Abstract
Background
Gemcitabine plus platinum (GEM-P) combination chemotherapy is standard treatment for first-line advanced cholangiocarcinoma (aCC). GEM-P first-line therapy reports a progression-free survival (PFS) of 8 months and overall survival (OS) of 11.7 months. Treatment in the second-line setting is less clear. Five-year survival for aCC remains dismal at 5-10%. The purpose of this study was to describe the outcomes with second-line systemic treatment at our institution.
Methods
This study was a single institution retrospective chart review of aCC patients who initiated second-line systemic treatment during 1/1/2009 to 12/31/2012. The primary objective was to evaluate PFS with second-line systemic treatment. Secondary objectives were OS and disease control rate. Second-line systemic regimens were classified into four treatment groups: GEM-P, gemcitabine + fluoropyrimidine (GEM-FU), other FU combination (FU-combo), and others.
Results
Fifty-six patients were included and the majority had intrahepatic aCC. A total of 80% received first-line gemcitabine-based therapy. Second-line therapy consisted of GEM-P (19.6%), GEM-FU (28.6%), FU-combo (37.5%), and others (14.3%). Median PFS was 2.7-month (95% CI, 2.3-3.8 months) with a median OS of 13.8 months (95% CI, 12-19.3 months) and a disease control rate of 50%. No significant difference in survival was identified between the four treatment groups.
Conclusions
This study revealed a 2.7-month PFS, 50% disease control rate, and potential survival benefit with second-line treatment. Options for second-line systemic therapy include GEM-FU, FU-combo, GEM-P if not given in the first-line setting. Targeted therapy with erlotinib or bevacizumab could be considered in addition to chemotherapy.
Keywords: Cholangiocarcinoma, metastatic, chemotherapy, biotherapy, second-line
Introduction
Biliary tract cancers (BTC) are a heterogeneous group of rare malignancies that include gallbladder cancer, ampullary cancer, and intrahepatic, hilar, and extrahepatic cholangiocarcinoma. In the United States, there are estimated to be 10,310 new cases in 2013 with 3,230 estimated deaths (1). Cholangiocarcinomas, tumors that originate in the bile duct epithelium, are further sub-classified into intrahepatic, extrahepatic, and hilar depending on the location of the primary tumor in the biliary tree. Due to the rarity of these malignancies, trials often combine these heterogeneous BTC, making it difficult to determine patient outcomes and therapeutic decisions specifically for cholangiocarcinoma.
Curative treatment has historically been with surgical resection, with extrahepatic cholangiocarcinoma having the highest chance for resectability (2,3). For extrahepatic cholangiocarcinoma patients who have undergone curative resection, the 5-year survival rate ranges from 16-50% (2). Unfortunately, the majority of cholangiocarcinoma cases are unresectable portending a poor prognosis. In these patients, overall 5-year survival rates are reported at 5-10% (3). Systemic chemotherapy is considered the mainstay of treatment for unresectable cholangiocarcinoma. Gemcitabine plus platinum (GEM-P) is commonly selected as first-line treatment in advanced cholangiocarcinoma (aCC) based on the results of the ABC-02 trial. This was a phase III study that compared gemcitabine plus cisplatin versus gemcitabine monotherapy in patients with unresectable, recurrent, or metastatic BTC (intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, gallbladder cancer, or ampullary cancer) (4). The median PFS was 8 months in the doublet chemotherapy group versus 5 months in the gemcitabine only group (P<0.001) and the median survival was 11.7 versus 8.1 months, respectively (P<0.001). Treatment options for aCC after first-line progression are less defined. The purpose of this retrospective study was to evaluate the outcomes with second-line systemic therapy.
Patient population and methods
We performed a retrospective chart review of aCC patients who received second-line systemic treatment during 1/1/2009 to 12/31/2012 at UT MD Anderson Cancer Center (MDACC). The primary outcome was median progression-free survival (PFS), defined as the start of second-line systemic treatment to radiographic progression or last follow-up. Secondary objectives included disease control rate (complete response + partial response + stable disease evaluated at first restaging imaging) and overall survival (OS). OS was defined as the date of diagnosis to death or last follow-up.
