Abstract
Purpose
High-dose interferon (IFN) for 1 year (HDI) is the US Food and Drug Administration–approved adjuvant therapy for patients with high-risk melanoma. Efforts to modify IFN dose and schedule have not improved efficacy. We sought to determine whether a shorter course of biochemotherapy would be more effective.
Patients and Methods
S0008 (S0008: Chemotherapy Plus Biological Therapy in Treating Patients With Melanoma) was an Intergroup phase III trial that enrolled high-risk patients (stage IIIA-N2a through IIIC-N3), randomly assigning them to receive either HDI or biochemotherapy consisting of dacarbazine, cisplatin, vinblastine, interleukin-2, IFN alfa-2b (IFN-α-2b) and granulocyte colony-stimulating factor given every 21 days for three cycles. Coprimary end points were relapse-free survival (RFS) and overall survival (OS).
Results
In all, 432 patients were enrolled. Grade 3 and 4 adverse events occurred in 57% and 7% of HDI patients and 36% and 40% of biochemotherapy patients, respectively. At a median follow-up of 7.2 years, biochemotherapy improved RFS (hazard ratio [HR], 0.75; 95% CI, 0.58 to 0.97; P = .015), with a median RFS of 4.0 years (95% CI, 1.9 years to not reached [NR]) versus 1.9 years for HDI (95% CI, 1.2 to 2.8 years) and a 5-year RFS of 48% versus 39%. Median OS was not different (HR, 0.98; 95% CI, 0.74 to 1.31; P = .55), with a median OS of 9.9 years (95% CI, 4.62 years to NR) for biochemotherapy versus 6.7 years (95% CI, 4.5 years to NR) for HDI and a 5-year OS of 56% for both arms.
Conclusion
Biochemotherapy is a shorter, alternative adjuvant treatment for patients with high-risk melanoma that provides statistically significant improvement in RFS but no difference in OS and more toxicity compared with HDI.
INTRODUCTION
Melanoma remains a highly curable malignancy when identified early. However, 20% to 30% of patients with T2 or thicker primary melanoma will be found to have regional lymph node involvement (American Joint Committee on Cancer stage III), which is associated with at least a 30% risk of subsequent distant metastasis and death.1 Adjuvant interferon (IFN) has been evaluated in multiple phase III trials in an attempt to delay or prevent subsequent metastasis and death. High-dose IFN (HDI) for 1 year has been used in the treatment or control arm in three phase III US Intergroup trials (Eastern Cooperative Oncology Group; E-1684, E-1690, E-1694) for patients with high-risk primary T4 melanomas or regional nodal involvement.2–4 HDI was associated with relapse-free survival (RFS) benefit in each study. Overall survival (OS) benefit was seen in E-1684 and E-1694 but not in E-1690.
Numerous additional phase III trials have been completed that compared observation with IFN for patients with high-risk melanoma by using different IFN doses, schedules, and durations in an attempt to identify a similar or greater benefit than the standard 1-year HDI regimen. Several meta-analyses of those trials have been published, demonstrating consistent RFS benefit and modest OS benefit for the use of IFN compared with observation.5–7 To date, no trials have demonstrated that any regimen has a greater benefit than HDI in terms of either RFS or OS. Efforts to add other antineoplastic agents to IFN to enhance treatment effectiveness have been difficult because of the toxicity and duration of IFN therapy.
Interleukin-2 (IL-2) has been identified as an effective treatment for patients with stage IV melanoma and, when administered at high doses, it is associated with durable complete remissions in approximately 6% to 10% of patients in collected phase II studies.8 The standard US Food and Drug Administration–approved schedule and dose of IL-2 has been difficult to administer outside of selected tertiary care centers that have considerable experience in its use. Numerous phase II studies in the late 1980s and early 1990s demonstrated the feasibility of combining IL-2 with available chemotherapy agents in both inpatient and outpatient regimens in what was subsequently referred to as IL-2–based biochemotherapy. The majority of biochemotherapy regimens evaluated also included low-dose IFN.9–13 By the late 1990s, meta-analysis of more than 7,000 patients who had stage IV melanoma and were in phase II studies demonstrated the highest response rates and response durations for biochemotherapy regimens in which chemotherapy (usually including cisplatin and dacarbazine DTIC was combined with IL-2 and IFN.14
Legha et al15,16 evaluated biochemotherapy combining cisplatin, vinblastine, and dacarbazine chemotherapy with IL-2 and IFN, which seemed promising with high response rates and response durability. Modifications to reduce toxicity were developed by McDermott et al17 and were assessed in a cooperative group setting in patients with unresectable stage III or IV melanoma but they were not superior to chemotherapy alone.18 Biochemotherapy was compared with HDI in the adjuvant setting by using a regimen that could be completed in 9 weeks as opposed to 1 year of HDI treatment or up to 5 years for pegylated IFN adjuvant therapy.19 Patients with stage IIIA-N2a and above were included as an appropriate risk group for a treatment regimen with potentially greater toxicity and benefit.
