Abstract
Purpose
Pancreatic cancer is rapidly fatal with median survival of only 6 months (mo). Quality-of-life (QoL) was analyzed prospectively in a phase 2 study of gemcitabine (G), capecitabine (C) and bevacizumab (B) in APC patients.
Methods
A total of 50 patients with APC received B 15 mg/kg, C 1,300 mg/m2 daily for 2 weeks and G 1,000 mg/m2 weekly 2 times; cycles were repeated every 21 days. Endpoints: progression free survival (PFS), overall survival (OS) and assessment of QoL prior to each cycle using the European organization for research and treatment of cancer (EORTC) PAN-26 QoL questionnaire. An exact 95% confidence interval (CI) (Clopper-Pearson method) was used to assess rate of improved QoL (defined as >5% decrease in two consecutive scores compared with baseline).
Results
Patient characteristics- Stage IIB/III/IV: 3/5/42; Sex: 28 M/22 F; Median age: 64 years. QoL in patients- improved: 56%, no improvement: 24%; unevaluable: 20%. Median PFS: 5.8 mo, OS: 9.8 mo. QoL improvement rate: 28/40=0.7 (95% CI: 0.53-0.83) in evaluable patients. Using QoL improvement rate, no significant difference was seen in patients with OS ≥6 mo compared to OS <6 mo. However QoL scores at 3 and 6 weeks from start of treatment correlated strongly with ≥6 mo survival (P value 0.0092 and 0.0081, respectively).
Conclusions
Baseline score and change in QoL scores of patients on G, C and B were not predictive of survival ≥6 mo. Post treatment scores at 3 and 6 weeks from start of therapy however, were predictive of survival ≥6 mo suggesting the potential predictive value of this tool for use in future studies.
Keywords: Quality of life (QoL); pancreatic cancer; biomarkers; neoplasm, European organization for research and treatment of cancer (EORTC); palliative care; supportive oncology; outcomes; pancreas
Introduction
Pancreatic cancer is the fourth leading cause of cancer death in men and women in the United States (1). This cancer is characterized by aggressiveness and high mortality rates that nearly parallel its incidence. This is a challenging disease in many ways. Due to the anatomical location of the pancreas, initial signs of cancer are easily missed by both the patient and the doctor. To date there is no screening method for early detection. As a result, at diagnosis, 30% of patients with pancreatic cancer are unresectable stage 3 locally advanced (2). While there have been some advances in the treatment options of pancreatic cancer, there has only been a dismal increase from 2% to 6% in 5-year pancreatic cancer survival rates from 1975-2008 (1).
When success of treatment options and their impact on traditional outcomes such as progression free survival (PFS) or overall survival (OS) is so limited, the focus of treatment should shift towards better quality-of-life (QoL).
Our study has found that demonstration of an improved QoL, using a well validated tool, of patients while on treatment can predict which patients will have prolonged survival at a stage earlier than most other prognostic/predictive biomarkers currently used in APC. This is a step beyond simply incorporating QoL as an endpoint in cancer trials.
Methods
A total of 50 patients with advanced pancreatic cancer were enrolled in a phase II study of bevacizumab 15 mg/kg, capecitabine 1,300 mg/m2 daily for 2 weeks and gemcitabine 1,000 mg/m2 weekly 2 times; cycles were repeated every 21 days.
All patients provided written informed consent before study enrollment. Adult patients with previously untreated metastatic or locally advanced unresectable pancreatic cancer, Eastern Cooperative Oncology Group (ECOG) PS of 0 or 1, normal blood counts (leucocytes >3,000 per μL, neutrophils >1,500 per μL, platelets >100,000 per μL) and chemistries (bilirubin <2 mg per 100 mL, AST/ALT <5 times upper limits of normal, creatinine <1.5 mg per 100 mL) were included. Prior adjuvant therapy was permitted if completed >6 months before enrollment. Exclusion criteria included proteinuria, pregnancy, lactation, bleeding diathesis, uncontrolled hypertension or cardiovascular disease, brain metastases or recent surgery.