Eligible patients were those who had histopathologically confirmed aCC (including unknown primary with pathology consistent with cholangiocarcinoma), documented progression on first-line therapy, and follow-up reimaging studies after starting second-line therapy at MDACC. Exclusion criteria included localized treatment for aCC prior to second-line therapy or consolidative chemoradiation, mixed histology tumors, and a history of another malignancy. Second-line systemic therapy was classified into four treatment groups: gemcitabine + platinum (GEM-P), gemcitabine + fluoropyrimidine (GEM-FU), FU combination (FU-combo) such as FU plus oxaliplatin or 5-fluorouracil (5-FU) plus irinotecan, and other. Other was defined as targeted therapy or single agent chemotherapy +/- targeted therapy.
Data collection points consisted of patient demographics (age, sex), site of disease (intra, extra, or hilar), date of diagnosis, first-line systemic treatment, progression date of first-line treatment, type and initiation date of second-line systemic treatment, type of chemotherapy regimen, progression date of second-line treatment, baseline carbohydrate antigen 19-9 (CA 19-9), and date of death or last follow-up.
Statistical analysis
For statistical methods, the distribution of each continuous variable was summarized by its mean, standard deviation, median, and range. The distribution of each categorical variable was summarized in terms of its frequencies and percentages. Kaplan-Meier curves were used to estimate the time to event difference regarding treatment regimen (5). The Cox proportional hazards regression model was used to evaluate each variable effect on time to event (6). All computations were carried out in SAS version 9.3.
Results
Fifty-six patients met criteria for analysis with 95% having intrahepatic cholangiocarcinoma. Baseline demographics are summarized in Table 1. Eighty percent of patients received gemcitabine based first-line treatment: GEM-P (64.3%), GEM-P + erlotinib (14.3%) and gemcitabine monotherapy (1.8%). Median follow-up was 38 months. Second-line systemic therapy included GEM-P (19.6%), GEM-FU (28.6%), FU-combo (37.5%), and other (14.3%). Regimens in the other group consisted of capecitabine +/- bevacizumab, gemcitabine +/- bevacizumab or erlotinib, erlotinib + bevacizumab, or erlotinib monotherapy. Baseline characteristics did not significantly differ between the four treatment groups. Overall median PFS was 2.7 months (95% CI, 2.3-3.8). Disease control rate was 50% with a median OS of 13.8 months (95% CI, 12-19.3). OS and PFS for each treatment group are summarized in Table 2. No significant difference in PFS or OS was identified between the four treatment groups (Figures 1,2). For patients who had disease control with second-line therapy, the median duration of disease control was 6.11 months.
Table 1. Patient demographics.
| Demographics | Number (percent or range) |
|---|---|
| Gender | |
| Male | 33 (58.9%) |
| Female | 23 (41.1%) |
| Disease site | |
| Intrahepatic | 53 (94.6%) |
| Extrahepatic | 1 (1.9%) |
| Hilar | 2 (3.7%) |
| 1st line chemotherapy | |
| Gemcitabine + cisplatin | 36 (64.3%) |
| Gemcitabine + cisplatin + erlotinib | 8 (14.3%) |
| Gemcitabine | 1 (1.8%) |
| Other* | 11 (19.6%) |
| Median baseline CA 19-9 at diagnosis | 179.3 U/mL (1.2-15,998) |
| Median CA 19-9 at 2nd line treatment initiation | 146.4 U/mL (1.9-8,666) |
| Median CA 19-9 at 2nd line progression | 210.9 U/mL (1.1-29,409) |
*Other regimens: FOLFOX, FOLFIRINOX, gemcitabine + capecitabine, carboplatin + paclitaxel; CA 19-9, carbohydrate antigen 19-9.