PATIENTS AND METHODS
Patient Eligibility
Eligible patients had histologically proven melanoma of cutaneous origin or from an unknown primary (mucosal and uveal primaries were excluded) and were stage IIIA-N2a through stage IIIC-N3, which included any one of the following: an ulcerated cutaneous primary with one or more tumor-positive sentinel lymph nodes, a nonulcerated cutaneous primary with two or more tumor-positive sentinel lymph nodes, any cutaneous primary (or an unknown primary) presenting with regional lymph node macrometastasis, any cutaneous primary (or an unknown primary) with satellite or in-transit metastasis with or without regional lymph node involvement, or any cutaneous primary with regional nodal recurrence. Gross or microscopic extracapsular extension of nodal tumor was allowed. Patients were eligible at the time of initial diagnosis of the primary disease or on subsequent regional nodal or in-transit recurrence. Tissue confirmation by hematoxylin and eosin–stained slides was required. Patients with multiple regional nodal basins involved were eligible. Patients must have been registered within 56 days of lymphadenectomy and/or resection of satellite or in-transit metastasis.
Patients must have undergone wide excision of the cutaneous primary with pathologically negative margins and a complete regional lymphadenectomy and could have no clinical, radiologic, or pathologic evidence of residual or metastatic melanoma. Patients were not permitted to have received prior radiation therapy, chemotherapy, or immunotherapy with IFN or IL-2 for any type of cancer. No planned concomitant therapy, including radiotherapy, was allowed.
Patients must have been 10 years of age or older at the time of registration, with a Zubrod performance status of 0 or 1 and adequate hepatic, renal, and hematologic function assessed by laboratory parameters. A chest x-ray or computed tomography scan of the chest that was negative for metastasis was required within 4 weeks of the definitive surgery. Patients with a history of cardiac or pulmonary disease and all patients older than age 50 years were required to pass a cardiac stress test and to demonstrate adequate pulmonary function (forced expiratory volume at one second of > 2.0 L or 75% of predicted volume). Patients must have been willing and able to discontinue all antihypertensive medications if they were randomly assigned to biochemotherapy.
Pregnant or nursing women were excluded, and all men and women of reproductive age were required to use an effective contraceptive method. A negative pregnancy test within 14 days of the start of treatment was required for all women of reproductive age. No prior malignancies were allowed except adequately treated basal cell or squamous cell skin cancers, in situ cervical cancer, adequately treated stage I or II cancers from which the patient was in complete remission, or any other cancer from which the patient was disease free for 5 years or more. All patients provided informed written consent.
Patient stratification was performed on the basis of the number of involved nodes (one to three versus four or more, including matted nodes or satellite/in-transit metastasis with no lymph node involvement), the degree of lymph node involvement (micrometastasis or none [satellite/in-transit metastasis only] v macrometastasis), and ulceration of the primary tumor (yes v no v unknown).
Study Design and Treatments
The patients were randomly assigned 1:1 between the two treatments arms by the Southwest Oncology Group Statistical Center on the basis of stratification factors (Fig 1). Patients in arm A received high-dose IFN alfa-2b (IFN-α-2b) 20 MU/m2 per day intravenously (IV) 5 days per week for 4 weeks followed by 10 MU/m2 subcutaneously three times per week for 48 weeks. The use of corticosteroids and other immunosuppressive medications during treatment was not permitted.
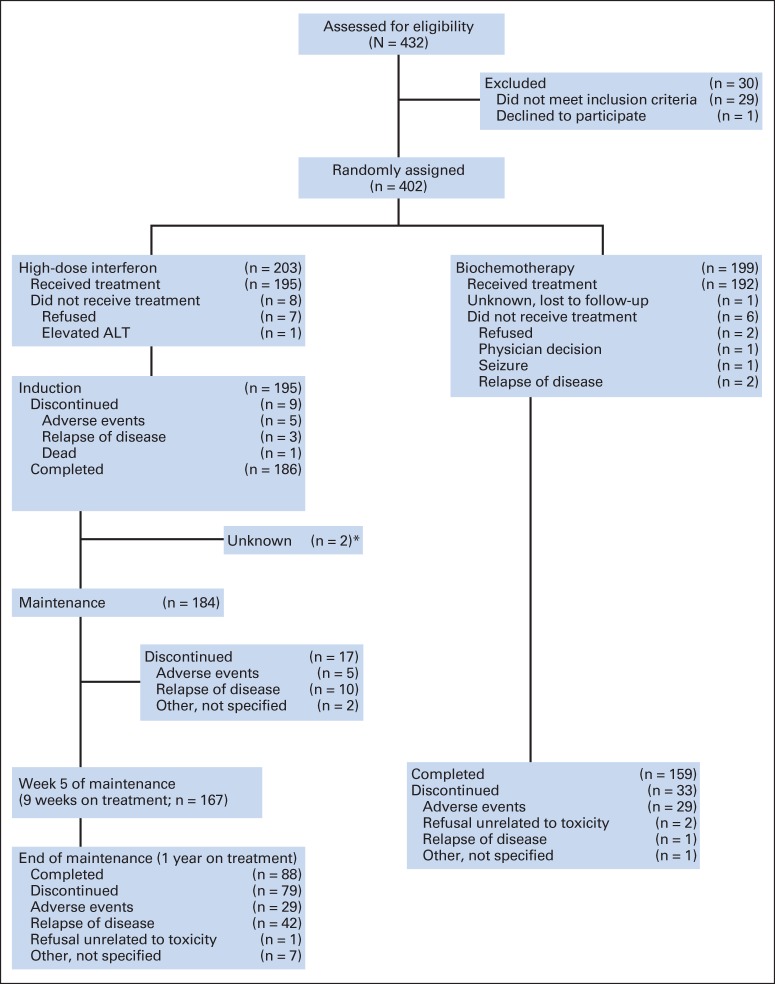
Fig 1.
