Abstract
As the latest addition to the sub-strain specific WHO Reference Reagents of BCG vaccine, an international collaborative study was completed to evaluate the suitability of a candidate BCG Moreau-RJ sub-strain as a WHO Reference Reagent of BCG vaccine. This follows the recent replacement of the WHO 1st International Reference Preparation for BCG vaccine, by three sub-strain specific WHO Reference Reagents of BCG vaccine (Danish 1331, Tokyo 172-1 and Russian BCG-I) in order to complete the coverage of most predominant sub-strains used for BCG vaccine production and distribution for use worldwide. The study used cultural viable count and modified ATP assays to quantify the preparation and multiplex PCR to confirm the identity of the sub-strain. The establishment of this WHO Reference Reagent of BCG vaccine of Moreau-RJ sub-strain was approved by the WHO Expert Committee on Biological Standardization meeting in October 2012. This preparation is available for distribution by NIBSC-MHRA, UK. The data from real-time stability monitoring demonstrated that these Reference Reagents of BCG vaccine are very stable in storage condition at −20 °C. They serve as the valuable source of BCG Reference Reagents for use as comparators (1) for viability assays (such as cultural viable count and modified ATP assays); (2) for in vivo assays (such as the absence of virulent mycobacteria, dermal reactivity and protection assays) in the evaluation of candidate TB vaccines in non-clinical models; (3) for identity assays using molecular biology techniques.
Keywords: WHO, Reference reagent, BCG, Vaccine, Viability, ATP, Stability
1. Introduction
Based on the recommendations from international experts in three WHO consultation meetings [1–3] on BCG vaccine, the WHO 1st International Reference Preparation (IRP) for BCG vaccine established in 1965 has been replaced with sub-strain specific BCG Reference Reagents (RRs). They are the BCG Danish 1331, Russian BCG-I and Tokyo 172-1 and they are available for distribution from NIBSC-MHRA (http://www.nibsc.org; NIBSC code: 07/270, 07/272, 07/274 respectively) since 2010. These preparations represent some of the predominant sub-strains used for BCG vaccine production and distribution for use worldwide. Attempts to source the Moreau sub-strain, which would have completed the worldwide coverage, were not successful at the time. The required material was subsequently sourced and the candidate preparation was ampoule-filled for preserving long-term stability. Reference preparations are essential to both vaccine manufacturers and National Control Laboratories in order to monitor quality control assay consistency. They may also be used as a comparator or reference in research and pre-clinical studies for the development and evaluation of new tuberculosis (TB) vaccines. An international collaborative study using two independent viability assays and an identity assay was carried out to evaluate the content and suitability of this candidate as WHO RR of BCG vaccine of Moreau RJ sub-strain.
BCG vaccine is a live attenuated strain of Mycobacterium bovis. Viability of the bacilli is critical for the stimulation of cellular immune responses that provide protection against M. tuberculosis; thus the effectiveness of the BCG vaccine. The cultural viable count assay is not strictly a measure of potency but it is commonly used as a surrogate marker for potency of BCG vaccines. In recent years, a modified ATP assay has been evaluated and adopted as an appropriate alternative method for estimating viability of BCG vaccines [4–7]. The multiplex PCR (mPCR) assay, a molecular biology technique, has been introduced as a quality control test for identity of BCG vaccine [8]. This is a useful method to distinguish between different sub-strains of BCG that are currently being used in vaccine production. Specific regions of BCG, RD1, 2, 8, 14 and 16 have been successfully employed to produce a fingerprint that differentiates between sub-strains. The SenX3-RegX3 mycobacterial two-component system (responsible for the virulence and phosphate dependant gene expression of M. tuberculosis) has also been identified as a target site for use in identifying BCG sub-strains [8]. This assay has been successfully evaluated in a collaborative study as a molecular identity test for different sub-strains of BCG vaccine [9].
