Abstract
Ulcerative colitis is a chronic inflammatory disease of the colon; as many as 25% of patients with this disease require hospitalization. The goals of hospitalization are to assess disease severity, exclude infection, administer rapidly acting and highly effective medication regimens, and determine response. During the hospitalization, patients should be given venous thromboembolism prophylaxis and monitored for development of toxic megacolon. Patients who do not respond to intravenous corticosteroids should be considered for rescue therapy with infliximab or cyclosporine. Patients who are refractory to medical therapies or who develop toxic megacolon should be evaluated promptly for colectomy. Patients who do respond to medical therapies should be discharged on an appropriate maintenance regimen when they meet discharge criteria. We review practical evidence-based management principles and propose a day-by-day algorithm for managing patients hospitalized for ulcerative colitis.
Introduction
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon that is usually manifest as diffuse, continuous, superficial inflammation of the colon. During periods of flare, some patients experience severe symptoms that require hospitalization. In severe cases, inflammation can become transmural, leading to deep ulcerations and risk of toxic megacolon. The estimated risk of a patient with UC requiring hospitalization for severe colitis ranges from 18% to 25% (1, 2). One study revealed that 9% of patients admitted with active UC undergo colectomy during that hospitalization (3). However, patients admitted with severe UC have a 27% rate of colectomy (4). The mortality of severe UC is 1% and the mortality of toxic colitis is much higher (1, 4). The total number of hospitalizations for UC in the United States increased by 52% from 1998 to 2007 (5). Although the treatment of severe colitis has recently been reviewed (6), specific detailed recommendations for management of individual patients are limited in the literature. We provide practical, evidence-based management principles for the care of patients hospitalized for UC.
Definitions
Severe Ulcerative Colitis
Several instruments can be used to define and quantify severe UC in clinical practice including the commonly used Truelove and Witts’ Index. (Table 1)(7). The Lichtiger Index (please refer to web supplement) (8) has been used to assess disease activity in clinical trials but does not correlate with other measures of disease activity or clinical outcomes. Patients with severe disease, as defined by Truelove and Witts’ Index, should be distinguished from outpatients with “moderate to severe disease unresponsive to conventional therapy” that were included in clinical trials of infliximab (9) and adalimumab (10).
Table 1.
Truelove and Witts Criteria for Evaluating the Severity of Ulcerative Colitis*
| Variable | Mild Disease | Severe Disease | Fulminant Disease |
|---|---|---|---|
| Stools (number/d) | <4 | >6 | >10 |
| Blood in stool | Intermittent | frequent | continuous |
| Temperature (°C) | Normal | >37.5 | >37.5 |
| Pulse (beats/min) | Normal | >90 | >90 |
| Hemoglobin | Normal | <75% of normal value | transfusion required |
| Erythrocyte sedimentation rate (mm/hr) | ≤30 | >30 | >30 |
| Colonic features on x-ray | air, edematous wall, thumbprinting | dilatation | |
| Clinical signs | abdominal tenderness | abdominal distention and tenderness |
Moderate disease includes features of both mild and severe disease
(7)
Indications for Hospitalization
Indications for hospitalization of patients with UC can include: severe disease, toxic megacolon, failure of outpatient medical therapy, complications of the disease (i.e. arterial or venous thromboembolism), complications related to medical therapy (i.e. opportunistic infections), and severe extraintestinal manifestations.
Goals of Hospitalization
The primary goals during hospitalization are to comprehensively assess disease activity, monitor for complications, and apply medical treatments and/or surgery to improve the patient’s symptoms. During the hospital admission, the medical team should determine the severity and anatomical extent of disease and assess for factors that might have led to disease exacerbation. Anatomic extent of disease is an important prognostic indicator: the presence of pancolitis is associated with more disease related complications, higher rates of failure of medical therapy, and a higher rate of colectomy (11–13). It is important to exclude concomitant infection with Clostridium difficile or cytomegalovirus (CMV). Rapidly acting and highly effective medication regimens such as intravenous steroids, infliximab, and cyclosporine should be administered while obtaining surgical consultation and following the patient closely to determine response to medical therapy and need for additional salvage medical therapy or colectomy.
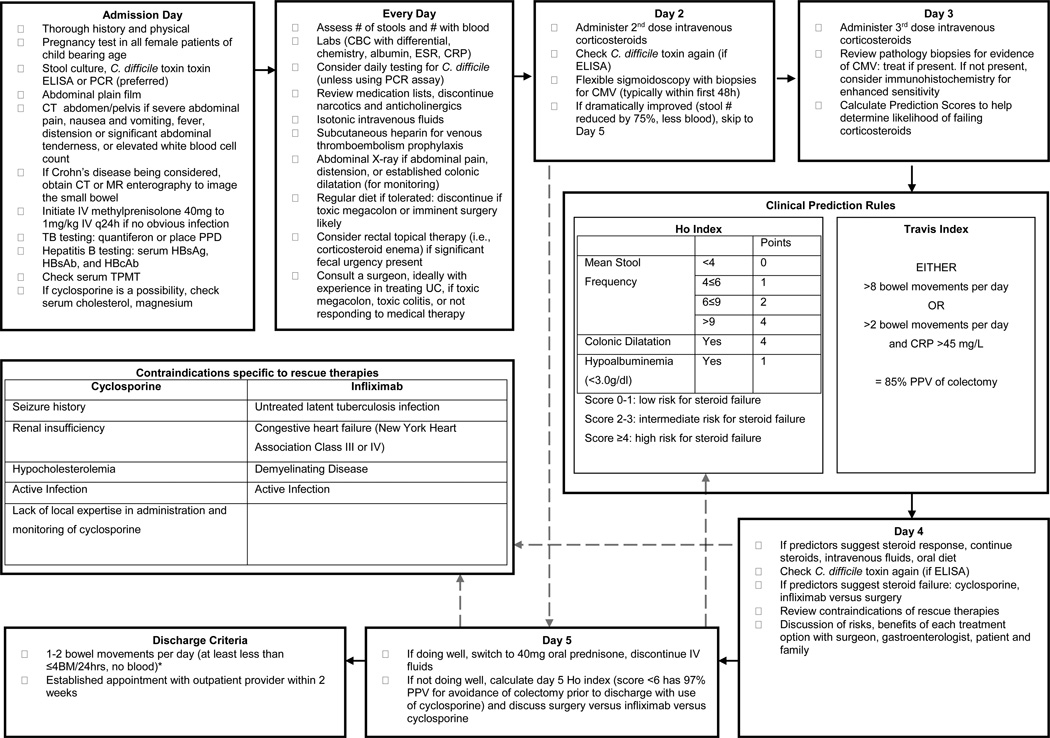
Many of the treatments for severe colitis initially described in 1974 by Truelove and Jewell (14) remain the mainstay of treatment today, including intravenous fluids, electrolyte supplements, transfusion as needed, and intravenous steroids. The goal of medical therapy is to markedly improve symptoms such as urgency, pain, and frequent or bloody stools and to transition the patient to an outpatient medication regimen. See Discharge Criteria, Follow-up and Prevention of Re-Hospitalization for proposed discharge criteria. Patients with massive hemorrhage, perforation, or toxic megacolon with impending perforation should not be considered for medical therapy, and should be immediately evaluated by a surgeon, ideally a colorectal surgeon or a general surgeon with experience in UC when available. When medical therapy is successful, patients should be expected to achieve or nearly achieve clinical remission before discharge. Some patients do not respond to medical therapy; for these cases, clinical judgment and clinical prediction rules should be used to assess prognosis (see Figure 1, which includes previously published clinical prediction rules within a proposed algorithm for the care of patients admitted with active UC). Lastly, attention should be paid to the prevention, diagnosis, and treatment of toxic megacolon; prevention of venous thromboembolism; and optimization of nutritional status.
Figure 1. Proposed algorithm for managing ulcerative colitis in the hospital (13,15,63).
*Exact number of acceptable bowel movements varies patient to patient but needs to be while tolerating a full diet and manageable for that patient.