CONSORT diagram. (*) These patients remained in follow-up but treatment status after induction not reported.
Patients in arm B received biochemotherapy consisting of cisplatin 20 mg/m2 administered as a 30-minute infusion on days 1 through 4, vinblastine 1.2 mg/m2 IV push immediately after cisplatin on days 1 through 4, dacarbazine 800 mg/m2 administered IV over 1 hour on day 1 only after vinblastine, IL-2 at 9 MU/m2 administered as a 96-hour continuous IV infusion on days 1 through 4, and IFN-α-2b at 5 MU/m2 administered on days 1 through 5; the dosing regimen continued on an outpatient basis on days 8, 10, and 12. Patients were admitted to the hospital for the first 5 days of each treatment cycle. Treatment was repeated every 21 days for a total of three cycles.
Biochemotherapy patients discontinued antihypertensive therapy 24 hours before the beginning of each treatment cycle. A triple-lumen catheter was placed at the beginning of each treatment cycle and removed at the time of discharge. Patients received either cephalexin or ciprofloxacin 250 mg orally twice per day on days 1 through 17 of each treatment cycle. Patients were prehydrated before each cisplatin dose with 1,000 mL of dextrose 5% (D5) half- normal saline with 8 meq/L of MgSO4 given over 3 hours once per day and then maintained on D5 half-normal saline with 20 meq/L of KCl at 100 mL per hour. Granulocyte colony-stimulating factor 5 μg/kg per day subcutaneously was administered on days 7 through 16 (or until absolute neutrophil count exceeded 10,000/dL). Aggressive antiemetic therapy (ondansetron 32 mg IV or equivalent) was administered every day during therapy and was continued for several days after discharge in patients with persistent nausea or vomiting. Steroids were not permitted. Acetaminophen, ranitidine, and naproxen were provided prophylactically, and antipruritics, antidiarrheals, and anxiolytics were administered as needed.
Dose Modification
Dose modifications were defined on the basis of adverse events, by using the National Cancer Institute Common Toxicity Criteria.
IFN arm.
Separate dose modification schemes were defined for weeks 1 to 4 and weeks 5 to 52. A patient requiring dose modifications during the first 4 weeks started week 5 treatment at full dose. Grade 2 anemia, arrhythmia, intractable nausea, vomiting or diarrhea, increased creatinine, grade 4 neutropenia, and any nonhematologic grade 3 toxicity required that IFN be held until a return to the institutional norms. IFN was resumed at a 33% dose reduction for the first interruption for toxicity and at a 66% reduction for the second interruption for toxicity. Dose re-escalation was not permitted. A third interruption for toxicity required removal of the patient from study treatment.
Biochemotherapy arm.
Patients developing grade 3 toxicity while receiving inpatient therapy (days 1 through 5) had treatment held until toxicity returned to grade 2 or lower. Therapy was then restarted at full doses of chemotherapy and at a 50% dose reduction for both IL-2 and IFN. If a portion of an IL-2 infusion or a dose of IFN was held, it was not re-administered. All dose reductions were permanent. If grade 3 or 4 toxicity developed despite a 50% dose reduction of IL-2 and IFN, no further IL-2 or IFN was administered in that or subsequent treatment cycles. If any grade 3 toxicity occurred during week 2 of any cycle, the IFN was held for the remainder of that cycle. Subsequent IFN was administered at full dose. Exceptions to this approach included the management of hypotension, nephrotoxicity, hematologic toxicity, nausea and vomiting, and neurotoxicity (Appendix Tables A1 and A2, online only).
Statistical Methods
OS was defined as time from study registration to death, with patients last known to be alive censored at date of last contact. RFS was defined as time from registration until the first observation of recurrent disease or death as a result of any cause, with patients last known to be alive without recurrence censored at date of last contact. Survival after relapse was measured from the date of first recurrence until death as a result of any cause, with patients last known to be alive censored at date of last contact.
OS and RFS were coprimary end points. OS was used for sample size and power estimation. On the basis of historical data, the 5-year survival rate for the control arm was assumed to be approximately 40%. The design called for 410 eligible patients enrolled over 3 years with an additional 3 years of follow-up to observe approximately 113 deaths on the control arm, assuming exponential survival distributions. With these assumptions, the power to detect a survival increase was approximately 91% and 80% if the true hazard ratios (HRs) were 1.53 and 1.42, respectively.