Pretreatment evaluations included complete history and physical exam, complete blood count, chemistry including liver function tests, prothrombin time, pregnancy test for women and 12-lead electrocardiography. Urine protein/creatinine ratio was measured at baseline and every 6 weeks. History and physical exam were performed every 3 weeks. Complete blood count, serum CA 19-9 level and serum chemistries (including liver function tests) were measured on day 1 of each treatment cycle. Computed tomography scans to assess tumor size and response were obtained every 6 weeks.
Gemcitabine was administered in a dose of 1,000 mg/m2 intravenously over 30 min on days 1 and 8; capecitabine 650 mg/m2 twice daily was administered on days 1-14 and bevacizumab 15 mg/kg was administered after gemcitabine on day 1 of a 21-day cycle. Treatment was continued until disease progression, death or toxicity. A maximum of 1 year of bevacizumab therapy was permitted. However, patients could receive gemcitabine and capecitabine beyond 1 year if indicated. Institutional review board approval was obtained for this study.
The PFS was defined as the length of time during and after treatment in which the patient remained alive with cancer without disease progression. OS was defined as the time from treatment initiation until demise. Responses were estimated using the response evaluation criteria in solid tumors (RECIST) (3). QoL was assessed using the EORTC PAN-26 QoL questionnaire which was administered at baseline and then after every treatment cycle.
An exact 95% confidence interval (CI) using the Clopper-Pearson method was given for the rate of improved quality of life. The definition of improved quality of life was as follows: a greater than 5% decrease in two consecutive scores compared with the baseline score. Two sample t-test was used to compare the two survival groups for baseline, 3-week and 6-week quality of life scores. Fisher’s exact test was used to compare the two survival groups for categorical variables. The estimated overall and PFS distributions were obtained using the Kaplan-Meier method. Ninety-five percent CI for the median overall and PFS were calculated using Greenwood’s formula. Statistical assessment of observed differences in the survival distributions between improved and un-improved quality of life groups was done using the log-rank test. A 0.05 nominal significance level was used in all testing. All statistical analyses were done using SAS (version 9.4).
Results
A total of 50 patients from three institutions were enrolled in this study between 7 September 2004 and 3 March 2007. The median follow-up duration was 8-9 months. Median age of the patients was 64 years (range, 38-83 years), 28 males and 22 females, 3/50 (6%) had locally advanced cancer while the remaining 47 (94%) had metastatic disease at the time of enrollment.
A total of 348 cycles were administered. Median number of cycles delivered was 6 (range, 1-18). Reasons for treatment discontinuation in all 50 patients were as follows: one patient completed the 1 year of bevacizumab (2%), 24 patients had disease progression (48%), 18 patients experienced toxicity of the drugs (36%) and 4 patients died while on treatment (8%). Of the last 3 patients (6%), 1 had symptomatic deterioration, 1 had open wounds and 1 was at the discretion of the investigator.
All 50 patients were included in an intention-to-treat survival and response analysis. The radiological responses were independently confirmed by the Response Review Committee. There was a response rate (RR) of 11/50 (22%) in this trial. RR was obtained by adding patients with complete response (CR) and partial response (PR). 30 patients (60%) had stable disease (SD), 5 patients (10%) had progressive disease (PD) and the remaining 4 patients (8%) had clinical disease progression. The median PFS was 5.8 months (95% CI: 4.2-7.8 months) and the median OS was 9.8 months (95% CI: 7.6-11.9 months). 1-year survival was 35.5% (95% CI: 21.7-49.5%) and 1-year PFS was 19% (95% CI: 9.4-31.6%).
Patients who suffered Grade 3/4/5 toxicities during the first two cycles of treatment, defined as neutropenia, thrombocytopenia, thromboembolic events, hypertension and hemorrhage, were divided into two groups according to 6-month survival as shown in Table 1. There was no significant difference between the frequency of grade 3/4/5 toxicities suffered by patients in the two survival groups after cycle 1 and cycle 2 of treatment, with P value of 0.6997 and 0.4660 respectively.