History and Physical Examination
Information collected on patients’ history should include a review of the initial diagnosis, anatomic extent of disease, current symptoms, presence or absence of extra-intestinal manifestations, medical treatment history, smoking history, and use of potentially exacerbating medications. Current symptoms should be characterized in terms of bowel movements (number, consistency, presence of nocturnal bowel movements, presence of blood, and urgency), abdominal pain, bloating, nausea and vomiting, fever, and weight loss. The patient’s vital signs should be examined for evidence of hypovolemia or sepsis. The abdominal examination should include an assessment of the patient’s bowel sounds, identification of abdominal distension, and determination of the degree and location of any abdominal tenderness, including the presence of guarding or rebound. In patients treated with high-dose corticosteroids and/or narcotic analgesics, as well as in elderly patients, the abdominal exam may be less reliable. Many of these symptoms and signs are non-specific, so one should consider other diagnoses that could present with symptoms and signs that are similar to that of a UC flare, such as other gastrointestinal diseases (appendicitis, diverticulitis, adhesion-related small-bowel obstruction, infectious colitis, and medication induced colitis) or non-gastrointestinal diseases. Some symptoms and signs that indicate non-gastrointestinal diseases include flank pain or tenderness (renal disease), or vaginal discharge (gynecologic disease).
Diagnostic Testing
Figure 1 presents a proposed algorithm for the care of patients admitted with active UC, along with a list of laboratory tests that should be performed at admission and throughout hospitalization. Initial laboratory tests should be performed to assess disease severity (complete blood count with differential, chemistry, erythrocyte sedimentation rate, and levels of albumin and C reactive protein) and identify co-infections (stool culture and testing for C difficile). Forty to fifty percent of patients hospitalized for severe UC fail intravenous steroid therapy, therefore tests required to initiate rescue therapy with infliximab and azathioprine or cyclosporine and azathioprine should be performed at admission. These tests include assays for thiopurine methyltransferase (TPMT) enzyme activity or genotype (in anticipation of azathioprine therapy); tests for latent Mycobacterium tuberculosis infection (chest x-ray and purified protein derivative or Quantiferon), hepatitis B surface antigen, surface antibody and core antibody (in anticipation of infliximab); and assays for serum concentrations of cholesterol and magnesium (in anticipation of cyclosporine or tacrolimus).
Although not all patients require computed tomography (CT) scan, a plain film of the abdomen should be considered for all patients, to screen for a dilated colon or free air. Abdomino-pelvic CT scans should be considered for patients with severe abdominal pain or tenderness, nausea and vomiting, fever, distension, or increased white blood cell count.
Endoscopy
Patients hospitalized with severely active UC should be assessed by early flexible sigmoidoscopy, to assess disease extent and severity, to identify C difficile infection, and to collect biopsy samples for analysis of CMV infection. Endoscopic findings of deep ulcerations could indicate the presence of CMV, Crohn’s disease, or severe UC. Deep ulcers and extensive disease are associated with failure of medical treatment and a higher rate of colectomy (12, 13, 15). Typically, flexible sigmoidoscopy should be performed early after admission (within the first 48 h), to assess disease severity and identify CMV infection.
The safety of colonoscopy during an acute flare of UC has been demonstrated (12, 16, 17), however, caution should be taken when a patient has a severely ulcerated or distended colon to avoid precipitating a perforation or toxic megacolon. Endoscopy is generally contraindicated in the presence of toxic megacolon. A large retrospective study revealed that there was a higher risk of perforation during colonoscopy of IBD inpatients, compared with healthy controls (1% vs 0.6%, respectively) (18). Such patients might have a higher risk for perforation, therefore terminal ileal intubation with biopsies should not be a priority.
Medical and Surgical Treatment of Severe UC
Medications
Mesalamine and Other 5-Aminosalicylate (5-ASA)-Based Medications
The value of continuing mesalamine therapy in patients hospitalized for severe UC is limited. Some experts advocate a trial of stopping mesalamine and other 5-ASAs because of the possibility of a paradoxical worsening of diarrhea either, from a hypersensitivity reaction or a drug-induced exacerbation of colitis that can be indistinguishable from a flare (19–22).
Corticosteroids
Patients should be considered for hospitalization and treatment with intravenous steroids when they have failed to respond to oral prednisone, 40–60 mg/day, or have severe UC. The recommended steroid dosing regimen for hospitalized patients with UC is methylprednisolone (40 mg to 1 mg/kg), administered once daily as an intravenous bolus. Once transitioned to oral steroids, the optimal dose of prednisone is 40 mg/day, which is more effective than 20 mg/day and similar in efficacy to 60 mg/day but with fewer side effects (23). A small randomized controlled trial of oral prednisolone comparing 40 mg, once daily to 10 mg, 4 times daily in patients with active proctocolitis revealed no difference in response rates or side effects between the groups (24). Although the optimal taper strategy is unknown, a commonly used taper is to give patients prednisone, 40 mg/day for 2–4 weeks, and then taper the dose by 5 mg per week, to a daily dose of 20 mg, and then by 2.5–5 mg per week, until prednisone is discontinued.
There have been no randomized controlled trials of intravenous corticosteroids for the treatment of severe UC. A systematic review of 32 cohort studies and controlled trials of intravenous steroids in UC from 1974 to 2006 showed that 581 of 1991 patients (27%) required colectomy and 22 patients (1%) died (4). Clinical remission generally occurs in steroid-sensitive cases within 5–7 days (25), however only ~60% of patients hospitalized for severe UC respond to intravenous corticosteroids (14, 26–28).
Thiopurine Immunosuppressives (Azathioprine and 6-Mercaptopurine)
The active metabolites for the thiopurine agents have a half-life of 3–5 days, requiring 2–4 weeks to reach steady state and up to 8–10 weeks to reach maximal clinical effect. They therefore have little utility as inductive agents in hospitalized patients with severe UC, but can be useful as an adjunctive agent for infliximab and as a maintenance agent following treatment with cyclosporine or tacrolimus. They are often initiated or continued in hospitalized patients with UC who previously received 1 of these treatment regimens. The activity or genotype of TPMT should be checked before initiating thiopurine immunosuppresive therapy; it will determine the suggested starting dose (29).
Cyclosporine
The calcineurin inhibitor cyclosporine is a rapidly acting immunosuppressive agent effective for severely active UC. Contraindications to using cyclosporine include hypocholesterolemia (risk of seizure), infection, and significant renal insufficiency. A small placebo-controlled trial demonstrated that intravenous cyclosporine was effective in hospitalized patients with severe steroid-refractory UC (82% in cyclosporine arm vs 0% in placebo arm) (30), however at 6 months, only 45% had avoided colectomy (31). Other studies have shown that monotherapy with intravenous cyclosporine (4 mg/kg) is comparable to treatment with intravenous steroids (32) or intravenous steroids in addition to cyclosporine (33). One recent efficacy trial demonstrated that intravenous cyclosporine was not superior to infliximab in patients that failed treatment with intravenous steroids (34). In a review of randomized controlled trials of cyclosporine for severe UC in the hospital setting, responses ranged from 64% to 84% (35); however, over a longer term (5–7 years of follow up), 38%–78% of patients still required colectomy (36–40). Relapse rates are higher among patients who previously failed maintenance therapy with azathioprine or 6-mercaptopurine (36, 39).
Cyclosporine is given as a continuous infusion of 2 mg/kg over 24 h, with a target whole-blood cyclosporine A concentration (HPLC or monoclonal radioimmunoassay) of ~200–250 ng/ml (41). Patients typically respond within 7 days; lack of response within that time frame should prompt a colectomy. If a patient responds to intravenous cyclosporine, they should eventually be discharged on oral cyclosporine (Sandimune or Neoral or Gengraf) at a dose that is approximately 2-fold the total daily dose that they received intravenously, with a target trough concentration of 200–250ng/ml. Oral cyclosporine therapy should overlap with either azathioprine or 6-mercaptopurine therapy for 2–3 months before it is tapered for maintenance of remission. Outpatients that do not respond to, or are intolerant of, thiopurines should not receive salvage cyclosporine therapy, because cyclosporine is given with the ultimate goal of transitioning to a thiopurine therapy for long-term maintenance after induction of remission. There are no studies of oral cyclosporine for maintenance of remission in patients with UC.
Infliximab
Infliximab is an immunoglobulin (Ig)G1 monoclonal antibody to tumor necrosis factor (TNF); it is effective for treatment of outpatients with moderately to severely active UC (9). Two placebo-controlled trials demonstrated that infliximab is effective in hospitalized patients with severely active UC who fail intravenous steroids (42, 43). Contraindications to infliximab are listed in Figure 1. Two small controlled trials have indicated the similar efficacies of infliximab and intravenous steroids in hospitalized patients with severely active UC (44, 45). One recent efficacy trial demonstrated that intravenous cyclosporine was not superior to infliximab in patients who failed intravenous steroids (34).