As in a previous collaborative study [10], three independent methods were used to evaluate the suitability of BCG Moreau-RJ sub-strain as a WHO Reference Reagent. Its content was defined as number of Colony Forming Units (CFU) and amount of ATP (ng) per ampoule. Multiplex PCR was used to identify the BCG sub-strain. The study report was approved by the WHO Expert Committee on Biological Standardization (ECBS) in October 2012 and this WHO Reference Reagent of BCG vaccine of Moreau RJ sub-strain has been made available for distribution since 2013. As these BCG Reference Reagents are live preparations, their stability in terms of viability has been monitored in NIBSC annually to ensure these preparations maintain their viability within an acceptable range at time of distribution.
2. Materials and methods
2.1. Study materials
The BCG vaccine preparation of Moreau-RJ sub-strain was obtained lyophilized and sterile-filled in ampoules at commercial manufacturing facility with Good Manufacturing Practices (GMP). Five thousand ampoules were generously donated by a well-established BCG vaccine manufacturer (Fundacao Ataulpho de Pavia, Brazil) to WHO. This preparation (NIBSC code: 10/272) was shipped in dry ice and is stored at −20 °C at NIBSC. For the collaborative study, all BCG samples and ATP standard (BioThema, Sweden), mPCR primer set and DNA base pair markers were shipped on dry ice and the participants were advised to store them at −20 °C until testing. For real-time stability monitoring, all four WHO BCG RRs of BCG vaccines were used (NIBSC code: 07/270, 07/272, 07/274, 10/272).
2.2. Participants of collaborative study
The BCG Moreau-RJ samples were sent to 16 participants in 13 different countries. These include 7 BCG vaccine manufacturers and 9 national control laboratories worldwide. Fifteen of the participating laboratories agreed to perform the cultural viable count assay for the estimation of CFU, 10 agreed to perform the modified ATP assay and 13 agreed to perform the mPCR assay. All participants are experienced in cultural viable count assay for lyophilized BCG preparations but familiarity with the modified ATP and mPCR assays is varied. Many of the participants have been involved in a previous collaborative study which involved the use of these techniques. For this report, a code number was allocated at random to each participant, not necessarily representing the order of the participant list (Appendix I).
2.3. Study design and testing protocols
Participants were requested to test 10 ampoules of BCG Moreau-RJ vaccine preparation in their established routine in-house method for the cultural viable count assay, 10 ampoules in the modified ATP assay and 2 ampoules in the mPCR assay.
For the cultural viable count assay the study design recommended the 10 ampoules of BCG sample should be tested in at least two to three independent experiments using different batches of solid medium preparation. No pooling of reconstituted BCG ampoules was permitted for this study and each ampoule was tested individually. Three 1:2 serial dilutions (with the optimal dilution as the middle of the serial dilutions) were prepared from each reconstituted ampoule. Each diluted suspension was tested in triplicate, resulting in three readings per dilution and a total of nine readings per ampoule. After approximately 21 days incubation at 37 °C the average CFU counts were calculated, recorded and sent to NIBSC for collation and statistical analysis.
Laboratories participating in the modified ATP assay estimated the content of ATP in 10 lyophilized BCG Moreau RJ samples following the protocol provided. The 10 ampoules of BCG were tested in at least two to three independent experiments, as in the cultural viable count assay. Lyophilized BCG samples were reconstituted with 1 ml Dubos medium (SSI Diagnostica, Denmark) or other suitable culture medium; and the BCG suspensions were incubated at 37 °C for 22–26 h. Three 1:2 serial dilutions were prepared from each overnight BCG culture in pre-warmed medium (undiluted, 1:2 and 1:4). The procedures of ATP extraction and estimation were the same as described previously [10]. Results were recorded and data sent to NIBSC for collation and statistical analysis.