Fisher's exact test was used to assess differences in categorical variables between the arms. OS and RFS were estimated by using the Kaplan-Meier method. Cox models were used for multivariable regression modeling of OS and RFS.
RESULTS
Study Population
Between September 2000 and November 2007, 432 patients registered for this study. Of those patients, 212 were randomly assigned to HDI and 220 to biochemotherapy. Twenty-nine patients were deemed ineligible (nine HDI and 20 biochemotherapy), and one patient withdrew consent immediately after registration, leaving 203 eligible patients in the HDI arm and 199 in the biochemotherapy arm. Reasons for ineligibility included incorrect stage (n = 19), inadequate surgery (n = 7), not having melanoma (n = 2), and having a mucosal primary (n = 1).
Patient demographics and clinical characteristics were well balanced between the arms. Table 1 outlines those features for both groups. The median age was 47 years, 70% of patients were male, 96% were white, and 76% had one to three tumors with lymph node involvement or satellite/in-transit metastases without lymph node involvement. A majority of the enrolled patients (57%) had macroscopic nodal involvement at presentation, and 41% had documented ulceration of their primary lesion. There were no significant differences between the two arms on any of these factors.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | HDI (n = 203) |
Biochemotherapy (n = 199) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | |||||
| Median | 48 | 46 | .58 | ||
| Range | 12-73 | 10-74 | |||
| Sex | |||||
| Male | 141 | 69 | 141 | 71 | .83 |
| Female | 62 | 31 | 58 | 29 | |
| Race/ethnicity | |||||
| White | 195 | 96 | 190 | 95 | .64 |
| Other | 8 | 4 | 10 | 5 | |
| No. of nodes | |||||
| One to three or S/IT only | 154 | 76 | 151 | 76 | 1.00 |
| Four or more or one or more with S/IT | 49 | 24 | 48 | 24 | |
| Nodal involvement | |||||
| Micro only* | 86 | 43 | 88 | 44 | .84 |
| Any macro† | 117 | 57 | 111 | 56 | |
| Ulceration | |||||
| Yes | 84 | 41 | 82 | 41 | .89 |
| No | 66 | 34 | 69 | 35 | |
| Unknown | 53 | 25 | 48 | 24 | |
Abbreviations: HDI, high-dose interferon; S/IT, satellite/in-transit metastasis.
Micro, lymph node micrometastasis.
Macro, lymph node macrometastasis.
Treatment Characteristics
All eligible patients were included in the treatment assessments. Table 2 summarizes treatment information. Eight patients (4%) in the HDI arm and four patients (2%) in the biochemotherapy arm refused their assigned treatment. One additional patient assigned to the biochemotherapy arm was lost to follow-up, and it is unknown whether or not that patient received any protocol treatment. In the HDI arm, 88 patients (43%) completed 52 weeks of therapy compared with 159 patients (80%) in the biochemotherapy arm who completed 9 weeks of therapy (P < .001). Relapse of disease accounted for 55 patients (27%) not completing the HDI regimen compared with two patients (1%) who relapsed during biochemotherapy (P < .001). Thirty-nine patients (19%) in the HDI arm stopped treatment because of adverse effects compared with 29 patients (15%) in the biochemotherapy arm (P = .23). Other or unknown reasons for stopping treatment occurred in 13 patients (6%) in the HDI arm and five patients (2%) in the biochemotherapy arm.
Table 2.
Treatment Summary
| Variable | HDI (n = 203) |
Biochemotherapy (n = 199) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Completed therapy | 88 | 43 | 159 | 80 | < .001 |
| Relapse | 55 | 27 | 2 | 1 | < .001 |
| Adverse effects | 39 | 19 | 29 | 15 | .23 |
| Other or unknown reason | 12 | 6 | 5 | 2 | .14 |
| Refused assigned therapy | 8 | 4 | 4 | 2 | .38 |
| Lost to follow-up | 1 | 1 | 0 | 0 | 1 |
Abbreviation: HDI, high-dose interferon.
Toxicity
Toxicity was assessed for 193 patients in the HDI arm and 185 in the biochemotherapy arm. Grade 3 or higher toxicity was seen in 64% of the patients (grade 3, 57%; grade 4, 7%) in the HDI arm and in 76% of the patients (grade 3, 36%; grade 4, 40%) in the biochemotherapy arm. The most common toxicities varied by arm (Table 3) and included neurologic, psychiatric, and hepatic toxicity with HDI and hematologic, GI, and metabolic toxicity as well as hypotension with biochemotherapy. There was one treatment-related death in each arm. In the HDI arm, one patient was found apneic at home after the end of week 2. One patient in the biochemotherapy arm also died at home of unknown causes after the completion of 9 weeks of treatment.
Table 3.