The dosing regimen for induction therapy with infliximab is intravenous administration of 5 mg/kg at weeks 0, 2, and 6. Infliximab (5–10 mg/kg) can be administered subsequently every 8 weeks to maintain remission. A comparative effectiveness trial in outpatients with steroid-refractory UC demonstrated that combination therapy with infliximab and azathioprine was more effective than either agent alone (46), so combination therapy is preferred. No similar trials have been performed on inpatients. If one chooses to treat a patient with a thiopurine, they should be tested for TPMT at the time of admission, because of prolonged testing turn-around times. Although earlier data indicated an increased risk of post-operative complications in patients treated with infliximab (47–49), several more-recent studies found no increased post-operative outcomes after infliximab use (50–54).
A Note on Rescue Therapies
When a patient has no contraindications to either cyclosporine or infliximab and the center has adequate expertise with both, risks and benefits should be discussed with the patient. Cyclosporine therapy has 1%–2% mortality. A potential benefit of rescue therapy with infliximab, compared with cyclosporine, is that infliximab can be continued as maintenance therapy in patients who respond. Patients who receive rescue therapy with infliximab or cyclosporine should also start or continue taking azathioprine or 6-mercaptopurine. Patients who fail to respond to rescue therapy within 7–10 days should undergo colectomy rather than treatment with another rescue therapy. Switching from one rescue therapy to another has been reported to achieve remission in 30%–40% of patients but has been associated with serious adverse events and infections in 16%–20%, from excessive immunosuppression, and some patients have died (55, 56).
Clinical Prediction Rules
It is useful to attempt to predict which patients will and will not respond to intravenous steroid therapy. Several investigators have developed prediction rules to estimate the risk of colectomy (see Figure 1). One prospective study of 51 consecutive episodes of severe UC reported that more than 8 bowel movements/day after 3 days of treatment (or >2 bowel movements/day with a level of C-reactive protein >45mg/L, or of a score >4) had a 85% positive predictive value for colectomy. The study also estimated a 60% rate of colectomy if, at day 7 of treatment, patients have more than 3 bowel movements/day or if blood is still visible in the stool (15). This index has also been prospectively validated in a pediatric cohort (57) and has been used in practice as well as in clinical trials. An alternative predictive index exists that uses the same parameters (58) (59). A newer prediction score known as the Ho Index gives points for colonic dilation, albumin, and stool frequency; a sum greater than 3 has an 80% positive predictive value for colectomy on day 3 of intravenous corticosteroid use (13). This score was based on a retrospective review of 167 patients with severe UC seen consecutively in 1 medical center and has not been prospectively validated. The Ho Index has also been used to predict avoidance of colectomy with use of cyclosporine therapy among patients that failed at least 5 days of corticosteroid use (60). It should be noted that clinical prediction calculations should not replace clinical judgment. Instead, these rules provide evidence-based percentages to assist the care team in setting expectations regarding the possibility of colectomy.
Surgery
Indications for surgery in hospitalized patients include longstanding disease refractory to medical therapy or emergent, severe disease or fulminant colitis that does not respond to medical therapy, toxic megacolon, perforation, and refractory hemorrhage (61). In patients with UC, perforation can occur in the absence of colonic dilation and can present without classic signs of peritonitis (62). Surgical consultation is highly recommended for patients admitted with severe colitis, because 27% will require colectomy. It is important to try to identify patients who are likely to require surgery because delay in surgery can worsen outcomes (63–65). There has been no demonstrable reduction in the colectomy rate during the last 30 years (4).
The standard of care in the non-urgent elective setting is total proctocolectomy, with or without a restorative procedure to preserve fecal continence, through creation of an ileoanal J pouch from the terminal ileum. This operation can be performed open, laparoscopically, or robotically. The ranges of morbidity and mortality for this surgery are 19%–27% and 0.2%–0.4%, respectively (62, 66). Total proctocolectomy with end ileostomy might be preferred for patients with significant medical comorbidities or distal rectal cancer, who are 60 years old or greater, or with pre-existing fecal incontinence (61).
Patients that require emergent surgery typically undergo restorative proctocolectomy in 3 stages. In the first stage, a total or subtotal abdominal colectomy with end ileostomy leaving a rectal or rectosigmoid stump as a Hartmann’s pouch is performed. Some surgeons bring the rectal stump up to the skin as a mucus fistula, and others advocate bringing the recto-sigmoid stump to the subcutaneous tissue at the lower end of wound, so that stump dehiscence results in wound infection rather than an intra-abdominal leak with abscess and peritonitis (61, 62). The goal in these cases is to expeditiously treat the emergent condition, allowing the patient to recover (systemically and nutritionally) and eventually undergo a restorative procedure or a completion proctectomy with end ileostomy. If the patient is a candidate for restorative proctectomy, the procedure is completed in 2 additional stages. In the second stage, the patient undergoes a completion proctocolectomy with ileoanal J pouch and a diverting loop ileostomy. In the third stage, the loop ileostomy is reversed. The goal of the 3-stage procedure is to reduce the risk of abdominal sepsis and leakage (62).
The potential risks of surgery include hemorrhage, infection, small-bowel obstruction, intra-abdominal or pelvic sepsis/abscess, anastomotic stricture, pouchitis, cuffitis, fistulas, reduced female fertility, erectile and sexual dysfunction, and need for surgical revision or excision of the pouch (61, 62).
C difficile
Patients with IBD are at increased risk of developing C difficile infection and incidence rates nearly doubled in patients with UC from 1998 to 2004 (67, 68). One study from Japan found that 40% of patients with symptoms of a UC flare were infected with C difficile (69). Hospitalized patients with UC who become infected with C difficile have a more aggressive disease course, longer and more costly hospital stays, and colectomy rates of approximately 20% (67, 70). Mortality is greater among patients hospitalized with IBD and C difficile infection than patients with IBD without C difficile infection (an adjusted odds ratio of 4.7) or patients with C difficile infection without IBD (an odds ratio of 2.2).
Although prior antibiotic use is a strong risk factor for patients with C difficile infection, 39% of patients with concomitant IBD did not have antibiotic exposure within 2 previous months, in 1 cohort (71). Patients with IBD and C difficile infection also tended to be older and have more comorbidities than patients with IBD without C difficile infection (70). C difficile infection often occurs in stable patients in remission on patient receiving combination or immunomodulator monotherapy before their clinical deterioration. In fact, maintenance use of immunomodulators, but not biologics, was independently associated with infection by C difficile, in one study (71). C difficile infection can mimic and exacerbate IBD (67). Because of the associated poor outcomes, it is important to diagnose and treat the infection promptly.
An ELISA of stool samples for C difficile toxins has a higher yield with repeated testing (71); newer and possibly more sensitive PCR-based assays need to be studied for patients with UC to assess whether a reduced frequency of testing has sufficient sensitivity to detect the infection in these patients (72). The endoscopic appearance of C difficile infection is different in patients with IBD, compared to controls (71). Pseudomembranes are seen in ~50% of all patients with C difficile infection (67). One cohort of patients with IBD and C difficile infection had no typical features on endoscopy or histology. The authors of this study advocated sending stool recovered during colonoscopy for C difficile testing in all patients with active colitis (71).
There are no guidelines for treatment of C difficile infection that are specific to patients with IBD. Not specific to patients with IBD, the Infectious Diseases Society of America suggests that patients with C difficile infection undergo treatment with metronidazole, as first-line therapy, unless the C difficile infection is severe, complicated, or is a second recurrence, in which case oral vancomycin should be given (72). No trials have studied oral vancomycin in patients with IBD, but because of increasing rates of metronidazole failure and arguably because of a higher likely severity of disease in this subgroup, some experts have recommended treating hospitalized patient with IBD with oral vancomycin as first-line therapy. One retrospective analysis of this practice associated lower rates of colectomy with a change from metronidazole to oral vancomycin as the initial treatment regimen for patients with IBD and C difficile infection (from 45.5% to 3.5%, from 2004 to 2006) (73). One possible approach would be to initiate oral vancomycin either empirically (in patients where the infection is highly suspected) (67), or immediately after establishing that they are infected with C difficile.
Patients with IBD and concomitant C difficile infection often require added immunosuppression in the near future (74). A recent randomized trial revealed that treatment with fidaxomicin was noninferior to treatment with vancomycin and was superior to vancomycin for reducing the rate of recurrent infection with some strains of C difficile, although patients known to have UC or Crohn’s disease were excluded from the study (75). Recurrence rates following treatment of C difficile infection are high; 1 study revealed a 59% recurrence rate within 1 month of treatment in patients with IBD in a small cohort, 26% of which required subsequent colectomy (67). Recurrent C difficile infection can be treated by repeating the initially prescribed regimen and if a second recurrence occurs, by a prolonged, high-dose, and/or tapering doses of oral vancomycin (72).
