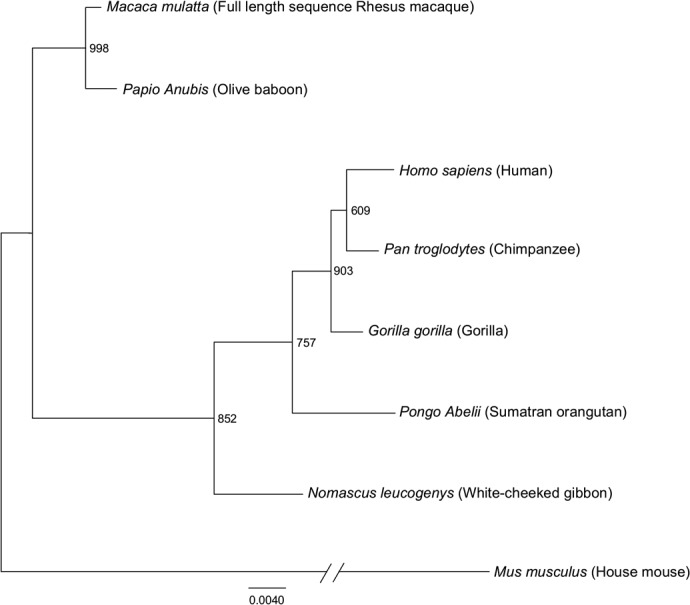
The phylogenetic relationship of the full-length IFN-ε mRNA sequences derived from the rhesus macaque obtained in this study, other nonhuman primates, mice, and humans was analyzed using the maximum likelihood method based on the JTT matrix-based model. The tree is drawn to scale, with branch lengths measured in the number of nucleotide substitutions per site. The tree with the highest log likelihood is shown. The percentage of the trees in which the associated taxa are clustered together is indicated on the branches (out 1000 bootstrap replicates). The mouse sequence was used as an out-group and redrawn to condense the tree. The tree was generated in PhyML, and the text was modified in FigTree.
DISCUSSION
Here, we report for the first time the expression of IFN-ε in epithelial cells lining the jejunum, rectum, bronchioles (lung), and both female and male genital tract tissues of rhesus macaques. Previous studies in mice demonstrated that IFN-ε was expressed by mucosal epithelial cells only in the female reproductive tract and could protect against HSV2 and C. muridarum infections [11]. Furthermore, before this study, there was only a predicted rhesus macaque IFN-ε mRNA sequence, which did not include the 5′ UTR region. In this study, we show that the rhesus macaque IFN-ε 5′ UTR region is 96% identical to that of humans and other nonhuman primates. In addition, with the exception of only the HNF transcription factor-binding site, the transcription factor-binding sites are conserved among the rhesus macaque, human, and nonhuman primate IFN-ε 5′ UTRs, including in the progesterone transcription factor-binding site. Previous studies have shown that mouse and human IFN-ε transcription was increased in response to higher estrogen levels within female genital tract tissues [11]. Our finding of IFN-ε expression in the lungs and gastrointestinal tract and the conservation of several transcription factor-binding sites may suggest a tissue-specific mechanism for regulating the expression of IFN-ε, as sex hormones, such as estrogen or others, are likely to be largely absent in these tissues [11]. The dichotomy of the absence of IFN-ε transcriptional up-regulation in response to viral infection and the fact that it can provide protection against viral infection [11, 12] remain poorly understood, although one plausible explanation is that the IFN-ε-protective effect may be dependent on other cofactors expressed in cells or specific tissues that are inducible in response to pathogens.
Of note, the full-length macaque IFN-ε mRNA sequenced in this study revealed high homology (94% identity) in the coding region with that of humans and other nonhuman primates. Therefore, it is plausible that IFN-ε would have similar function across these species. Further investigation would be needed to address this question, as well as the function and protective effects of IFN-ε in the gut and lung tissues and between female and male reproductive tissues, as these mucosal sites have drastic anatomic, physiological, and microbiological differences. The examination of the breadth and potency of IFN-ε in mediating protection against different pathogens in vivo in different mucosal tissues is also needed.