Toxicity by Treatment Arm
| Toxicity | HDI (%) (n = 193) | Biochemotherapy (%) (n = 185) | P |
|---|---|---|---|
| Grade 4* | |||
| Hematologic | |||
| Leukopenia | 1 | 13 | < .01 |
| Neutropenia | 4 | 26 | < .01 |
| Thrombocytopenia | 0 | 14 | < .01 |
| Grade 3 to 4† | |||
| Gastrointestinal | |||
| Anorexia | 1 | 5 | .01 |
| Nausea | 5 | 28 | < .01 |
| Vomiting | 5 | 20 | .01 |
| Neurologic/psychological | |||
| Depression | 7 | 2 | .03 |
| Headache | 5 | 3 | .42 |
| Fatigue/lethargy | 20 | 11 | .05 |
| Metabolic | |||
| Hypocalcemia | 0 | 8 | < .01 |
| Dermatologic | |||
| Rash | 2 | 5 | .11 |
| Hepatic | |||
| Increased aspartate aminotransferase | 9 | 4 | .02 |
| Increased alanine aminotransferase | 16 | 4 | < .01 |
| Cardiovascular | |||
| Hypotension | 0 | 9 | < .01 |
Abbreviation: HDI, high-dose interferon.
Percentage of patients experiencing a maximum of grade 4 toxicity when it occurred ≥ 5% of the time in either arm.
Percentage of patients experiencing a maximum of either grade 3 or 4 toxicity when it occurred ≥ 5% of the time in either arm.
Efficacy
The efficacy analysis was updated with a cutoff date of February 7, 2013. The median follow-up of patients on study was 7.2 years. There were 98 deaths in the HDI arm and 91 deaths in the biochemotherapy arm. There were 129 RFS events (relapse or death) in the HDI arm and 107 RFS events in the biochemotherapy arm.
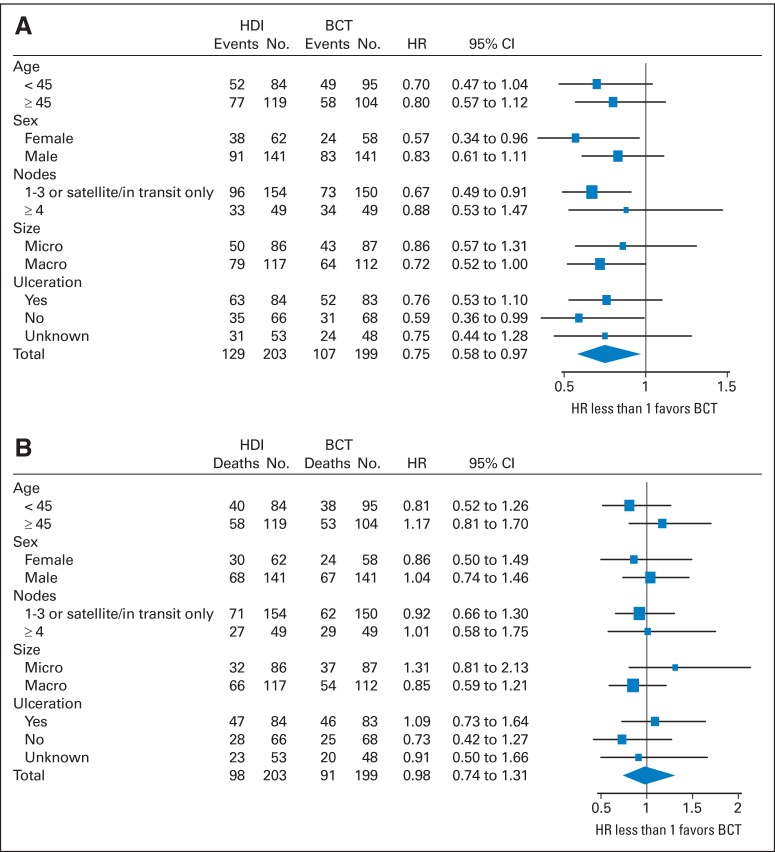
The median RFS for HDI patients was 1.9 years, and for biochemotherapy patients, it was 4.0 years (HR, 0.75; 95% CI, 0.58 to 0.97; two-sided P = .029; Fig 2). The 5-year RFS for the HDI arm was 39% (95% CI, 32% to 46%), and for the biochemotherapy arm, it was 48% (95% CI, 41% to 55%). The forest plot for patient characteristics and tumor stratification factors is presented in Figure 3. In that analysis, biochemotherapy patients who were female, had one to three positive nodes or satellite/in-transit metastases without nodal involvement, or had macroscopic nodal presentation had significantly improved RFS compared with HDI patients.
Fig 2.
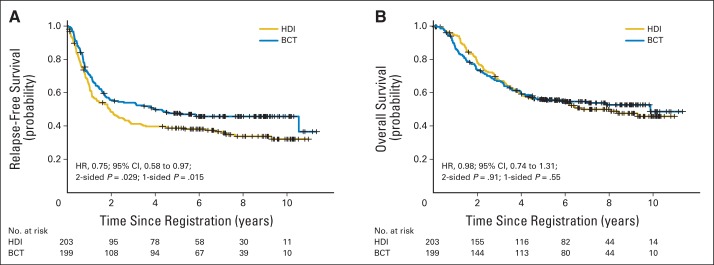
(A) Relapse-free survival and (B) overall survival. BCT, biochemotherapy; HDI, high-dose interferon; HR, hazard ratio.