Participants were requested to use their own in-house method to extract and purify DNA from two ampoules of BCG Moreau-RJ samples to be used in two independent mPCR assays. The mPCR assay protocol was provided to all participants and as described previously [9]. Horizontal gel electrophoresis was used to resolve the PCR products. A 50 bp DNA ladder was used as a marker on the gel. The PCR product profiles were visualized using the participants’ in-house method and electronic images were sent to NIBSC for collation and analysis.
2.4. Stability studies – thermal and real-time
The cultural viable count assay was used to monitor the thermal stability of the live BCG vaccine preparation and was performed at NIBSC only. An accelerated degradation study was not used for this live preparation as incubation temperatures greater than 37 °C for a period longer than 4 weeks can kill most of the live bacilli in the preparation. A slightly modified method used for temperature stability, as stated in both WHO Recommendations [4] and European Pharmacopoeia monograph for BCG vaccine, freeze-dried [5] was used instead to determine the thermal stability of the lyophilized BCG vaccine preparation. Five ampoules each of the BCG Moreau-RJ preparation were incubated at 4 °C or 37 °C for a period of 4 weeks prior to performing the cultural viable count assay. These results were then compared with those from ampoules stored at −20 °C as recommended storage temperature for this preparation.
Real-time stability study is performed by NIBSC. The viability in terms of CFUs in cultural viable count assay of all four Reference Reagents of BCG vaccine stored at −20 °C, will be monitored for 10 years of shelf life annually to ensure the viability of these Reference Reagents is maintained within the acceptable range (as estimated from collaborative studies) at time of distribution.
2.5. Statistical analysis
All of the results from the cultural viable count assay were converted to CFU per ampoule. The mean CFU per ampoule was calculated from the mean estimates of the colony counts of each dilution [10] following the WHO/TB/Technical Guide/77.9 (in vitro assays of BCG products, unpublished working document in 1977). The choice of formula reflects the appropriate weight given to the number of colonies counted for a test BCG sample at each dilution level. Any of the ampoules within a laboratory's results that were found to be outliers using an in-house program [11] and Grubbs’ test [12] were excluded from further statistical analysis.
For the modified ATP assays, standard curves were generated by linear regression of log10 light emission reading (response) on log10 concentration of ATP standard. Responses for the test ampoules were converted to pmol ATP/100 μl using the fitted regression lines. The results were then converted to ng ATP/ampoule.
The overall mean of laboratory means was calculated as the final estimate for the preparation for both the cultural viable count and modified ATP assays. An estimate of uncertainty combining the standard deviation (SD) of the mean (reflecting variability between laboratories) with the pooled laboratory SD (reflecting between-ampoule homogeneity and variability between assays) was used to calculate an expanded uncertainty corresponding to a 95% level of confidence.
3. Results
3.1. Cultural viable count assay for estimation of CFU
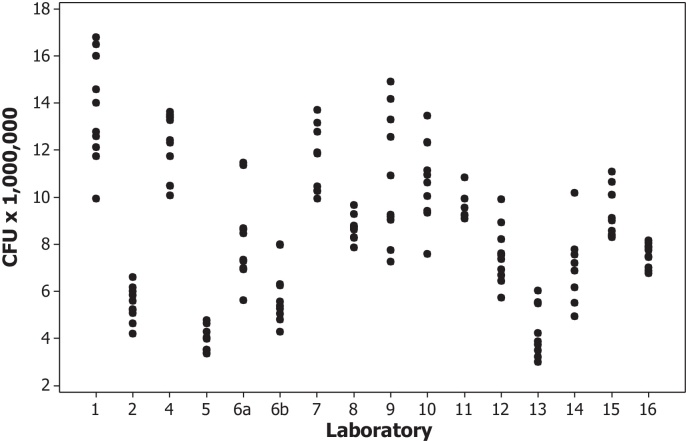
Sixteen complete data sets were received from 15 participants. Table 1 summarizes the mean CFU of the Moreau-RJ sub-strain preparation, SD and coefficient of variation (CV) of individual ampoule estimates for each laboratory and the type of solid culture media used. Two sets of data (6a and b) were provided from Laboratory 6 as two different culture media were used for the viable count assay. Data from one ampoule within Laboratory 7 was excluded as an outlier using Grubbs’ test [12] and was not used in further analysis. Data obtained from Laboratory 3 was omitted from this study as only mean CFU estimates were provided, there was no information on which solid media had been used and no optimal count ‘ω’ value for their cultural viable count assay was given. The distribution of mean CFU from all 10 ampoules of the BCG preparation performed by each participating laboratory is shown in Fig. 1.