Table 2. Second-line systemic treatment groups.
| Group | Number of patients (%) | Median PFS (months; CI) | Median OS (months; CI) |
|---|---|---|---|
| GEM-P | 11 (19.6) | 3.8 (2.3-8.2) | 12.3 (10-40.7) |
| GEM-FU | 16 (28.6) | 2.6 (1.8-3.9) | 15.1 (8.6-22) |
| FU-combo | 21 (37.5) | 2.5 (1.8-4.6) | 14.7 (9.6-23.5) |
| Other* | 8 (14.3) | 2.8 (2-9) | 20.9 (1.8-N/A) |
*GEM-P, gemcitabine plus platinum; GEM-FU, gemcitabine plus fluoropyrimidine; FU-combo, fluoropyrimidine combination; CI, confidence interval.
Figure 1.
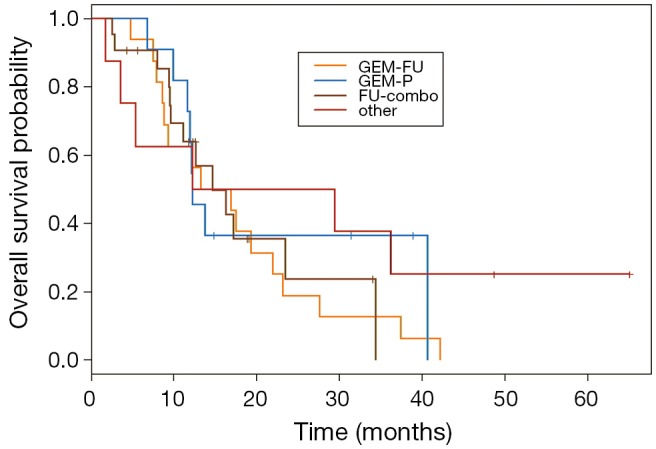
OS Kaplan Meier for four second-line systemic groups. OS, overall survival. GEM-FU, gemcitabine plus fluoropyrimidine; GEM-P, gemcitabine plus platinum; FU-combo, fluoropyrimidine combination.
Figure 2.
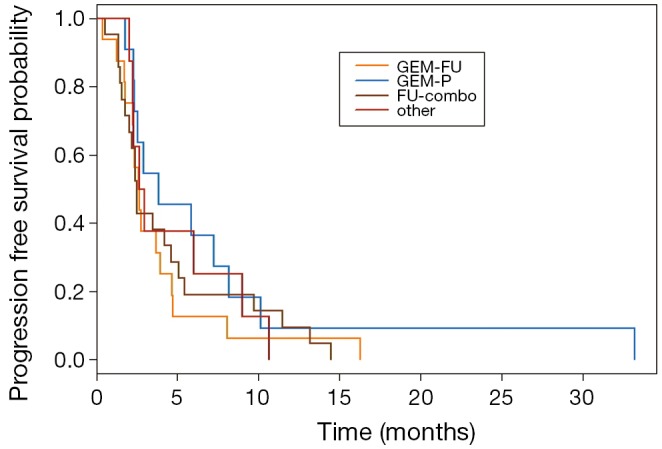
PFS Kaplan Meier for four second line systemic groups. PFS, progression-free survival. GEM-FU, gemcitabine plus fluoropyrimidine; GEM-P, gemcitabine plus platinum; FU-combo, fluoropyrimidine combination.
OS of our patients who received second-line systemic therapy was higher in patients who achieved disease control while on first-line systemic treatment compared to those who progressed at their first restaging evaluation (median OS of 22.02 months compared to 9.98 months; P<0.001) (Figure 3). There was also a trend towards an improvement in PFS with second-line therapy (median PFS 3.47 vs. 2.51 months; P=0.18) in patients that had response or stable disease while on first-line systemic treatment (Figure 4). In addition, a higher CA19-9 at the start of second-line treatment was associated with significantly poorer PFS (P=0.03) and OS (P<0.01).
Figure 3.