Fig 3.
Forest plots for (A) relapse-free survival and (B) overall survival. BCT, biochemotherapy; HDI, high-dose interferon; HR, hazard ratio; macro, lymph node macrometastasis; micro, lymph node micrometastasis.
The median OS for HDI patients was 6.7 years, and for biochemotherapy patients, it was 9.9 years (HR, 0.98; 95% CI, 0.74 to 1.31; two-sided P = .55; Fig 2). The 5-year OS for both arms was 56% (95% CI, 49% to 63% for both). There is no subset for which there was a statistically significant OS benefit for one treatment arm compared with the other (Fig 3). Appendix Table A1 and Table A2 summarize results from multivariable regression models for OS and RFS, with similar results for the forest plot and univariable analysis.
DISCUSSION
The results of Intergroup studies E-1684, E-1690, and E-16942–4 established HDI as the adjuvant therapy standard of care for patients with high-risk melanoma. Additional phase III trials have evaluated modifications to the dose, schedule, and/or duration of IFN administration without improving the outcomes.20–22 To the best of our knowledge, this randomized trial is the first to compare a multiagent regimen against HDI in patients with high-risk melanoma and the first to demonstrate a statistically significant RFS benefit for any treatment regimen over HDI. The 25% relative improvement in RFS for biochemotherapy corresponded to a 9% absolute increase in freedom from relapse at 5 years. The median RFS of 4 years represents a value not previously observed in any adjuvant therapy trial for patients with high-risk melanoma. Of note, however, there was no corresponding improvement in OS for the biochemotherapy arm, and even with continued follow-up, it seems unlikely that a significant difference will emerge. Subset analysis (Fig 3) does not identify a group that has a particular OS benefit.
The toxicity of treatment differed substantially by treatment arm because of the specific adverse effects of the drugs involved as well as the time on treatment (9 weeks for biochemotherapy and 52 weeks for HDI). There was more grade 4 toxicity associated with biochemotherapy than with HDI (40% v 7%), but grade 3 and 4 toxicity combined was similar: 76% for biochemotherapy versus 64% for HDI. The majority of toxicity associated with biochemotherapy was hematologic and was of short duration. Discontinuation of treatment because of adverse effects is an equally important assessment of toxicity and perhaps a more legitimate measure when comparing two different treatment programs. The rate for discontinuation because of toxicity was 19% for HDI and 15% for biochemotherapy, a nonsignificant difference.
It has been 30 years since the first HDI adjuvant melanoma cooperative group trial began. Other doses, schedules, combinations, and durations of IFN have failed to improve on that important advance. This study is the first to demonstrate an improvement in RFS compared with HDI. Toxicities for biochemotherapy and HDI are different but comparable in magnitude, particularly when discontinuation for toxicity is considered. The lack of an OS difference may be attributable to greater efficacy of salvage therapies in the HDI arm as well as differences in the location and timing of first relapses (data not shown). Because this trial did not involve a no-treatment arm, we cannot assess whether OS for both arms differed from what might have been achieved with surgery alone.
On the basis of the results of this Intergroup randomized trial, biochemotherapy may be considered an alternative for the adjuvant therapy in high-risk melanoma in appropriately selected patients by physicians at centers experienced in the use of this regimen. The results provide evidence that drugs with different mechanisms of action can be safely combined to delay disease recurrence in the adjuvant setting. Many promising new drugs and combinations have emerged in recent years. Anti-CTLA4 and anti-PD-1 antibodies,23–26 and targeted mutant BRAF and MEK inhibitors27,28 have demonstrated significant value in the management of patients with metastatic melanoma. Combinations of these drugs are in development and may allow us to selectively replace less effective components of this biochemotherapy regimen with more effective ones. This regimen provides timely information as new drugs enter this field and a foundation on which those future efforts can proceed.
Supplementary Material
Acknowledgment
Supported in part by Public Health Service Cooperative Agreement Grants No. CA32102, CA38926, CA14028, CA27057, CA20319, CA13612, CA37981, CA46282, CA22433, CA04919, CA45377, CA58861, CA46368, CA76447, CA35262, CA86780, CA45808, CA35261, CA35176, CA35178, CA58658, CA67575, CA46113, CA74811, CA12644, CA35090, CA35281, CA76429, CA67663, CA58686, CA11083, CA58416, CA45560, CA21115, CA80775, CA39229 CA31946, CA007190, and CA98543 awarded by the National Cancer Institute, Department of Health and Human Services, and in part by Novartis Pharmaceuticals.
Presented in part as oral presentation at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012.
Glossary Terms
- CTLA4 (CD152):
receptor on activated T cells that binds B7 molecules with a higher affinity than CD28, downregulating T-cell responses by inhibiting CD28 signaling.
- dacarbazine DTIC:
an alkylating agent that is used commonly for the treatment of melanoma, Hodgkin lymphoma, and soft-tissue sarcomas.
- IFN-α-2b (interferon alfa-2b):
recombinant interferon alfa that is commercially prepared from a bacterial fermentation of E. coli bearing an expression vector containing the interferon alfa-2b (IFN-α-2b) gene from human leukocytes.