Table 1. Survival and toxicities in patients in cycle 1 and cycle 2.
| Cycle | Survival | Level | Grade 3/4/5 toxicity |
Overall, n (%) | P value | |
|---|---|---|---|---|---|---|
| No, n (%) | Yes, n (%) | |||||
| 1 | OS 6 mo | <6 mo | 12 (32.4) | 2 (20.0) | 14 (29.8) | 0.6997 |
| ≥6 mo | 25 (67.6) | 8 (80.0) | 33 (70.2) | |||
| 2 | OS 6 mo | <6 mo | 10 (30.3) | 2 (16.7) | 12 (26.7) | 0.466 |
| ≥6 mo | 23 (69.7) | 10 (83.3) | 33 (73.3) | |||
OS, overall survival; mo, months.
QoL was assessed using the EORTC PAN-26 QoL questionnaire which was administered at baseline and then after every treatment cycle. The lower the score, the better the quality of life. QoL was considered improved if there was a >5% decrease in two consecutive scores compared with baseline, unimproved if none or ≤5% decrease and not-evaluable if less than three questionnaires were filled. A total of 28 patients showed improvement (56%), 12 patients showed no improvement or unimproved (24%) and 10 patients were not evaluable (20%).
Therefore among the 40 patients whose QoL could be assessed, the improvement rate was 0.7% (95% CI: 0.53-0.83) with P value (one sample proportion test comparing with 0.5 or 50% improvement rate) of 0.017. Among ‘improved’ individuals mean duration (until the score less than 5% decrease after showing the first improvement) was 3.0 survey times (median 2.0, SD 1.53) and mean number of showing the score greater than 5% decrease throughout the study was 5.7 survey times (median 5.0, SD 3.6). Average time between surveys was 22.45 days (Median 21.93). Thus, 3.2 survey times can be translated to 71.84 days.
Median PFS for patients with unimproved QoL was 6.6 months (95% CI: 2.2-8.3) and for patients with improved QoL it was 7.1 months (95% CI: 4.5-9.8) with log rank test P value of 0.641 (Figure 1A).
Figure 1.
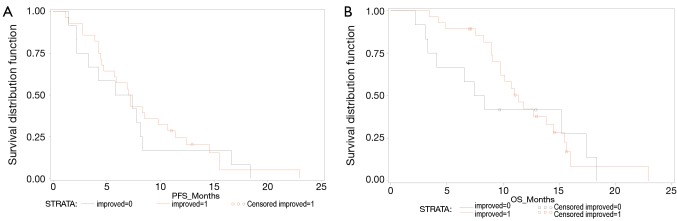
(A) Progression free survival (PFS) curve. Unimproved (black), improved (red); (B) Overall survival (OS) curve. Unimproved (black), improved (red).
Median OS for patients with unimproved QoL was 7.9 months (95% CI: 3.1-17.4) and for patients with improved QoL it was 11.3 months (95% CI: 9.1-14.5) with log rank test P value of 0.5501 (Figure 1B).
QoL plot was formulated as shown in Figure 2, representing data from the 46 patients who had an evaluable response to treatment. Of note, the QoL score of 96 is worst and 0 is best.
Figure 2.