CMV
Patients admitted to the hospital with an acute flare of colitis should to be evaluated for the possibility of a concurrent infection with CMV—especially if they are receiving immunosuppressive medications that can reactivate latent infections (76). CMV infection (detection on objective tests, not necessarily with associated symptoms) is common in the general population and is not necessarily indicative of active disease. It is unclear whether CMV infection in the colon in patients with severe UC is pathogenic, a marker of more severe disease, or simply an innocent bystander. A review of the literature indicated that the presence of CMV antigens does not necessarily increase disease severity, and that CMV infection is reactivated in patients with severe UC but does not affect prognosis (77). The prevalence of CMV infection is difficult to estimate because of the heterogeneity of data and because most studies have been retrospective. Among specimens collected from colectomies, the CMV infection rate was as high as 22% (78). A higher prevalence of CMV was reported among steroid-refractory patients (33%–36%) (79, 80), but it is not clear whether these cases resulted from steroid-induced reactivation of CMV or the CMV was a marker of disease severity. Rates of colectomy among patients with CMV infection have been decreasing, according to recent studies. It is unusual to detect CMV infection in patients with mild to moderate UC (81, 82). Furthermore, cyclosporine, high-dose steroids, and tacrolimus can cause CMV to rapidly replicate and/or systemically disseminate (83).
CMV-induced colitis typically affects immunocompromised individuals, although it has been observed in patients that have not received steroids or immunosuppression, including patients with IBD (76). CMV reactivation has not been associated with the use of infliximab and, interestingly, TNF promotes replication of CMV; the absence of TNF has been associated with viral latency in vitro (77, 84). The relationship between CMV and UC is complex, however, in that there are occasional reported cases of colitis and documented cases of CMV infection that improved during treatment with corticosteroids and did not require antiviral agents (80, 85). Furthermore, CMV has been detected in histologic specimens taken from patients without active colitis (78). Because of the high rate of colectomy and morbidity associated with CMV infection in patients with active UC, patients with biopsies that test positive for CMV are typically treated with antiviral agents, despite the uncertainty about how much of the colitis can be attributed to the CMV infection.
Tests for CMV infection include endoscopic, histologic, serologic, viral culture, antigen detection, and DNA analyses. Of note, fewer than 60% of IBD patients with CMV colitis have antigenemia (86). On the contrary, although DNA tests are sensitive, their specificity is questionable—cutoff values for determining whether or not a patient is infected with CMV have not been established or validated (76). Quantitative real-time PCR assays are more sensitive than antigenemia tests or histologic analysis in detecting CMV in samples from inflamed colon (86), but results do not always correlate with those from immunohistochemical analysis; real-time PCR might be so sensitive that it detects clinically insignificant reactivation of CMV.
When a patient is admitted with UC, it is reasonable to measure serum levels of CMV IgM and IgG, which together have a strong negative predictive value. Patients with active UC should be evaluated by endoscopy for CMV infection when they are admitted to the hospital, without waiting for the results of the serum antibody tests. Endoscopic features of CMV are non-specific but include deep ulcerations, patchy erythema, exudates, microerosions, diffuse edema, and even pseudotumors. Histologic examination reveals cytomegalic cells with large eosinophilic cowdry type A intranuclear inclusions, occasionally surrounded by a clear halo and smaller cytoplasmic inclusions. The detection of of CMV on H&E can be improved with use of immunohistochemical assays that use monoclonal antibodies against CMV immediate early antigen, which detect CMV infection with 93% sensitivity. CMV infection affects the right colon alone in 30% of cases (76). While awaiting pathology results, a negative result from a test for CMV antibodies (IgG) can be used to exclude CMV infection.
There have been no randomized clinical trials to investigate whether gancyclovir can be used to treat CMV infection in patients with severe or steroid-refractory UC. Guidelines from the American College of Gastroenterology do not make a specific recommendation regarding treatment, but instead state that “treatment with gancyclovir may lead to clinical improvement” (87). The European Crohn’s and Colitis Organization, however, recommends: “[i]n case of severe colitis with CMV detected in the mucosa during immunomodulator therapy, antiviral therapy should be initiated and discontinuation of immunomodulators considered until colitis symptoms improve. In case of systemic CMV infection immunomodulator therapy must be discontinued” (88).
Regardless, the low risk of antiviral therapy prompts most providers to treat CMV infections in patients with active colitis. One approach would be to consider reducing immunosuppression or tapering steroids and administering intravenous gancyclovir (5 mg/kg), twice daily for 14 days, followed by (oral) valgancyclovir (450 mg), twice daily for 28 days, although the efficacy of oral valgancyclovir treatment for CMV colitis is not well established. The most important adverse effect of gancyclovir is neutropenia. Colitis remission rates after antiviral therapy for documented CMV infection in IBD patients ranged from 67% to 100% in a review of several small studies (76).
Toxic Megacolon
The most severe form of colitis is fulminant colitis (89). Toxic megacolon (Table 2), a potential complication of fulminant colitis, is a clinical diagnosis based on features of toxic colitis and colonic dilatation (61, 90). The traditional definition includes at least 3 of the following: temperature >38.6°C, heart rate >120 beats per minute, white blood cell counts >10.5×103/mm3, and anemia, plus at least 1 of the following: dehydration, altered mental status, electrolyte disturbances, and hypotension (91).
Table 2.
Summary of Toxic Megacolon
| Definition | Clinical Diagnosis: Toxic colitis + Colonic dilatation |
| Traditional Definition {{157 Jalan, K.N. 1969}} At least 3 of the following:
| |
| Predisposing Factors | C. difficile infection, barium enema, narcotic antidiarrheals, anticholinergics, loperamide, diphenoxylate, narcotics |
| Other Clinical Features | Abdominal distension and tenderness, decreased or absent bowel sounds. Dilated colon seen on radiographs. |
| Diagnostics |
|
| Management |
Surgical
|
One percent to 2% of patients with severe UC progress to toxic colitis and/or megacolon. A prospective study found that 7.9% of patients admitted with UC to have toxic megacolon (92). Thirty percent of patients with toxic megacolon present within 3 months of diagnosis (93). It is important to test patients for C difficile infection. Although plain films are easy to repeat on a daily basis, they are less sensitive than CT for evaluating the extent and severity of colitis, determining the presence of colonic dilatation, and assessing for perforation (94).
Medical and surgical expertise are each required to manage patients with toxic megacolon. Barium enema, narcotic antidiarrheals, anticholinergics, loperamide, diphenoxylate, and narcotics should be avoided because they have been associated with the development of toxic megacolon. Frequent physical examinations, laboratory tests, and daily or twice daily abdominal X-rays are important if toxic colitis or megacolon is suspected. Patients should be transfused and receive intravenous fluids, electrolytes, and total parenteral nutrition, as indicated. Broad-spectrum antibiotics are often used in the management of toxic megacolon, due to the potential for microperforation. Nasogastric tube decompression is not helpful for colonic decompression (95). Patients with marked distension should be instructed to roll around in bed or lie in the knee-elbow position every 30 minutes, as these maneuvers have been shown to reduce colonic gas and bowel distension (96, 97). Although toxic megacolon is not necessarily an absolute indication for surgery, many advocate that surgery be performed immediately (98, 99). One reason for this recommendation is that many patients who initially respond to medical therapy will subsequently require colectomy.
Pain Management
The primary method of pain control in patients with severe UC is treatment of their underlying disease. Use of narcotics should be avoided in patients hospitalized for UC because they might precipitate megacolon, although 1 study found that 70.1% of hospitalized patients with IBD received narcotics (100). A retrospective review of patients with UC exposed to narcotics during hospitalization did not report higher rates of colectomy (101), although prospective studies are needed to confirm this finding. Narcotics are associated with increased infectious complications and mortality in patients with IBD (102).
Oral analgesics such as tramadol and acetaminophen can be used but may be insufficient to achieve a level of analgesia acceptable to the patient. Non-steroidal anti-inflammatory drugs should be avoided because of their association with disease exacerbation. Severe pain related to UC could represent transmural inflammation and its persistence, despite medical therapy, often warrants surgery.
Nutrition
Most patients hospitalized with severe UC should continue to receive a normal diet. Two randomized controlled trials have demonstrated that bowel rest does not affect the outcome of severe UC in patients treated with intravenous prednisone (103, 104). Patients with toxic colitis or megacolon should be made nil-per-os because of the potential for imminent surgical intervention. Peripheral or central intravenous nutrition should be instituted if there is evidence of malnutrition, which has been associated with increased length of stay, total hospital charges, and in-hospital mortality in patients with IBD (105). The goal of intravenous nutrition is to replace nutritional deficits rather than for any primary therapeutic benefit. Hypoalbuminemia is associated with higher post-operative complications and is often a contraindication to surgery that requires anastomosis without a protective ileostomy (106).