Table 1.
Summary of results of cultural viable count assays from participating laboratories using various solid culture media. The mean content of BCG Moreau-RJ sub-strain preparation is presented as million CFU/ampoule.
| Laboratory | Culture medium used | Mean | SD | CV |
|---|---|---|---|---|
| 1 | Middlebrook | 11.71 | 2.26 | 19.3% |
| 2 | Löwenstein–Jensen | 3.47 | 0.72 | 20.9% |
| 4 | Löwenstein–Jensen | 10.36 | 1.34 | 12.9% |
| 5 | Ogawa | 1.91 | 0.53 | 27.5% |
| 6a | Löwenstein–Jensen | 6.29 | 1.89 | 30.1% |
| 6b | Middlebrook | 3.90 | 1.26 | 32.4% |
| 7 | Dubois oleic acid | 8.64 | 1.42 | 16.5% |
| 8 | Löwenstein–Jensen | 6.70 | 0.51 | 7.6% |
| 9 | Ogawa | 8.84 | 2.74 | 31.0% |
| 10 | Löwenstein–Jensen | 8.73 | 1.72 | 19.7% |
| 11 | Löwenstein–Jensen | 7.66 | 0.66 | 8.6% |
| 12 | Löwenstein–Jensen | 5.55 | 1.17 | 21.0% |
| 13 | Löwenstein–Jensen | 2.42 | 1.12 | 46.2% |
| 14 | Ogawa | 5.04 | 1.62 | 32.1% |
| 15 | Löwenstein–Jensen | 7.38 | 1.03 | 14.0% |
| 16 | Ogawa | 5.54 | 0.50 | 9.1% |
| Mean | 6.51 | |||
| SD of mean | 0.72 (11.1%) | |||
| Pooled between-ampoule SD | 1.44 (22.1%) | |||
| Combined uncertainty | 1.60 (24.6%) | |||
| Expanded uncertainty (95% confidence) | 3.10–9.92 |
Key: SD, standard deviation; CV, coefficient of variation.
Fig. 1.
The distribution of CFU counts per ampoule of BCG Moreau-RJ sub-strain preparation performed in participating laboratories, excluding one outlier from Laboratory 7.
3.2. Modified ATP assay for estimation of ATP content
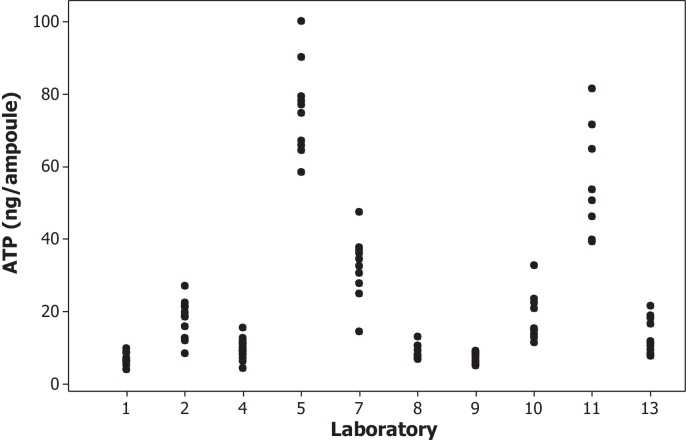
Ten data sets were received from the participants. Details of the modified ATP assay conditions used by participating laboratories in this study are listed in Table 2. Results from two ampoules within Laboratory 11 were excluded as outliers as they were greater than seven-fold higher than the mean result obtained for the other ampoules. Table 2 also shows the mean ATP content for the BCG Moreau-RJ preparation (ng/ampoule), SD and CV of the 10 individual ampoule estimates for each laboratory. The results from Laboratories 5, 7 and 11 were shown to be significantly different (higher) from those of the other participants by analysis of variance using Duncan's multiple comparisons tests. Fig. 2 shows the distribution of ATP content of the BCG preparation performed in participating laboratories, excluding two outliers from Laboratory 11.