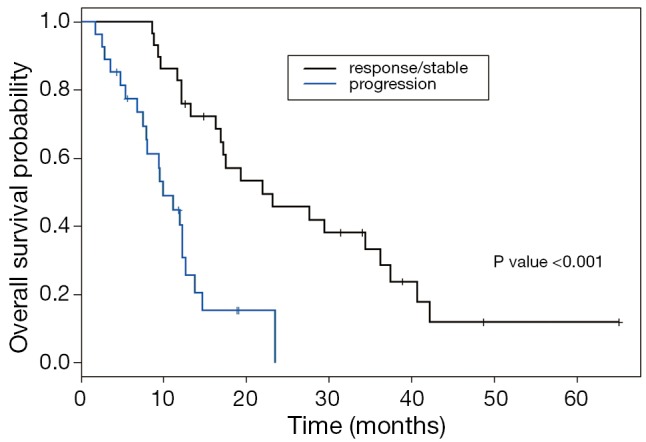
Overall OS Kaplan Meier based on first-line response or progression. OS, overall survival.
Figure 4.
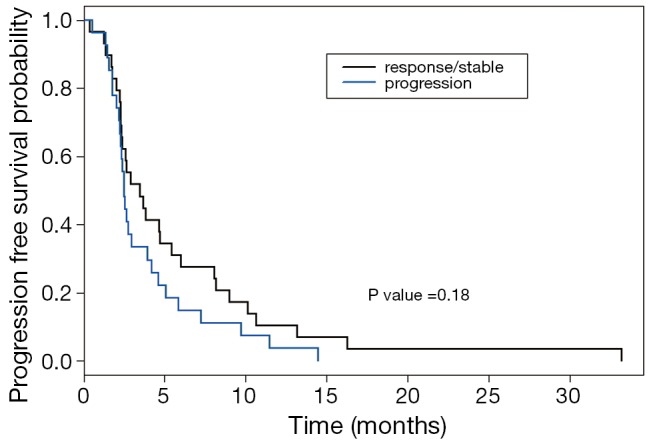
Overall PFS Kaplan Meier based on first-line response or progression. PFS, progression-free survival.
Discussion
Our study revealed a median PFS of 2.7 months, 50% disease control rate, and a potential survival benefit with second-line systemic therapy. No statistical difference was observed between the four treatment groups which may be in part due to the small patient population available for analysis and the various second-line therapies used in our retrospective review. Patients who had improved survival outcomes with second-line therapy in our study were those who had disease control with first-line systemic treatment, suggesting that the tumor biology of these patients were more favorable. Further, our study found that a higher CA 19-9 at the start of second line therapy correlated with worse outcomes. This finding is consistent with existing data regarding CA 19-9 as a potential prognostic marker for BTC (7-9).
Current data in the field of biliary cancers consists of prospective and retrospective evaluations that include all BTC (cholangiocarcinoma, gallbladder cancer, and ampullary cancer), thus making it difficult to delineate specific treatment guidelines for these individual rare tumors. A recent retrospective study evaluated 378 patients with advanced BTC (gallbladder, cholangiocarcinoma, and ampullary cancer) in which 25% received second-line chemotherapy (10). The authors found that those who received second-line chemotherapy were more likely younger patients and had longer PFS on first-line therapy. Regimens identified in the second-line setting were similar to those identified in our study (i.e., GEM-P, 5-FU therapy, other). The authors found similar outcomes with regards to disease control and PFS (disease control: 43%; median PFS =2.8 months). While this study reported an OS of 7.5 months, survival was measured from the start of second-line treatment while our study revealed an OS of 13.8 months measured from the date of diagnosis.