Venous Thromboembolism Prophylaxis
Active UC with bloody bowel movements is not a contraindication to venous thromboembolism prophylaxis with low molecular weight heparin. IBD is recognized as a hypercoagulable state (107) and both venous and arterial clots, which can occur in usual or unusual sites and are at risk for embolization. IBD is associated with an approximate 3-fold increase in risk of venous thromboembolism, and the risks seems to be higher during a flare (a hazard ratio of 8.4) (108). Prophylaxis-dosed anti-coagulation is therefore recommended for patients hospitalized with IBD, although there has been no data from prospective studies to demonstrate that this intervention is effective (109, 110).
Discharge Criteria, Follow-Up, and Preventing Re-Hospitalization
There are no validated discharge criteria for patients hospitalized for active UC. It is reasonable to delay discharge until a patient has markedly improved (ideally defined as ≤1–2 non-bloody bowel movements/day, certainly not more than 3–4 bowel movements/day), has transitioned to an appropriate outpatient regimen of medications, and is able to tolerate oral hydration and nutrition. Clinical improvement cannot be assessed if the patient is not eating a normal or nearly normal diet.
Communication with the patient is paramount. It is critical to convey the plan for medical therapy, possible side effects, warning signs that should trigger a return to the hospital, contact information for the IBD treatment team, and the follow-up plan upon discharge.
Conclusions
UC is a chronic condition with a relapsing and remitting course that often results in hospitalization. Severity can be assessed and the extent of disease can be determined by conducting a thorough history and physical examination, laboratory tests, and endoscopy and imaging analyses when applicable. After exclusion of C difficile infection, the primary treatment is administration of intravenous corticosteroids while preventing and monitoring for complications such as toxic megacolon and deep-vein thrombosis. Inadequate response to steroids should prompt the use of infliximab or cyclosporine, usually in combination with azathioprine. Patients who are not responding to medical therapy should be referred for colectomy. Patients can be considered for discharge once they have had a marked improvement in bowel habits with resolution of rectal bleeding and have a clear follow-up plan.
Supplementary Material
Acknowledgments
Dr. Sandborn reports having received consulting fees from Abbott, ActoGeniX NV, AGI Therapeutics Inc, Alba Therapeutics Corp, Albireo, Alfa Wasserman, Amgen, AM-Pharma BV, Anaphore, Astellas, Athersys Inc, Atlantic Healthcare Ltd, Aptalis, BioBalance Corp, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Celek Pharmaceuticals, Cellerix SL, Cerimon Pharmaceuticals, ChemoCentryx, CoMentis, Cosmo Technologies, Coronado Biosciences, Cytokine Pharmasciences, Eagle Pharmaceuticals, EnGene Inc, Eli Lilly, Enteromedics, Exagen Diagnostics Inc, Ferring Pharmaceuticals, Flexio Therapeutics Inc, Funxional Therapeutics Ltd, Genzyme Corp, Gilead Sciences, Given Imaging, GSK, Human Genome Sciences, Ironwood Pharmaceuticals, KaloBios Pharmaceuticals, Lexicon Pharmaceuticals, Lycera Corp, Meda Pharmaceuticals, Merck Research Laboratories, Merck Serono, Millenium Pharmaceuticals, Nisshin Kyorin Pharmaceuticals, Novo Nordisk, NPS Pharmaceuticals, Optimer Pharmaceuticals, Orexigen Therapeutics Inc, PDL Biopharma, Pfizer, Procter and Gamble, Prometheus Laboratories, ProtAb Ltd, Purgenesis Technologies Inc, Relypsa Inc, Roche, Salient Pharmaceuticals, Salix Pharmaceuticals, Santarus, Schering Plough, Shire Pharmaceuticals, Sigmoid Pharma Ltd, Sirtris Pharmaceuticals, SLA Pharma UK Ltd, Targacept, Teva Pharmaceuticals, Therakos, Tilliotts Pharma AG, TxCell SA, UCB Pharma, Viamet Pharmaceuticals, Vascular Biogenics Ltd, Warner Chilcott UK Ltd and Wyeth; research grants from Abbott, Bristol-Myers Squibb, Genentech, GSK, Janssen, Milennium Pharmaceuticals, Novartis, Pfizer, Procter and Gamble, Shire Pharmaceuticals and UCB Pharma; payments for lectures/speakers bureau from Abbott, Bristol-Myers Squibb and Janssen; and holds stock/stock options in Enteromedics. Dr. McLemore reports having been a consultant to Intuitive Surgical, Ethicon Endo-Surgery, and Applied Medical. Dr. Docherty reports having been a consultant to Par Pharmaceutical. Dr. Patel reports being on the Abbott Labs Speakers Bureau.
Footnotes
Author contributions:
Suresh Pola performed the primary literature search, created the figures, and wrote, edited, and submitted the manuscript. Derek Patel & William Sandborn assisted with the literature search, writing of the manuscript and editing of manuscript and figures. Elisabeth Mclemore and Sonia Ramamoorthy assisted with the literature search and reviewed and edited the manuscript. Marianne Fahmy, Jesus Rivera-Nieves, John T Chang, Elisabeth Evans, Michael Docherty, and Mark Talamini assisted by reviewing and editing the manuscript.
For the remaining authors, no conflicts of interest exist.
References
- 1.Edwards FC, Truelove SC. The course and prognosis of ulcerative colitis. Gut. 1963 Dec;4:299–315. doi: 10.1136/gut.4.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinesen LC, Walsh AJ, Protic MN, Heap G, Cummings F, Warren BF, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010 Oct;4(4):431–437. doi: 10.1016/j.crohns.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Kohn A, Fano V, Monterubbianesi R, Davoli M, Marrollo M, Stasi E, et al. Surgical and nonsurgical hospitalization rates and charges for patients with ulcerative colitis in italy: A 10-year cohort study. Dig Liver Dis. 2011 Dec 22; doi: 10.1016/j.dld.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: A systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007 Jan;5(1):103–110. doi: 10.1016/j.cgh.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. A nationwide analysis of changes in severity and outcomes of inflammatory bowel disease hospitalizations. J Gastrointest Surg. 2011 Feb;15(2):267–276. doi: 10.1007/s11605-010-1396-3. [DOI] [PubMed] [Google Scholar]
- 6.Bitton A, Buie D, Enns R, Feagan BG, Jones JL, Marshall JK, et al. Treatment of hospitalized adult patients with severe ulcerative colitis: Toronto consensus statements. Am J Gastroenterol. 2011 Nov 22; doi: 10.1038/ajg.2011.386. [DOI] [PubMed] [Google Scholar]
- 7.Hanauer SB. Inflammatory bowel disease. N Engl J Med. 1996 Mar 28;334(13):841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- 8.Lichtiger S, Present DH. Preliminary report: Cyclosporin in treatment of severe active ulcerative colitis. Lancet. 1990 Jul 7;336(8706):16–19. doi: 10.1016/0140-6736(90)91521-b. [DOI] [PubMed] [Google Scholar]
- 9.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005 Dec 8;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012 Feb;142(2):257, 65.e1–65.e3. doi: 10.1053/j.gastro.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993 Jun;38(6):1137–1146. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- 12.Carbonnel F, Lavergne A, Lemann M, Bitoun A, Valleur P, Hautefeuille P, et al. Colonoscopy of acute colitis. A safe and reliable tool for assessment of severity. Dig Dis Sci. 1994 Jul;39(7):1550–1557. doi: 10.1007/BF02088063. [DOI] [PubMed] [Google Scholar]
- 13.Ho GT, Mowat C, Goddard CJ, Fennell JM, Shah NB, Prescott RJ, et al. Predicting the outcome of severe ulcerative colitis: Development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther. 2004 May 15;19(10):1079–1087. doi: 10.1111/j.1365-2036.2004.01945.x. [DOI] [PubMed] [Google Scholar]
- 14.Truelove SC, Jewell DP. Intensive intravenous regimen for severe attacks of ulcerative colitis. Lancet. 1974 Jun 1;1(7866):1067–1070. doi: 10.1016/s0140-6736(74)90552-2. [DOI] [PubMed] [Google Scholar]