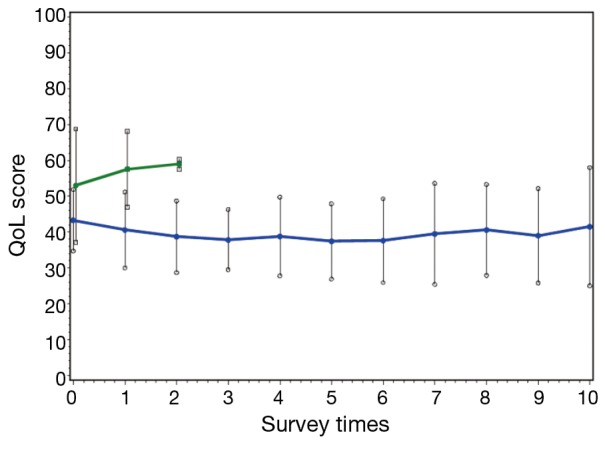
Quality-of-life (QoL) plot. At baseline (time 0): progressive disease (PD) (green) represents 5 pts, and complete response (CR) + partial response (PR) + stable disease (SD) (blue) represents 41 pts, total of 46 pts data. Vertical lines for each point indicate standard deviation. For Green, the last point (at 2) has only 2 pts. For Blue, the last point (at 10) has about 10 pts data. Average days between surveys are 22.5 days.
QoL analysis: (total 40 evaluable patients)
Using rate of QoL improvement, no significant difference was seen in patients with OS ≥6 months compared to OS <6 months (P=0.1680), as shown in Table 2.
Table 2. QoL analysis.
| Survival | Level | QoL |
Overall | P value | |
|---|---|---|---|---|---|
| Improved | Unimproved | ||||
| OS 6 mo | <6 mo | 3 | 4 | 7 | 0.168 |
| ≥6 mo | 25 | 8 | 33 | ||
| Total | 28 | 12 | 40 | ||
QoL, quality-of-life; OS, overall survival; mo, months.
Score comparison by visits: (note: The lower score, the better QoL)
QoL scores at initial visit were not related to survival, however QoL scores at visit 2 and visit 3 correlated strongly with ≥6 month survival and achieved statistical significance (Visit 2: P=0.0092; Visit 3: P=0.0081), as shown in Table 3 and Figure 3A-C.
Table 3. QoL scores at subsequent clinic visits, correlated with ≥6 mo survival.
| Visit | 95% two-sided CL | QoL scores |
P value | |
|---|---|---|---|---|
| ≥6 mo survival | <6 mo survival | |||
| 1 (initial day of treatment) | Lower | 0.088 | ||
| Mean | 39.314 | 39.705 | ||
| Std Dev | 6.0914 | 9.8603 | ||
| Mean estimate | ||||
| Mean | 42 | 53.857 | ||
| Std Dev | 7.5746 | 15.302 | ||
| Upper | ||||
| Mean | 44.686 | 68.009 | ||
| Std Dev | 10.019 | 33.695 | ||
| Std Err | 1.3186 | 5.7835 | ||
| Total | 33 | 7 | ||
| 2 (3 weeks into treatment) | Lower | 0.0092 | ||
| Mean | 35.635 | 45.359 | ||
| Std Dev | 8.6039 | 6.6266 | ||
| Mean estimate | ||||
| Mean | 39.759 | 56.5 | ||
| Std Dev | 10.842 | 10.616 | ||
| Upper | ||||
| Mean | 43.883 | 67.641 | ||
| Std Dev | 14.663 | 26.037 | ||
| Std Err | 2.0133 | 4.334 | ||
| Total | 29 | 6 | ||
| 3 (6 weeks into treatment) | Lower | 0.0081 | ||
| Mean | 33.124 | 40.988 | ||
| Std Dev | 6.6836 | 5.8808 | ||
| Mean estimate | ||||
| Mean | 36.481 | 49.429 | ||
| Std Dev | 8.487 | 9.1261 | ||
| Upper | ||||
| Mean | 39.839 | 57.869 | ||
| Std Dev | 11.631 | 20.096 | ||
| Std Err | 1.633 | 3.4493 | ||
| Total | 27 | 7 | ||
QoL, quality-of-life; mo, months; CL, confidence limits.
Figure 3.
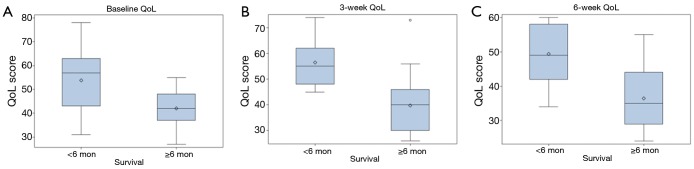
A Quality-of-life (QoL) scores at visit 1 (initial day of treatment); (B) QoL scores at visit 2 (3 weeks into treatment); (C) QoL scores at visit 3 (6 weeks into treatment).
