Abstract
Introduction
Lymphoma of the mucosa-associated lymphoid tissue (MALT) has been used to describe a marginal zone B-cell lymphoma derived from gastrointestinal lymphoid tissue. mucosa-associated lymphoid tissue lymphoma (MALToma) of the ileum are extremely rare and only few reports with endoscopic features have been reported.
Case study
We present a 55-year-old male patient with history of abdominal pain and loss of appetite since 2½ years. Abdomino-pelvic ultrasonography was normal, but computed tomography (CT) scan of the abdomen showed, dilated segment of ileum containing both contrast and debris. He underwent segmental resection of ileum associated with stricture site, histopathology of which revealed MALToma of ileum. Patient was subsequently treated with low dose chemotherapy and strictly followed up.
Discussion
Primary treatment possibility should be considered as the treatment of H. pylori infection while surgical resection for superficial lesions followed by low dose chemotherapy is recommended. The present case report explore MALToma of the GI tract, its diagnostic criterions, role of radiological and pathological tools, various investigative techniques and role of surgery and chemotherapy in such cases.
Keywords: Mucosa-associated lymphoid tissue lymphoma (MALToma) of ileum, MALT lymphomas (MALToma) of the stomach, anastomosis
Introduction
Since its first proposal in 1983 by Isaacson and Wright, the concept of mucosa-associated lymphoid tissue (MALT) has become established as a distinct clinical pathologic entity (1,2). Extranodal marginal zone lymphoma of MALT lymphoma is a rare variant of non-Hodgkin’s lymphoma and is characterized by the presence of clear cytoplasm or “cytoplasm inclusion bodies” that displace the nucleus to the periphery of the cell, giving a signet ring cell appearance. It is mainly characterized by morphologically heterogenous small B-cells including marginal (centrocyte like) cells, cell resembling monocytoid cells, small lymphocytes, scattered immunoblasts, centroblast-like cells, invasion of lymphoma cells around the epithelium (lympho-epithelial lesions) and proliferation of plasma cells in the lamina propria of the mucosa (3,4). Clinical presentation of MALToma is often insidious but its clinical behavior has been reported as favorable as a low-grade lesion and disease tends to remain localized for a continued aeon of time (5). Ileal involvement is an extremely rare entity and presentation within an area of focal anti-mesenteric ileal wall dilatation has been reported only in infinitesimal cases in the literature. There is a very little known about the pathogenesis of the MALToma but the overall 5-year survival for MALToma is documented as 81% (3).
Case report
A 55-year-old male patient presented with a history of abdominal pain and loss of appetite since 2½ years. Pain was associated with off and on vomiting and constipation. Patient was chronic bidi smoker but non-alcoholic with a history of significant sun exposure throughout his life. He had a history of significant weight loss. There was no significant past, medical or surgical history. General physical and systemic examination was normal. Complete hemogram and routine blood biochemistry parameters of the patient were within normal limits. A chest radiograph did not indicate any metastatic nodules. Abdomino-pelvic ultrasonography of the patient was normal. A computed tomography (CT) scan of the abdomen following an episode of acute abdominal pain and vomiting demonstrated a 3.8 cm dilated segment of ileum containing both contrast and debris. Patient underwent segmental resection of ileum including the stricture site with end-to-end anastomosis.
Histological examination
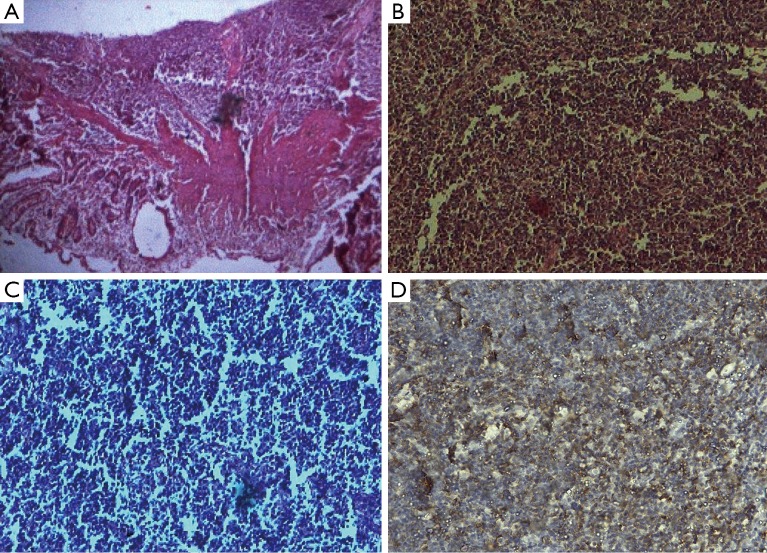
Histopathology of the resected segment revealed extensive infiltration by small atypical monomorphic lymphoid cells, infiltration extending up to mucosa, sub-mucosa and muscularis layer suggesting non-Hodgkin lymphoma-MALToma. Immunohistochemistry showed positivity for CD20 (cyclin D20) and negative CD1, CD3, CD5, CD1 and Bcl2 (Figure 1).
Figure 1.