- 15.Travis SP, Farrant JM, Ricketts C, Nolan DJ, Mortensen NM, Kettlewell MG, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996 Jun;38(6):905–910. doi: 10.1136/gut.38.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terheggen G, Lanyi B, Schanz S, Hoffmann RM, Bohm SK, Leifeld L, et al. Safety, feasibility, and tolerability of ileocolonoscopy in inflammatory bowel disease. Endoscopy. 2008 Aug;40(8):656–663. doi: 10.1055/s-2008-1077445. [DOI] [PubMed] [Google Scholar]
- 17.Alemayehu G, Jarnerot G. Colonoscopy during an attack of severe ulcerative colitis is a safe procedure and of great value in clinical decision making. Am J Gastroenterol. 1991 Feb;86(2):187–190. [PubMed] [Google Scholar]
- 18.Navaneethan U, Parasa S, Venkatesh PG, Trikudanathan G, Shen B. Prevalence and risk factors for colonic perforation during colonoscopy in hospitalized inflammatory bowel disease patients. J Crohns Colitis. 2011 Jun;5(3):189–195. doi: 10.1016/j.crohns.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Sandborn WJ. Therapeutic approaches to the treatment of ulcerative colitis. In: Targan S, Shanahan F, Karp L, editors. Inflammatory Bowel Disease. Translating basic science into clinical practice. Wiley-Blackwell; 2010. p. 415. [Google Scholar]
- 20.Sturgeon JB, Bhatia P, Hermens D, Miner PB., Jr Exacerbation of chronic ulcerative colitis with mesalamine. Gastroenterology. 1995 Jun;108(6):1889–1893. doi: 10.1016/0016-5085(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 21.Kapur KC, Williams GT, Allison MC. Mesalazine induced exacerbation of ulcerative colitis. Gut. 1995 Dec;37(6):838–839. doi: 10.1136/gut.37.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta MK, Pollack S, Hutchings JJ. Mesalamine induced symptom exacerbation of ulcerative colitis: Case report and brief discussion. World J Gastrointest Pharmacol Ther. 2010 Dec 6;1(6):132–134. doi: 10.4292/wjgpt.v1.i6.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baron JH, Connell AM, Kanaghinis TG, Lennard-Jones JE, Jones AF. Out-patient treatment of ulcerative colitis. comparison between three doses of oral prednisone. Br Med. J. 1962 Aug 18;2(5302):441–443. doi: 10.1136/bmj.2.5302.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell-Tuck J, Bown RL, Lennard-Jones JE. A comparison of oral prednisolone given as single or multiple daily doses for active proctocolitis. Scand J Gastroenterol. 1978;13(7):833–837. doi: 10.3109/00365527809182199. [DOI] [PubMed] [Google Scholar]
- 25.Meyers S, Lerer PK, Feuer EJ, Johnson JW, Janowitz HD. Predicting the outcome of corticoid therapy for acute ulcerative colitis. results of a prospective, randomized, double-blind clinical trial. J Clin Gastroenterol. 1987 Feb;9(1):50–54. doi: 10.1097/00004836-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Truelove SC, Willoughby CP, Lee EG, Kettlewell MG. Further experience in the treatment of severe attacks of ulcerative colitis. Lancet. 1978 Nov 18;2(8099):1086–1088. doi: 10.1016/s0140-6736(78)91816-0. [DOI] [PubMed] [Google Scholar]
- 27.Jarnerot G, Rolny P, Sandberg-Gertzen H. Intensive intravenous treatment of ulcerative colitis. Gastroenterology. 1985 Nov;89(5):1005–1013. doi: 10.1016/0016-5085(85)90201-x. [DOI] [PubMed] [Google Scholar]
- 28.Gustavsson A, Halfvarson J, Magnuson A, Sandberg-Gertzen H, Tysk C, Jarnerot G. Long-term colectomy rate after intensive intravenous corticosteroid therapy for ulcerative colitis prior to the immunosuppressive treatment era. Am J Gastroenterol. 2007 Nov;102(11):2513–2519. doi: 10.1111/j.1572-0241.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 29.Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011 Mar;89(3):387–391. doi: 10.1038/clpt.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtiger S, Present DH, Kornbluth A, Gelernt I, Bauer J, Galler G, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994 Jun 30;330(26):1841–1845. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 31.Kornbluth A, Lichtiger S, Present D, Hanauer S. Long-term results of oral cyclosporin in patients with severe ulcerative colitis: A double-blind, randomized, multi-center trial. Gastroenterology. 1994;106:A714. [Google Scholar]
- 32.Svanoni F, Bonassi U, Bagnolo F, Caporuscio S. Effectiveness of cyclosporine A (CsA) in the treatment of active refractory ulcerative colitis (UC) Gastroenterology. 1998;1998:A1096. [Google Scholar]
- 33.D'Haens G, Lemmens L, Geboes K, Vandeputte L, Van Acker F, Mortelmans L, et al. Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology. 2001 May;120(6):1323–1329. doi: 10.1053/gast.2001.23983. [DOI] [PubMed] [Google Scholar]
- 34.Laharie D, Bourreille A, Branche J, Allez M, Bouhnik Y, Filippi J, et al. Cyclosporin versus infliximab in severe acute ulcerative colitis refractory to intravenous steroids: A randomized trial. Gastroenterology. 140(5) Supplement 1:S-112. [Google Scholar]
- 35.Pham CQ, Efros CB, Berardi RR. Cyclosporine for severe ulcerative colitis. Ann Pharmacother. 2006 Jan;40(1):96–101. doi: 10.1345/aph.1G374. [DOI] [PubMed] [Google Scholar]
- 36.Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: A five-year experience. Am J Gastroenterol. 1999 Jun;94(6):1587–1592. doi: 10.1111/j.1572-0241.1999.01149.x. [DOI] [PubMed] [Google Scholar]
- 37.Arts J, D'Haens G, Zeegers M, Van Assche G, Hiele M, D'Hoore A, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004 Mar;10(2):73–78. doi: 10.1097/00054725-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Campbell S, Travis S, Jewell D. Ciclosporin use in acute ulcerative colitis: A long-term experience. Eur J Gastroenterol Hepatol. 2005 Jan;17(1):79–84. doi: 10.1097/00042737-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Moskovitz DN, Van Assche G, Maenhout B, Arts J, Ferrante M, Vermeire S, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol. 2006 Jun;4(6):760–765. doi: 10.1016/j.cgh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Actis GC, Fadda M, David E, Sapino A. Colectomy rate in steroid-refractory colitis initially responsive to cyclosporin: A long-term retrospective cohort study. BMC Gastroenterol. 2007 Mar 27;7:13. doi: 10.1186/1471-230X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Assche G, D'Haens G, Noman M, Vermeire S, Hiele M, Asnong K, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003 Oct;125(4):1025–1031. doi: 10.1016/s0016-5085(03)01214-9. [DOI] [PubMed] [Google Scholar]
- 42.Sands BE, Tremaine WJ, Sandborn WJ, Rutgeerts PJ, Hanauer SB, Mayer L, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: A pilot study. Inflamm Bowel Dis. 2001 May;7(2):83–88. doi: 10.1097/00054725-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Jarnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlen P, Granno C, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: A randomized, placebo-controlled study. Gastroenterology. 2005 Jun;128(7):1805–1811. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Armuzzi A, De Pascalis B, Lupascu A, Fedeli P, Leo D, Mentella MC, et al. Infliximab in the treatment of steroid-dependent ulcerative colitis. Eur Rev Med Pharmacol Sci. 2004 Sep-Oct;8(5):231–233. [PubMed] [Google Scholar]
- 45.Ochsenkuhn T, Sackmann M, Goke B. Infliximab for acute, not steroid-refractory ulcerative colitis: A randomized pilot study. Eur J Gastroenterol Hepatol. 2004 Nov;16(11):1167–1171. doi: 10.1097/00042737-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Panaccione R, Ghosh S, Middleton S, Marquez JR, Khalif I, Flint L, et al. Infliximab, azathioprine, or infliximab + azathioprine for treatment of moderate to severe ulcerative colitis: UC SUCCESS trial. Gastroenterology. 2011;140:S-134. Abstract 835. [Google Scholar]
- 47.Selvasekar CR, Cima RR, Larson DW, Dozois EJ, Harrington JR, Harmsen WS, et al. Effect of infliximab on short-term complications in patients undergoing operation for chronic ulcerative colitis. J Am Coll Surg. 2007 May;204(5):956–962. doi: 10.1016/j.jamcollsurg.2006.12.044. discussion 962-3. [DOI] [PubMed] [Google Scholar]