Discussion
This study found that gemcitabine, capecitabine and bevacizumab in patients with APC was associated with median PFS of 5.8 months, median OS of 9.8 months and improved QoL. Baseline score and change in QoL scores were not predictive of survival ≥6 months. However post treatment QoL scores at 3 and 6 weeks from start of therapy were predictive of survival ≥6 months suggesting the potential predictive value of this tool.
The results support our original hypothesis that better QoL can be associated with improved survival in patients with APC, more specifically once the patients have started receiving treatment. This provides the backbone for introducing QoL as a predictive biomarker in pancreatic cancer. Predictive markers are the most clinically informative, since they directly influence patient outcomes by optimizing therapy (4).
Traditionally the choice to undergo cytotoxic therapy in pancreatic cancer is based on analysis of various prognostic and predictive markers such as patient’s age, performance status, baseline albumin levels, WBC, BUN, bilirubin, AST, LDH, CRP and CA 19-9 (5-7). However, when there are many treatment options available, all with associated risks and toxicities, and the benefits to the patient remain unclear, as with APC, there is a need for alternate markers to further stratify these patients and help drive the decision of who would be a better candidate for chemotherapy. As an example, patients with ECOG PS of 0 and 1 have increased chances of favorable outcomes on chemotherapy as compared to patients with ECOG PS of ≥2. By the addition of QoL in our trial we were able to additionally classify prognosis even amongst patients with ECOG PS 0-1.
While increasingly sophisticated methods are being employed for such further stratifications, such as assessment of genetic mutations that may predict response to certain chemotherapies in APC, namely mutations in BRCA1, BRCA2 (8) and the PALB2 gene (9), QoL scoring remains a simple yet greatly underestimated tool for guiding therapy in patients with APC, and perhaps for all kinds of cancers. This is not entirely unexpected as QoL still struggles to find its place as a designated endpoint in cancer trials, let alone being taken a step further, as in our study, to guide patient management. In 2006, Panzini et al. analyzed 405 randomized, controlled clinical trials (RCCTs) according to the level of importance of QoL as a measure of outcome (primary, important and secondary) and found the disappointing conclusion that more attention to QoL in all components of RCCTs (design, choice of instruments, data management and processing) was required from both clinicians and statisticians (10).
The strengths of this study lie in the fact that this is a prospective, multicenter trial which utilized a well validated tool for measuring QoL, the European organization for research and treatment of cancer (EORTC) PAN 26 QoL questionnaire (11). Unlike other biomarkers derived from blood or radiological imaging, which may be invasive and costly, measurement of QoL is quick, free of cost and allows patients to contribute significantly to their own care. Furthermore, no other biomarkers can give reliable predictive information at such precise points in time and in such a short interval from the start of treatment, such as 3 and 6 weeks as demonstrated in our trial. Patients have typically already suffered toxicities and increased morbidity over months before traditional markers are able to predict unfavorable outcomes and treatment ceased.
As clinicians typically neglect QoL, they instead use surrogate markers such as toxicities to decide whom to exclude from treatment. As shown in our results, grade 3/4/5 toxicities suffered by patients was in no manner predictive of 6-month survival, highlighting this as a poor replacement of better predictive and prognostic tools available.