Table 2.
Summary of variations in key experimental steps and results of modified ATP assay from participating laboratories. The mean ATP content is presented as ng/ampoule.
| Laboratory | Culture medium used | No. of ampoules tested per experiment | Samples prepared per ampoule | Luminescence readings per sample | Mean | SD | CV |
|---|---|---|---|---|---|---|---|
| 1 | 7H9 | 1 or 3 | 3 | 3 | 7.06 | 1.76 | 24.9% |
| 2 | Dubos | 1, 2 or 3 | 3 | 3 | 18.03 | 5.31 | 29.4% |
| 4 | Sauton | 10 | 3 | 1 | 9.88 | 2.40 | 24.3% |
| 5 | 7H9 | 2 | 3 | 3 | 75.68 | 12.54 | 16.6% |
| 7 | Dubos | 3 or 4 | 3 | 3 | 32.42 | 8.77 | 27.0% |
| 8 | Dubos | 5 | 3 | 3 | 8.97 | 2.02 | 22.5% |
| 9 | 7H9 | 1 or 3 | 3 | 3 | 7.37 | 1.49 | 20.2% |
| 10 | Dubos | 2 | 3 | 3 | 18.38 | 6.57 | 35.8% |
| 11 | Dubos | 5 | 1 | 1 | 56.06 | 15.36 | 27.4% |
| 13 | Dubos | 2 or 3 | 3 | 3 | 13.07 | 4.93 | 37.7% |
| Mean | 24.69 | ||||||
| SD of mean | 7.41 (30.0%) | ||||||
| Pooled between-ampoule SD | 6.97 (28.2%) | ||||||
| Combined uncertainty | 10.18 (41.2%) | ||||||
| Expanded uncertainty (95% confidence) | 1.67–47.71 |
Key: SD, standard deviation; CV, coefficient of variation.
Fig. 2.
The distribution of ATP content of BCG Moreau-RJ sub-strain preparation performed in participating laboratories, excluding two outliers from Laboratory 11.
3.3. Multiplex PCR assay for identification
Thirteen participants returned mPCR results for the BCG Moreau-RJ preparation. A diluted (1:10) DNA extraction was recommended in the study protocol as sometimes the mPCR reaction of neat DNA extracted from lyophilized BCG vaccine results in PCR products that are too intense to resolve clearly in gel electrophoresis. This was not a problem in the present study. The five mPCR products from BCG Moreau-RJ sub-strain are expected as RD8 (472 bp), RD2 (315 bp), senX3-regX3 (276 bp), RD14 (252 bp), and RD1 (196 bp). Each participating laboratory successfully resolved all five mPCR products, presented in Fig. 3. The resolution of the gel image from Laboratory 14 was not as clear as the others. Ten participants had extracted and performed subsequent mPCR from two ampoules of the preparation. Laboratories 1 and 16 returned results from only one ampoule. Laboratory 2 had combined the contents of the ampoules prior to the extraction of the DNA.
Fig. 3.