- 48.Mor IJ, Vogel JD, da Luz Moreira A, Shen B, Hammel J, Remzi FH. Infliximab in ulcerative colitis is associated with an increased risk of postoperative complications after restorative proctocolectomy. Dis Colon Rectum. 2008 Aug;51(8):1202–1207. doi: 10.1007/s10350-008-9364-7. discussion 1207-10. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Wu Q, Wu K, Fan D. Meta-analysis: Pre-operative infliximab treatment and short-term post-operative complications in patients with ulcerative colitis. Aliment Pharmacol Ther. 2010 Feb 15;31(4):486–492. doi: 10.1111/j.1365-2036.2009.04204.x. [DOI] [PubMed] [Google Scholar]
- 50.Kunitake H, Hodin R, Shellito PC, Sands BE, Korzenik J, Bordeianou L. Perioperative treatment with infliximab in patients with crohn's disease and ulcerative colitis is not associated with an increased rate of postoperative complications. J Gastrointest Surg. 2008 Oct;12(10):1730–1736. doi: 10.1007/s11605-008-0630-8. discussion 1736-7. [DOI] [PubMed] [Google Scholar]
- 51.Ferrante M, D'Hoore A, Vermeire S, Declerck S, Noman M, Van Assche G, et al. Corticosteroids but not infliximab increase short-term postoperative infectious complications in patients with ulcerative colitis. Inflamm Bowel Dis. 2009 Jul;15(7):1062–1070. doi: 10.1002/ibd.20863. [DOI] [PubMed] [Google Scholar]
- 52.Coquet-Reinier B, Berdah SV, Grimaud JC, Birnbaum D, Cougard PA, Barthet M, et al. Preoperative infliximab treatment and postoperative complications after laparoscopic restorative proctocolectomy with ileal pouch-anal anastomosis: A case-matched study. Surg Endosc. 2010 Aug;24(8):1866–1871. doi: 10.1007/s00464-009-0861-0. [DOI] [PubMed] [Google Scholar]
- 53.Gainsbury ML, Chu DI, Howard LA, Coukos JA, Farraye FA, Stucchi AF, et al. Preoperative infliximab is not associated with an increased risk of short-term postoperative complications after restorative proctocolectomy and ileal pouch-anal anastomosis. J Gastrointest Surg. 2011 Mar;15(3):397–403. doi: 10.1007/s11605-010-1385-6. [DOI] [PubMed] [Google Scholar]
- 54.Bregnbak D, Mortensen C, Bendtsen F. Infliximab and complications after colectomy in patients with ulcerative colitis. J Crohns Colitis. 2012 Apr;6(3):281–286. doi: 10.1016/j.crohns.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Maser EA, Deconda D, Lichtiger S, Ullman T, Present DH, Kornbluth A. Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clin Gastroenterol Hepatol. 2008 Oct;6(10):1112–1116. doi: 10.1016/j.cgh.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 56.Leblanc S, Allez M, Seksik P, Flourie B, Peeters H, Dupas JL, et al. Successive treatment with cyclosporine and infliximab in steroid-refractory ulcerative colitis. Am J Gastroenterol. 2011 Apr;106(4):771–777. doi: 10.1038/ajg.2011.62. [DOI] [PubMed] [Google Scholar]
- 57.Turner D, Mack D, Leleiko N, Walters TD, Uusoue K, Leach ST, et al. Severe pediatric ulcerative colitis: A prospective multicenter study of outcomes and predictors of response. Gastroenterology. 2010 Jun;138(7):2282–2291. doi: 10.1053/j.gastro.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 58.Lindgren SC, Flood LM, Kilander AF, Lofberg R, Persson TB, Sjodahl RI. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol. 1998 Oct;10(10):831–835. doi: 10.1097/00042737-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Jarnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlen P, Granno C, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: A randomized, placebo-controlled study. Gastroenterology. 2005 Jun;128(7):1805–1811. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 60.Aceituno M, Garcia-Planella E, Heredia C, Zabana Y, Feu F, Domenech E, et al. Steroid-refractory ulcerative colitis: Predictive factors of response to cyclosporine and validation in an independent cohort. Inflamm Bowel Dis. 2008 Mar;14(3):347–352. doi: 10.1002/ibd.20322. [DOI] [PubMed] [Google Scholar]
- 61.Grucela A, Steinhagen RM. Current surgical management of ulcerative colitis. Mt Sinai J Med. 2009 Dec;76(6):606–612. doi: 10.1002/msj.20152. [DOI] [PubMed] [Google Scholar]
- 62.Cohen JL, Strong SA, Hyman NH, Buie WD, Dunn GD, Ko CY, et al. Practice parameters for the surgical treatment of ulcerative colitis. Dis Colon Rectum. 2005 Nov;48(11):1997–2009. doi: 10.1007/s10350-005-0180-z. [DOI] [PubMed] [Google Scholar]
- 63.Goligher JC, de Dombal FT, Graham NG, Watkinson G. Early surgery in the management of severe ulcerative colitis. Br Med J. 1967 Jul 22;3(5559):193–195. doi: 10.1136/bmj.3.5559.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goligher JC, Hoffman DC, de Dombal FT. Surgical treatment of severe attacks of ulcerative colitis, with special reference to the advantages of early operation. Br Med J. 1970 Dec 19;4(5737):703–706. doi: 10.1136/bmj.4.5737.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberts SE, Williams JG, Yeates D, Goldacre MJ. Mortality in patients with and without colectomy admitted to hospital for ulcerative colitis and crohn's disease: Record linkage studies. BMJ. 2007 Nov 17;335(7628):1033. doi: 10.1136/bmj.39345.714039.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Silva S, Ma C, Proulx MC, Crespin M, Kaplan BS, Hubbard J, et al. Postoperative complications and mortality following colectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2011 Nov;9(11):972–980. doi: 10.1016/j.cgh.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 67.Issa M, Ananthakrishnan AN, Binion DG. Clostridium difficile and inflammatory bowel disease. Inflamm Bowel Dis. 2008 Oct;14(10):1432–1442. doi: 10.1002/ibd.20500. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008 Jun;103(6):1443–1450. doi: 10.1111/j.1572-0241.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 69.Kaneko T, Matsuda R, Taguri M, Inamori M, Ogura A, Miyajima E, et al. Clostridium difficile infection in patients with ulcerative colitis: Investigations of risk factors and efficacy of antibiotics for steroid refractory patients. Clin Res Hepatol Gastroenterol. 2011 Apr;35(4):315–320. doi: 10.1016/j.clinre.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 70.Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with clostridium difficile in patients with inflammatory bowel disease. Gut. 2008 Feb;57(2):205–210. doi: 10.1136/gut.2007.128231. [DOI] [PubMed] [Google Scholar]
- 71.Issa M, Vijayapal A, Graham MB, Beaulieu DB, Otterson MF, Lundeen S, et al. Impact of clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345–351. doi: 10.1016/j.cgh.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 72.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, et al. Clinical practice guidelines for clostridium difficile infection in adults 2010 update by the society for healthcare epidemiology of america (SHEA) and the infectious diseases society of america (IDSA) Infect Control Hosp Epidemiol. 2010 May;31(5):431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 73.Issa M, Weber LR, Skaros S, Beaulieu DB, Emmons J, Knox JF, et al. Decreasing rates of colectomy despite high rates of hospitalization in clostridium difficile infected IBD patients: A tertiary referral center experience. Gastroenterology. 2007;132:A663. [Google Scholar]
- 74.Chiplunker A, Ananthakrishnan AN, Beaulieu DB, Naik AS, Zadvornova Y, Skaros S, et al. Long-term impact of clostridium difficile on inflammatory bowel disease. Gastroenterology. 2009;136(Suppl 1):S1145. [Google Scholar]
- 75.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, et al. Fidaxomicin versus vancomycin for clostridium difficile infection. N Engl J Med. 2011 Feb 3;364(5):422–431. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 76.Kandiel A, Lashner B. Cytomegalovirus colitis complicating inflammatory bowel disease. Am J Gastroenterol. 2006 Dec;101(12):2857–2865. doi: 10.1111/j.1572-0241.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 77.Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: Pathogen or innocent bystander? Inflamm Bowel Dis. 2010 Sep;16(9):1620–1627. doi: 10.1002/ibd.21275. [DOI] [PubMed] [Google Scholar]