- interleukin-2 (IL-2):
a cytokine that stimulates proliferation of activated T cells and, at high doses, is used as antitumor therapy in metastatic renal cell carcinoma.
- MEK (MAPK-ERK kinase):
a protein kinase activated by c-Raf through phosphorylation of specific serine residues. Activation of ERK by activated MEK may lead to translocation of ERK to the nucleus, resulting in the activation of specific transcription factors.
- PD-1:
programmed cell death protein 1 (CD279), a receptor expressed on the surface of activated T, B, and NK cells that negatively regulates immune responses, including autoimmune and antitumor responses.
Appendix
Table A1.
Multivariable Analysis, Overall Survival End Point
| Covariate | HR | 95% CI | P |
|---|---|---|---|
| Biochemotherapy (ref., HDI) | 0.99 | 0.75 to 1.32 | .96 |
| Age 45 years or older (ref., age younger than 45 years) | 1.09 | 0.81 to 1.46 | .56 |
| Male (ref., female) | 1.04 | 0.75 to 1.43 | .83 |
| Nodal involvement: any macro (ref., only micro)* | 1.59 | 1.15 to 2.19 | .005 |
| No. of nodes: four or one with S/IT (ref., one to three or S/IT only) | 1.64 | 1.17 to 2.27 | .004 |
| No ulceration (ref., ulceration) | 0.59 | 0.42 to 0.83 | .003 |
| Ulceration unknown (ref., ulceration)† | 0.51 | 0.34 to 0.75 | < .001 |
Abbreviations: HDI, high-dose interferon; HR, hazard ratio; ref., reference; S/IT, satellite/in-transit metastasis.
Macro, lymph node macrometastasis; micro, lymph node micrometastasis.
Unknown ulceration includes unknown primary.
Table A2.
Multivariable Analysis, Relapse-Free Survival End Point
| Covariate | HR | 95% CI | P |
|---|---|---|---|
| Biochemotherapy (ref., HDI) | 0.75 | 0.58 to 0.97 | .03 |
| Age 45 years or older (ref., age younger than 45 years) | 1.00 | 0.77 to 1.30 | 1.00 |
| Male (ref., female) | 1.21 | 0.90 to 1.62 | .21 |
| Nodal involvement: any macro (ref., only micro)* | 1.56 | 1.16 to 2.09 | .003 |
| No. of nodes: four or one with S/IT (ref., one to three or S/IT only) | 1.54 | 1.14 to 2.09 | .006 |
| No ulceration (ref., ulceration) | 0.58 | 0.43 to 0.79 | < .001 |
| Ulceration unknown (ref., ulceration)† | 0.49 | 0.35 to 0.70 | < .001 |
Abbreviations: HDI, high-dose interferon; HR, hazard ratio; ref., reference; S/IT, satellite/in-transit metastasis.
Macro, lymph node macrometastasis; micro, lymph node micrometastasis.
Unknown ulceration includes unknown primary.
Footnotes
Listen to the podcast by Dr Weber at www.jco.org/podcasts
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00006237.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Lawrence E. Flaherty, Merck (C), Novartis (C); Michael B. Atkins, Merck (C), Prometheus Laboratories (C); Frank G. Haluska, Schering-Plough (C); Jeffrey A. Sosman, Genentech (C), GlaxoSmithKline (C); John M. Kirkwood, Bristol-Myers Squibb (C), Merck (C), GlaxoSmithKline (C), Vical (C); Vernon K. Sondak, Merck (C), Bristol-Myers Squibb (C), GlaxoSmithKline (C) Stock Ownership: None Honoraria: Lawrence E. Flaherty, Merck, Novartis; Jeffrey A. Sosman, Genentech, GlaxoSmithKline; Vernon K. Sondak, Merck Research Funding: Lawrence E. Flaherty, Merck, Novartis; John A. Thompson, Schering-Plough, Chiron Pharmaceuticals; Jeffrey A. Sosman, Bristol-Myers Squibb, Genentech, Novartis; John M. Kirkwood, Prometheus Laboratories Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Lawrence E. Flaherty, Michael B. Atkins, Ralph J. Tuthill, Frank G. Haluska, Jeffrey A. Sosman, Bruce G. Redman, John M. Kirkwood, Vernon K. Sondak
Administrative support: Vernon K. Sondak
Provision of study materials or patients: Michael B. Atkins, John A. Thompson, Jeffrey A. Sosman
Collection and assembly of data: Ralph J. Tuthill, John A. Thompson, John T. Vetto, Frank G. Haluska, Alberto S. Pappo, Jeffrey A. Sosman, James Moon, Vernon K. Sondak
Data analysis and interpretation: Megan Othus, Michael B. Atkins, Jeffrey A. Sosman, Antoni Ribas, Vernon K. Sondak
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: First analysis of Intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 4.Kirkwood JM, Ibrahim JG, Sosman JA, et al. High-dose interferon alfa-2b significantly prolongs relapse-free and overall survival compared with the GM2-KLH/QS-21 vaccine in patients with resected stage IIB-III melanoma: Results of Intergroup trial E1694/S9512/C509801. J Clin Oncol. 2001;19:2370–2380. doi: 10.1200/JCO.2001.19.9.2370. [DOI] [PubMed] [Google Scholar]
- 5.Wheatley K, Ives N, Hancock B, et al. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomized trials. Cancer Treat Rev. 2003;29:241–252. doi: 10.1016/s0305-7372(03)00074-4. [DOI] [PubMed] [Google Scholar]