Previous studies have demonstrated that IFN-ε expression could not be induced by viral infection in vitro or in vivo. Consistent with previous in vitro and in vivo studies in mice [11, 12], IFN-ε expression was not altered significantly throughout the early course of infection, suggesting that rhesus macaque IFN-ε expression in the rectum is not influenced directly by SIV infection. One caveat of this study is the small sample size; therefore, future studies using larger numbers of rhesus macaques may be better suited to fully address whether SIV infection has any influence on rhesus macaque IFN-ε expression.
In conclusion, we report here, for the first time, the expression of IFN-ε in epithelial cells of multiple mucosal tissues in Indian rhesus macaques. In addition, we show that Indian rhesus macaque IFN-ε expression in the rectum is not altered after SIV infection.
Finally, rhesus macaque IFN-ε mRNA, both in the coding region and the 5′ UTR, is highly conserved compared with humans and other nonhuman primates. The findings reported here may aid future studies to address the role of IFN-ε in protecting against mucosal infections.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health Grants DK087625 (to Q.L.), P40 OD012217 (to M. I. Martinez, University of Puerto Rico), and R01AI094603 and R01AI084142 (to L.M.).
We thank members of the Q.L. lab for their insightful technical advice on this project. The authors thank Mark Lewis at Bioqual and for the support of T. A. Santiago, P. P. Maldonado, C. A. Sariol, and M. I. Martinez at the University of Puerto Rico for all of the macaque care and of S. Abdulhaqq and B. Ross at Wistar. We also thank Dr. Dong Wong for his assistance with statistical analysis of the data and Dr. Todd Wical for editing of this manuscript.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- DAB
- diaminobenzidine
- ENV
- envelope glycoprotein
- HNF-1
- hepatocyte NF 1
- HSV2
- herpes simplex 2 viruses
- HTLV
- human T cell leukemia virus
- IHCS
- immunohistochemical staining
- LP
- lamina propria
- Mak6
- cytokeratin marker
- MUSCLE
- multiple sequence comparison by log-expectation
- NCBI
- National Center for Biotechnology Information
- qPCR
- quantitative PCR
- qRT-PCR
- quantitative RT-PCR
- Rh
- recombinant human
- UTR
- untranslated region
- vRNA
- viral RNA
AUTHORSHIP
A.D. and Q.L. conceived of and designed the experiments and wrote the manuscript. G.K., Y.L., and F.M. provided aid in data analysis. W.L. and Z.Y. performed tissue collection. Z.Y. also provided aid for the qPCR assay design. M.L. and E.N.K. housed and cared for the animals. L.M. provided some of the samples used in this study and contributed to the discussion.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1. Stark G. R., Kerr I. M., Williams B. R., Silverman R. H., Schreiber R. D. (1998) How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264. [DOI] [PubMed] [Google Scholar]
- 2. Isaacs A., Lindenmann J. (1957) Virus interference. I. The interferon. Proc. R. Soc. London 147, 258–267.13465720 [Google Scholar]
- 3. Doyle S., Vaidya S., O'Connell R., Dadgostar H., Dempsey P., Wu T., Rao G., Sun R., Haberland M., Modlin R., Cheng G. (2002) IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17, 251–263. [DOI] [PubMed] [Google Scholar]
- 4. Yamamoto M., Sato S., Mori K., Hoshino K., Takeuchi O., Takeda K., Akira S. (2002) Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J. Immunol. 169, 6668–6672. [DOI] [PubMed] [Google Scholar]
- 5. Honda K., Takaoka A., Taniguchi T. (2006) Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25, 349–360. [DOI] [PubMed] [Google Scholar]
- 6. Costa-Mattioli M., Sonenberg N. (2008) RAPping production of type I interferon in pDCs through mTOR. Nat. Immunol. 9, 1097–1099. [DOI] [PubMed] [Google Scholar]
- 7. Hardy M. P., Owczarek C. M., Jermiin L. S., Ejdeback M., Hertzog P. J. (2004) Characterization of the type I interferon locus and identification of novel genes. Genomics 84, 331–345. [DOI] [PubMed] [Google Scholar]