Some physicians may argue that QoL remains a highly subjective measure and dependent on individual needs; for example lack of sexual drive scored more leniently by elderly patients compared to the younger. However it is to be noted that the landmark trial conducted by Burris et al. in 1996, which led to gemcitabine becoming the reference regimen for APC, used OS and improvement in tumor-related symptoms, including pain, as endpoints. The authors note that at the time of the study had a disease-specific QoL instrument been available, it could have given them a way to measure both disease-related as well as drug-related symptoms (12). More recently in 2011, QoL was again used as a measurable end point in a study comparing the combination chemotherapy consisting of oxaliplatin, irinotecan, fluorouracil, and leucovorin (FOLFIRINOX) to gemcitabine as first-line therapy in patients with metastatic pancreatic cancer (13). These trials prove that measurement of QoL is not only undoubtedly important but is also extremely feasible as they were conducted across multiple institutions.
Also, by placing the emphasis on better QoL physicians are making sure that patients are able to carry out their lives in a manner as comfortable as possible and can then address psychosocial aspects that often get neglected in cancer patients or end-of-life care. When physical symptoms and suffering are controlled, it is easier to address patients’ concerns regarding psychological integrity, their families and about finding meaning in their lives. Enhanced understanding of the common psychological concerns of patients with serious illness can improve not only the clinical care of the patient, but also the physician’s sense of satisfaction and meaning in caring for the dying (14).
We are in an era where there is emphasis on informed decision making based on all facts bring provided to the patient. Measuring outcomes with validated tools are essential to communicate the measured rather than perceived impact on QoL. Classically physician’s interpretation relies on frequency of side effects rather than the psychosocial impact the diagnosis, complications, available support and treatment have.
As for future directions, the predictive value of QoL scores need to be studied further in the context of multiple chemotherapy regimens compared against each other. This way, when scores in the first few weeks remain unimproved, clinicians can give patients the choice to either cease treatment or switch to a different regimen. The effect of the new treatment should then again be continually assessed and measured in terms of improved or unimproved QoL. This can be accomplished if future comparative studies of various chemotherapy regimens for pancreatic cancer are structured to incorporate analysis of QoL at different stages during the trial.
Acknowledgements
We thank Roswell Park Cancer Institute, Buffalo, NY. We acknowledge National Comprehensive Cancer Network and Genentech for study support.
Disclosure: The authors declare no conflict of interest.
References
- 1.American Cancer Society. Cancer Statistics 2013. Available online: http://www.cancer.org/research/cancerfactsstatistics/
- 2.Faisal F, Tsai HL, Blackford A, et al. Longer Course of Induction Chemotherapy Followed by Chemoradiation Favors Better Survival Outcomes for Patients With Locally Advanced Pancreatic Cancer. Am J Clin Oncol 2013. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [DOI] [PubMed] [Google Scholar]
- 4.Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol 2013;107:15-22. [DOI] [PubMed] [Google Scholar]
- 5.Pant S, Martin LK, Geyer S, et al. Baseline serum albumin is a predictive biomarker for patients with advanced pancreatic cancer treated with bevacizumab: a pooled analysis of 7 prospective trials of gemcitabine-based therapy with or without bevacizumab. Cancer 2014;120:1780-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas M, Heinemann V, Kullmann F, et al. Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. J Cancer Res Clin Oncol 2013;139:681-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stocken DD, Hassan AB, Altman DG, et al. Modelling prognostic factors in advanced pancreatic cancer. Br J Cancer 2008;99:883-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowery MA, Kelsen DP, Stadler ZK, et al. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist 2011;16:1397-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, et al. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther 2011;10:3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panzini I, Fioritti A, Gianni L, et al. Quality of life assessment of randomized controlled trials. Tumori 2006;92:373-8. [DOI] [PubMed] [Google Scholar]
- 11.Bassi C, Johnson C, Fitzsimmons D, et al. Quality of life assessment in pancreatic carcinoma: results of an European multicentric study. Chir Ital 1999;51:359-66. [PubMed] [Google Scholar]
- 12.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [DOI] [PubMed] [Google Scholar]
- 13.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [DOI] [PubMed] [Google Scholar]
- 14.Block SD. Perspectives on care at the close of life. Psychological considerations, growth, and transcendence at the end of life: the art of the possible. JAMA 2001;285:2898-905. [DOI] [PubMed] [Google Scholar]