- 6.Wheatley K, Ives N, Eggermont A, et al. Interferon-{alpha} as adjuvant therapy for melanoma: An individual patient's data meta-analysis of randomized trials. J Clin Oncol. 2007;25(suppl):478s. abstr 8526. [Google Scholar]
- 7.Mocellin S, Pasquali S, Rossi CR, et al. Interferon alpha adjuvant therapy in patients with high-risk melanoma: A systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 8.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty LE, Redman BG, Chabot GG, et al. A phase I-II study of dacarbazine in combination with outpatient interleukin-2 in metastatic malignant melanoma. Cancer. 1990;65:2471–2477. doi: 10.1002/1097-0142(19900601)65:11<2471::aid-cncr2820651113>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Atkins MB, O'Boyle KR, Sosman JA, et al. Multiinstitutional phase II trial of intensive combination chemotherapy for metastatic melanoma. J Clin Oncol. 1994;12:1553–1560. doi: 10.1200/JCO.1994.12.8.1553. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty LE, Robinson W, Redman BG, et al. A phase II study of dacarbazine and cisplatin in combination with outpatient administered interleukin-2 in metastatic malignant melanoma. Cancer. 1993;71:3520–3525. doi: 10.1002/1097-0142(19930601)71:11<3520::aid-cncr2820711110>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Thompson JA, Gold PJ, Markowitz DR, et al. Updated analysis of an outpatient chemoimmunotherapy regimen for treating metastatic melanoma. Cancer J Sci Am. 1997;3:S29–S34. [PubMed] [Google Scholar]
- 13.Keilholz U, Goey SH, Punt CJ, et al. Interferon alfa-2a and interleukin-2 with or without cisplatin in metastatic melanoma: A randomized trial of the European Organization for Research and Treatment of Cancer Melanoma Cooperative Group. J Clin Oncol. 1997;15:2579–2588. doi: 10.1200/JCO.1997.15.7.2579. [DOI] [PubMed] [Google Scholar]
- 14.Allen IE, Kupelnick B, Kumashiro M. Efficacy of interleukin-2 in the treatment of metastatic melanoma: Systematic review and metastasis-analysis. Cancer Therapeutics. 1998;1:168–173. [Google Scholar]
- 15.Legha SS, Ring S, Eton O, et al. Development and results of biochemotherapy in metastatic melanoma: The University of Texas M.D. Anderson Cancer Center experience. Cancer J Sci Am. 1997;3:S9–S15. [PubMed] [Google Scholar]
- 16.Legha SS, Ring S, Eton O, et al. Development of biochemotherapy regimen with concurrent administration of cisplatin, vinblastine, dacarbazine, interferon alfa, and interleukin-2 for patients with metastatic melanoma. J Clin Oncol. 1998;16:1752–1759. doi: 10.1200/JCO.1998.16.5.1752. [DOI] [PubMed] [Google Scholar]
- 17.McDermott DF, Mier JW, Lawrence DP, et al. A phase II pilot trial of concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin 2, and interferon alpha-2B in patients with metastatic melanoma. Clin Cancer Res. 2000;6:2201–2208. [PubMed] [Google Scholar]
- 18.Atkins MB, Hsu J, Lee S, et al. Phase III trial comparing concurrent biochemotherapy with cisplatin, vinblastine, dacarbazine, interleukin-2, and interferon alfa-2b with cisplatin, vinblastine, and dacarbazine alone in patients with metastatic malignant melanoma (E3695): A Trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2008;26:5748–5754. doi: 10.1200/JCO.2008.17.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggermont AM, Suciu S, Testori A, et al. Long term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol. 2012;30:3810–3818. doi: 10.1200/JCO.2011.41.3799. [DOI] [PubMed] [Google Scholar]
- 20.Cascinelli N, Belli F, MacKie RM, et al. Effect of long-term adjuvant therapy with interferon alpha-2a in patients with regional node metastases from cutaneous melanoma: A randomized trial. Lancet. 2001;358:866–869. doi: 10.1016/S0140-6736(01)06068-8. [DOI] [PubMed] [Google Scholar]
- 21.Eggermont AM, Suciu S, MacKie R, et al. Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): Randomised controlled trial. Lancet. 2005;366:1189–1196. doi: 10.1016/S0140-6736(05)67482-X. [DOI] [PubMed] [Google Scholar]
- 22.Eggermont AM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon alfa 2b versus observation alone in resected stage III melanoma: Final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–126. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 23.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilumumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert C, Thomas L, Bondarenko I, et al. Ipilumumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 25.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF v600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF v600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.