- 8. Delhaye S., Paul S., Blakqori G., Minet M., Weber F., Staeheli P., Michiels T. (2006) Neurons produce type I interferon during viral encephalitis. Proc. Natl. Acad. Sci. USA 103, 7835–7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peng F. W., Duan Z. J., Zheng L. S., Xie Z. P., Gao H. C., Zhang H., Li W. P., Hou Y. D. (2007) Purification of recombinant human interferon-epsilon and oligonucleotide microarray analysis of interferon-epsilon-regulated genes. Protein Expr. Purif. 53, 356–362. [DOI] [PubMed] [Google Scholar]
- 10. Sang Y., Rowland R. R., Hesse R. A., Blecha F. (2010) Differential expression and activity of the porcine type I interferon family. Physiol. Genomics 42, 248–258. [DOI] [PubMed] [Google Scholar]
- 11. Fung K. Y., Mangan N. E., Cumming H., Horvat J. C., Mayall J. R., Stifter S. A., De Weerd N., Roisman L. C., Rossjohn J., Robertson S. A., Schjenken J. E., Parker B., Gargett C. E., Nguyen H. P., Carr D. J., Hansbro P. M., Hertzog P. J. (2013) Interferon-epsilon protects the female reproductive tract from viral and bacterial infection. Science 339, 1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hermant P., Francius C., Clotman F., Michiels T. (2013) IFN-epsilon is constitutively expressed by cells of the reproductive tract and is inefficiently secreted by fibroblasts and cell lines. PLoS One 8, e71320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Day S. L., Ramshaw I. A., Ramsay A. J., Ranasinghe C. (2008) Differential effects of the type I interferons alpha4, beta, and epsilon on antiviral activity and vaccine efficacy. J. Immunol. 180, 7158–7166. [DOI] [PubMed] [Google Scholar]
- 14. Matsumiya T., Prescott S. M., Stafforini D. M. (2007) IFN-epsilon mediates TNF-alpha-induced STAT1 phosphorylation and induction of retinoic acid-inducible gene-I in human cervical cancer cells. J. Immunol. 179, 4542–4549. [DOI] [PubMed] [Google Scholar]
- 15. Sharkey D. J., Macpherson A. M., Tremellen K. P., Robertson S. A. (2007) Seminal plasma differentially regulates inflammatory cytokine gene expression in human cervical and vaginal epithelial cells. Mol. Hum. Reprod. 13, 491–501. [DOI] [PubMed] [Google Scholar]
- 16. Day S. L., Ramshaw I. A., Ramsay A. J., Ranasinghe C. (2008) Differential effects of the type I interferons alpha4, beta, and epsilon on antiviral activity and vaccine efficacy. J. Immunol. 180, 7158–7166. [DOI] [PubMed] [Google Scholar]
- 17. Li Q., Estes J. D., Schlievert P. M., Duan L., Brosnahan A. J., Southern P. J., Reilly C. S., Peterson M. L., Schultz-Darken N., Brunner K. G., Nephew K. R., Pambuccian S., Lifson J. D., Carlis J. V., Haase A. T. (2009) Glycerol monolaurate prevents mucosal SIV transmission. Nature 458, 1034–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li Q., Duan L., Estes J. D., Ma Z. M., Rourke T., Wang Y., Reilly C., Carlis J., Miller C. J., Haase A. T. (2005) Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434, 1148–1152. [DOI] [PubMed] [Google Scholar]
- 19. Li Q., Smith A. J., Schacker T. W., Carlis J. V., Duan L., Reilly C. S., Haase A. T. (2009) Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J. Immunol. 183, 1975–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edgar R. C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guindon S., Dufayard J. F., Lefort V., Anisimova M., Hordijk W., Gascuel O. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. [DOI] [PubMed] [Google Scholar]
- 22. Chenine A. L., Siddappa N. B., Kramer V. G., Sciaranghella G., Rasmussen R. A., Lee S. J., Santosuosso M., Poznansky M. C., Velu V., Amara R. R., Souder C., Anderson D. C., Villinger F., Else J. G., Novembre F. J., Strobert E., O'Neil S. P., Secor W. E., Ruprecht R. M. (2010) Relative transmissibility of an R5 clade C simian-human immunodeficiency virus across different mucosae in macaques parallels the relative risks of sexual HIV-1 transmission in humans via different routes. J. Infect. Dis. 201, 1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Edgar R. C. (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.