- 78.Maconi G, Colombo E, Zerbi P, Sampietro GM, Fociani P, Bosani M, et al. Prevalence, detection rate and outcome of cytomegalovirus infection in ulcerative colitis patients requiring colonic resection. Dig Liver Dis. 2005 Jun;37(6):418–423. doi: 10.1016/j.dld.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 79.Cottone M, Pietrosi G, Martorana G, Casa A, Pecoraro G, Oliva L, et al. Prevalence of cytomegalovirus infection in severe refractory ulcerative and crohn's colitis. Am J Gastroenterol. 2001 Mar;96(3):773–775. doi: 10.1111/j.1572-0241.2001.03620.x. [DOI] [PubMed] [Google Scholar]
- 80.Criscuoli V, Casa A, Orlando A, Pecoraro G, Oliva L, Traina M, et al. Severe acute colitis associated with CMV: A prevalence study. Dig Liver Dis. 2004 Dec;36(12):818–820. doi: 10.1016/j.dld.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 81.Domenech E, Vega R, Ojanguren I, Hernandez A, Garcia-Planella E, Bernal I, et al. Cytomegalovirus infection in ulcerative colitis: A prospective, comparative study on prevalence and diagnostic strategy. Inflamm Bowel Dis. 2008 Oct;14(10):1373–1379. doi: 10.1002/ibd.20498. [DOI] [PubMed] [Google Scholar]
- 82.Orvar K, Murray J, Carmen G, Conklin J. Cytomegalovirus infection associated with onset of inflammatory bowel disease. Dig Dis Sci. 1993 Dec;38(12):2307–2310. doi: 10.1007/BF01299914. [DOI] [PubMed] [Google Scholar]
- 83.Pereyra F, Rubin RH. Prevention and treatment of cytomegalovirus infection in solid organ transplant recipients. Curr Opin Infect Dis. 2004 Aug;17(4):357–361. doi: 10.1097/01.qco.0000136933.67920.dd. [DOI] [PubMed] [Google Scholar]
- 84.Lavagna A, Bergallo M, Daperno M, Sostegni R, Costa C, Leto R, et al. Infliximab and the risk of latent viruses reactivation in active crohn's disease. Inflamm Bowel Dis. 2007 Jul;13(7):896–902. doi: 10.1002/ibd.20131. [DOI] [PubMed] [Google Scholar]
- 85.Leveque N, Brixi-Benmansour H, Reig T, Renois F, Talmud D, Brodard V, et al. Low frequency of cytomegalovirus infection during exacerbations of inflammatory bowel diseases. J Med Virol. 2010 Oct;82(10):1694–1700. doi: 10.1002/jmv.21877. [DOI] [PubMed] [Google Scholar]
- 86.Yoshino T, Nakase H, Ueno S, Uza N, Inoue S, Mikami S, et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007 Dec;13(12):1516–1521. doi: 10.1002/ibd.20253. [DOI] [PubMed] [Google Scholar]
- 87.Kornbluth A, Sachar DB Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2010 Mar;105(3):501–523. doi: 10.1038/ajg.2009.727. quiz 524. [DOI] [PubMed] [Google Scholar]
- 88.Rahier JF, Ben-Horin S, Chowers Y, Conlon C, De Munter P, D'Haens G, et al. European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009 Jun;3(2):47–91. doi: 10.1016/j.crohns.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 89.Sands BE. Fulminant colitis. J Gastrointest Surg. 2008 Dec;12(12):2157–2159. doi: 10.1007/s11605-008-0661-1. [DOI] [PubMed] [Google Scholar]
- 90.Gan SI, Beck PL. A new look at toxic megacolon: An update and review of incidence, etiology, pathogenesis, and management. Am J Gastroenterol. 2003 Nov;98(11):2363–2371. doi: 10.1111/j.1572-0241.2003.07696.x. [DOI] [PubMed] [Google Scholar]
- 91.Jalan KN, Sircus W, Card WI, Falconer CW, Bruce CB, Crean GP, et al. An experience of ulcerative colitis. I. toxic dilation in 55 cases. Gastroenterology. 1969 Jul;57(1):68–82. [PubMed] [Google Scholar]
- 92.Latella G, Vernia P, Viscido A, Frieri G, Cadau G, Cocco A, et al. GI distension in severe ulcerative colitis. Am J Gastroenterol. 2002 May;97(5):1169–1175. doi: 10.1111/j.1572-0241.2002.05691.x. [DOI] [PubMed] [Google Scholar]
- 93.Sheth SG, LaMont JT. Toxic megacolon. Lancet. 1998 Feb 14;351(9101):509–513. doi: 10.1016/S0140-6736(97)10475-5. [DOI] [PubMed] [Google Scholar]
- 94.Imbriaco M, Balthazar EJ. Toxic megacolon: Role of CT in evaluation and detection of complications. Clin Imaging. 2001 Sep-Oct;25(5):349–354. doi: 10.1016/s0899-7071(01)00330-8. [DOI] [PubMed] [Google Scholar]
- 95.Autenrieth DM, Baumgart DC. Toxic megacolon. Inflamm Bowel Dis. 2011 Aug 29; doi: 10.1002/ibd.21847. [DOI] [PubMed] [Google Scholar]
- 96.Panos MZ, Wood MJ, Asquith P. Toxic megacolon: The knee-elbow position relieves bowel distension. Gut. 1993 Dec;34(12):1726–1727. doi: 10.1136/gut.34.12.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Present DH, Wolfson D, Gelernt IM, Rubin PH, Bauer J, Chapman ML. Medical decompression of toxic megacolon by "rolling". A new technique of decompression with favorable long-term follow-up. J Clin Gastroenterol. 1988 Oct;10(5):485–490. doi: 10.1097/00004836-198810000-00004. [DOI] [PubMed] [Google Scholar]
- 98.Katzka I, Katz S, Morris E. Management of toxic megacolon: The significance of early recognition in medical management. J Clin Gastroenterol. 1979 Dec;1(4):307–311. doi: 10.1097/00004836-197912000-00005. [DOI] [PubMed] [Google Scholar]
- 99.D'Amico C, Vitale A, Angriman I, Ruffolo C, D'Amico F, Valente D, et al. Early surgery for the treatment of toxic megacolon. Digestion. 2005;72(2–3):146–149. doi: 10.1159/000088369. [DOI] [PubMed] [Google Scholar]
- 100.Long MD, Barnes EL, Herfarth HH, Drossman DA. Narcotic use for inflammatory bowel disease and risk factors during hospitalization. Inflamm Bowel Dis. 2011 Jul 7; doi: 10.1002/ibd.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lian L, Fazio VW, Hammel J, Shen B. Impact of narcotic use on the requirement for colectomy in inpatients with ulcerative colitis. Dis Colon Rectum. 2010 Sep;53(9):1295–1300. doi: 10.1007/DCR.0b013e3181e7562c. [DOI] [PubMed] [Google Scholar]
- 102.Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Diamond RH, Chen DM, et al. Serious infections and mortality in association with therapies for crohn's disease: TREAT registry. Clin Gastroenterol Hepatol. 2006 May;4(5):621–630. doi: 10.1016/j.cgh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 103.Dickinson RJ, Ashton MG, Axon AT, Smith RC, Yeung CK, Hill GL. Controlled trial of intravenous hyperalimentation and total bowel rest as an adjunct to the routine therapy of acute colitis. Gastroenterology. 1980 Dec;79(6):1199–1204. [PubMed] [Google Scholar]
- 104.McIntyre PB, Powell-Tuck J, Wood SR, Lennard-Jones JE, Lerebours E, Hecketsweiler P, et al. Controlled trial of bowel rest in the treatment of severe acute colitis. Gut. 1986 May;27(5):481–485. doi: 10.1136/gut.27.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nguyen GC, Munsell M, Harris ML. Nationwide prevalence and prognostic significance of clinically diagnosable protein-calorie malnutrition in hospitalized inflammatory bowel disease patients. Inflamm Bowel Dis. 2008 Aug;14(8):1105–1111. doi: 10.1002/ibd.20429. [DOI] [PubMed] [Google Scholar]
- 106.Nisar PJ, Appau KA, Remzi FH, Kiran RP. Preoperative hypoalbuminemia is associated with adverse outcomes after ileoanal pouch surgery. Inflamm Bowel Dis. 2011 doi: 10.1002/ibd.21842. n/a, n/a. [DOI] [PubMed] [Google Scholar]
- 107.Hatoum OA, Binion DG. The vasculature and inflammatory bowel disease: Contribution to pathogenesis and clinical pathology. Inflamm Bowel Dis. 2005 Mar;11(3):304–313. doi: 10.1097/01.mib.0000160772.78951.61. [DOI] [PubMed] [Google Scholar]
- 108.Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: A cohort study. Lancet. 2010 Feb 20;375(9715):657–663. doi: 10.1016/S0140-6736(09)61963-2. [DOI] [PubMed] [Google Scholar]
- 109.Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008 Sep;103(9):2272–2280. doi: 10.1111/j.1572-0241.2008.02052.x. [DOI] [PubMed] [Google Scholar]
- 110.Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: An epidemiological review. Am J Gastroenterol. 2011 Apr;106(4):713–718. doi: 10.1038/ajg.2011.53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.