Photomicrograph Hematoxylin-Eosin (H&E) stain. Original magnification ×100 (A); and magnification ×400 (B,C)—with extensive infiltration by small atypical monomorphic lymphoid cells characteristics of MALToma, infiltrating up to mucosa, sub-mucosa and muscularis layer at periphery and immunohistochemical study illustrating the neoplastic cells wide spread positivity for tumor cells positive for CD20 (D). MALToma, mucosa-associated lymphoid tissue lymphoma.
The patient received six-cycle of three-weekly post-operative chemotherapy with standard CHOP regimen (cyclophosphamide: 750 mg/m2, doxorubicin hydrochloride: 50 mg/m2, vincristine sulphate: 1.4 mg/m2 and prednisolone: 100 mg) (5,6). After one year of post-treatment follow up, patient is asymptomatic and his clinical, biochemical and radiological examination were unremarkable.
Discussion
Usually stomach is the most frequent site for MALTomas. For patients with MALToma a full systemic staging workup is indicated. Though the majority of cases are localized, it is important to conduct a thorough staging evaluation since up to one-third of patients have disseminated disease at diagnosis. Because MALToma can involve such a wide variety of organs, the treatment strategy has to be tailored to each specific site. Sometimes the disease may not form a visible tumor mass. Therefore, if MALToma is considered in the differential diagnosis for a patient undergoing endoscopy, multiple random systematic biopsies within the stomach and adjacent areas are warranted to optimize diagnostic accuracy for the specific site. In addition, the biopsy samples should be sent for immunophenotypic analysis (7). There is no specific marker for MALToma presently but main tumor cells of MALToma are: CD20+, CD5–, CD10–, CD23–, CD43±. During differential diagnosis, lack of CD5 and CD10 is useful in doing the distinction from mantle cell lymphoma and follicular lymphoma. The absence of characteristic markers for those neoplasms is warranted during differential diagnosis with other small B-cell lymphomas (6).
One riveting aspect of gastric MALToma is its link to a bacterial infection as an etiologic factor. Infection by Helicobacter pylori, among one of the common pathogen seen in the stomach, leads to formation of lymphoid tissue within the stomach (7). Other observations accept led to the antecedent that H. pylori act as an antigenic stimulus leading to the formation of acquired gastric lymphoid tissue, which ultimately evolves into blast transformation and tumor cell proliferation. The T-cell dependence explains, in part, about tendency of gastric MALToma to remain localized to the stomach as the tumor cells demand the manner of gastric T-cells for growth stimulation.
Given the growing evidence of the efficacy of antibiotic therapy, it is equitable to treat H. pylori positive patients with standard triple course regimen containing antibiotics; these patients require post-treatment close follow-up strictly for any possible lymphoma relapse. Patients, who relapse after the triple course therapy, generally are treated with radiotherapy. In spite of the fact that the behaviors of MALToma being similar to the normal variant lymphoma, it is important the distinction of the different entities (6). Many reports have approved the accord that for absolute diagnosis, careful follow-up examinations such as laboratory analyses, histopathology examination with immunohistochemical study is required. Fortunately, despite the rarity of this condition, the treatment for MALToma is similar to other lymphoma, depending on location and stage of the tumor. A solitary focally dilated segment of ileal wall may be neoplastic in attributes and surgical resection and chemotherapy needs to be considered (8).
Conclusions
MALToma is the third most common non-Hodgkin’s lymphoma subtype, MALToma of the ileum, however, is rare. Primary treatment possibility should be considered as the treatment of H. pylori, which plays an important role in pathogenesis of MALToma. The diagnosis is only made intra-operatively for intestinal obstruction making timely clinical diagnosis challenging. Patients require extended follow-up due to the potential for local relapse. Surgical resection for superficial lesions is an attractive option as it is associated with very low morbidity and should be followed by locally directed low dose chemotherapy considering its neoplastic nature.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Isaacson P, Wright DH, Jones DB. Malignant lymphoma of true histiocytic (monocyte/macrophage) origin. Cancer 1983;51:80-91. [DOI] [PubMed] [Google Scholar]
- 2.Du MQ, Isaccson PG. Gastric MALT lymphoma: from aetiology to treatment. Lancet Oncol 2002;3:97-104. [DOI] [PubMed] [Google Scholar]
- 3.Storey R, Gatt M, Bradford I.Mucosa associated lymphoid tissue lymphoma presenting within a solitary anti-mesenteric dilated segment of ileum: a case report. J Med Case Rep 2009;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terada T.Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) of the ileum in a 35-year-old Japanese woman. Int J Clin Exp Pathol 2013;6:951-6. [PMC free article] [PubMed] [Google Scholar]
- 5.Makino Y, Suzuki H, Nishizawa T, et al. Ileal Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma with a Large-Cell Component That Regressed Spontaneously. Gut Liver 2010;4:117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katic V, Katic K, Vucic M, et al. The histopathology and immunohistology of gastric MALT lymphoma. Arch Oncol 2004;12:5-6. [Google Scholar]
- 7.Fung CY, Grossbard ML, Linggood RM, et al. Mucosa-associated lymphoid tissue lymphoma of the stomach: long term outcome after local treatment. Cancer 1999;85:9-17. [DOI] [PubMed] [Google Scholar]
- 8.Wotherspoon AC. Extragastric MALT lymphoma. Gut 2002;51:148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]