Prospective salvage-line studies in BTC are summarized in Table 3. FU therapy, gemcitabine based therapy, sunitinib, and imatinib are among those studied in the second-line setting (11-20). Median time to progression (TTP) seen with these regimens ranged from 1.6 to 5.5 months. As evidenced by these trials, the lack of a first-line chemotherapy standard in BTC prior to the ABC-02 trial presented a challenge for investigating second-line therapy. This also presented a limitation in our retrospective review. Currently, there are approximately 300 clinical trials for BTC; however, only a limited number focus on second-line treatment for advanced disease (21). Trials in the refractory setting include monotherapy or combination chemotherapy (capecitabine + mitomycin C; DHA-paclitaxel), chemotherapy + targeted therapy (SPI-1620 + docetaxel), and targeted therapy (sunitinib, carbozantinib, pazopanib + trametinib).
Table 3. Salvage therapy in biliary tract cancers (BTC).
| Regimen | Patient population | 1st line therapy | Outcomes |
|---|---|---|---|
| FOLFOX-4 (11) | 37 BTC | GEM-P | Median TTP: 3.1 months; disease control rate: 62.2% |
| FOLFIRI (12) | 44 pancreatic and BTC; 15 aCC; FOLFIRI given 1st or 2nd line; 21 patients received 2nd line | Gemcitabine; GEMOX; FOLFOX; Gem-5-FU | 2nd line outcomes: PFS, 3.5 months; OS, 6.2 months |
| Sunitinib (13) | 56 BTC | Gemcitabine; GEM-P; GEMOX; gemcitabine + carboplatin; 5-FU + cisplatin; 5-FU + carboplatin | TTP: 1.7 months, disease control rate: 50%, median OS: 4.8 months |
| Imatinib (14) | 9 BTC | FOLFIRI; capecitabine + cisplatin; gemcitabine + capecitabine; gemcitabine; GEMOX | Median TTP: 2.8 months, median OS: 4.9 months |
| S-1 (15) | 22 BTC | Gemcitabine | Disease control rate: 50%, median TTP: 5.4 months, median OS: 13.5 months |
| GEM-P (16) | 22 BTC | Gemcitabine + S-1 | Disease control rate: 70%, median TTP: 3.6 months, median OS: 5.9 months |
| Gemcitabine (17) | 32 BTC | 5-FU | Median TTP: 1.6 months, median OS: 4.1 months |
| S-1 (18) | 45 BTC;16 patients received as 2nd line | Gemcitabine | 2nd line outcomes: disease control rate, 43.8%; median TTP, 5.5 months; median OS, 8 months |
| conti-FAM (19) | 31 pancreatic and BTC; 11 aCC | Gemcitabine | Median TTP: 2.3 months, median OS: 6.7 months |
| Capecitabine plus celecoxib (20) | 35 pancreatic and BTC | Gemcitabine based therapy | Median PFS: 4.25 months, median OS: 4.75 months |
FOLFOX, 5-FU, leucovorin, oxaliplatin; BTC, biliary tract cancer; GEM-P, gemcitabine + cisplatin; TTP, time to progression; FOLFIRI, 5-FU, leucovorin, irinotecan; aCC, advanced cholangiocarcinoma; GEMOX, gemcitabine + oxaliplatin; GEM-5-FU, gemcitabine + 5-FU; PFS, progression-free survival; OS, overall survival; conti-FAM, continuous 5-FU, doxorubicin, and mitomycin-C.
Conclusions
More research in the aCC population is necessary to establish practice guidelines for active chemotherapy regimens that can improve patient outcomes in both front-line and second-line settings. With a lack of phase III data after first-line progression, selection of appropriate second-line systemic treatment for aCC is difficult. Based on our retrospective study, potential options include GEM-FU, FU-combo, or GEM-P if not received in the first-line setting. Targeted therapy, including erlotinib or bevacizumab, may also be considered in addition to chemotherapy. Prospective randomized clinical trials are needed to confirm the results of our study and further investigate therapeutic options in this population. Trials exploring anti-VEGF therapy, anti-EGFR therapy, multikinase inhibitors, mitogen-activated extracellular kinases (MEK) inhibitors, and other chemotherapy combinations are underway. Healthcare providers are anxiously awaiting the outcomes associated with these agents to help improve the management of cholangiocarcinoma.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Siegel R, Naishadham D, Jemal A.Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 2.NCCN guidelines Heptobiliary Cancers. Version 2.2014. Available online: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
- 3.Ramírez-Merino N, Aix SP, Cortés-Funes H. Chemotherapy for cholangiocarcinoma: An update. World J Gastrointest Oncol 2013;5:171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457-81. [Google Scholar]
- 6.Cox DR. Regression models and life tables (with discussion). Journal of the Royal Statistical Society 1972;34:187-220. [Google Scholar]
- 7.Harder J, Kummer O, Olschewski M, et al. Prognostic relevance of carbohydrate antigen 19-9 levels in patients with advanced biliary tract cancer. Cancer Epidemiol Biomarkers Prev 2007;16:2097-100. [DOI] [PubMed] [Google Scholar]
- 8.Chung MJ, Lee KJ, Bang S, et al. Preoperative serum CA 19-9 level as a predictive factor for recurrence after curative resection in biliary tract cancer. Ann Surg Oncol 2011;18:1651-6. [DOI] [PubMed] [Google Scholar]
- 9.Singal AG, Rakoski MO, Salgia R, et al. The clinical presentation and prognostic factors for intrahepatic and extrahepatic cholangiocarcinoma in a tertiary care centre. Aliment Pharmacol Ther 2010;31:625-33. [DOI] [PubMed] [Google Scholar]
- 10.Walter T, Horgan AM, McNamara M, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer 2013;49:329-35. [DOI] [PubMed] [Google Scholar]
- 11.He S, Shen J, Sun X, et al. A phase II FOLFOX-4 regimen as second-line treatment in advanced biliary tract cancer refractory to gemcitabine/cisplatin. J Chemother 2014;26:243-7. [DOI] [PubMed] [Google Scholar]
- 12.Moretto R, Raimondo L, De Stefano A, et al. FOLFIRI in patients with locally advanced or metastatic pancreatic or biliary tract carcinoma: a monoinstitutional experience. Anticancer Drugs 2013;24:980-5. [DOI] [PubMed] [Google Scholar]
- 13.Yi JH, Thongprasert S, Lee J, et al. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur J Cancer 2012;48:196-201. [DOI] [PubMed] [Google Scholar]
- 14.Roth A, Schleyer E, Schoppmeyer K, et al. Imatinib mesylate for palliative second-line treatment of advanced biliary tract cancer: a bicentric phase II study. Onkologie 2011;34:469-70. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki T, Isayama H, Nakai Y, et al. Multicenter phase II study of S-1 monotherapy as second-line chemotherapy for advanced biliary tract cancer refractory to gemcitabine. Invest New Drugs 2012;30:708-13. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki T, Isayama H, Nakai Y, et al. Feasibility study of gemcitabine and cisplatin combination chemotherapy for patients with refractory biliary tract cancer. Invest New Drugs 2011;29:1488-93. [DOI] [PubMed] [Google Scholar]
- 17.Oh SY, Jeong CY, Hong SC, et al. Phase II study of second line gemcitabine single chemotherapy for biliary tract cancer patients with 5-fluorouracil refractoriness. Invest New Drugs 2011;29:1066-72. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T, Isayama H, Yashima Y, et al. S-1 monotherapy in patients with advanced biliary tract cancer. Oncology 2009;77:71-4. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Oh SY, Kim BG, et al. Second-line treatment with a combination of continuous 5-fluorouracil, doxorubicin, and mitomycin-C (conti-FAM) in gemcitabine-pretreated pancreatic and biliary tract cancer. Am J Clin Oncol 2009;32:348-52. [DOI] [PubMed] [Google Scholar]
- 20.Pino MS, Milella M, Gelibter A, et al. Capecitabine and celecoxib as second-line treatment of advanced pancreatic and biliary tract cancers. Oncology 2009;76:254-61. [DOI] [PubMed] [Google Scholar]
- 21.Biliary tract cancers. Available online: www.clinicaltrials.gov, accessed 2.28.14.