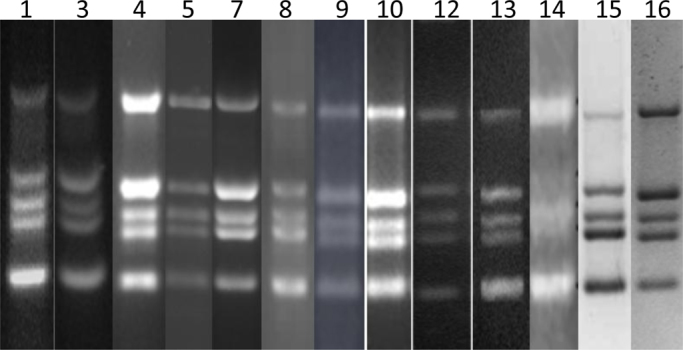
The multiplex PCR fingerprints of BCG Moreau-RJ sub-strain preparation performed in participating laboratories as numbered.
3.4. Stability study
The mean CFUs in thermal stability study were 10.80 (SD 2.84), 9.90 (SD 0.96) or 3.67 (SD 0.82) million per ampoule when this lyophilized preparation was stored at −20 °C, 4 °C or 37 °C, respectively. The % survival in CFU of the preparation from the thermal stability study was calculated by comparing it with the mean CFU of preparation stored at −20 °C. The % survival at 4 °C was 84.35% and at 37 °C was 33.98%.
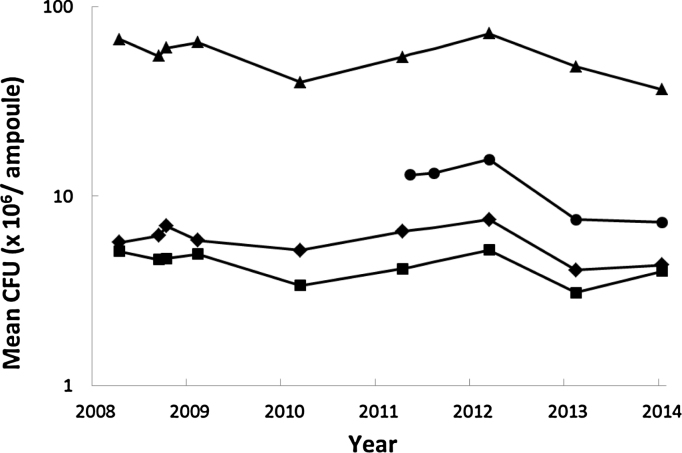
In real-time stability, the lower limits of CFU of these RRs are estimated from the expanded uncertainty (95% confidence) of this and previous collaborative studies on cultural viable count [10] and are 3.37, 29.60, 0.95 or 3.10 million per ampoule for Danish 1331, Tokyo 172-1, Russian BCG-I or Moreau-RJ, respectively. The trend of real time stability collected up to early 2014 is shown in Fig. 4. The current CFU results in 2014 of all four RRs are above the lower limits of the acceptable range, as 4.32, 36.56, 4.01 or 7.27 million per ampoule for Danish 1331, Tokyo 172-1, Russian BCG-I or Moreau-RJ, respectively.
Fig. 4.
Trend monitoring of real time stability data of WHO Reference Reagents of BCG vaccine of various sub-strains of (a) Danish 1331  , (b) Tokyo 172-1
, (b) Tokyo 172-1  , (c) Russian BCG-I
, (c) Russian BCG-I  , and (d) Moreau-RJ
, and (d) Moreau-RJ  , using the cultural viable count assay.
, using the cultural viable count assay.
4. Discussion
As in a previous collaborative study, two methodologies (cultural viable count and modified ATP assays) were used to assess the content of the BCG Moreau-RJ Reference Reagent preparation. The results estimated that there are 6.51 million CFU per ampoule with a SD of 0.72; and 24.69 ng ATP per ampoule with a SD of 7.41 for this preparation. There was a broad distribution of the mean CFU results received from all participants (Fig. 1). The expanded uncertainty (95% confidence) for this preparation is 3.10–9.92 million. The cultural viable count CFU results of lyophilized BCG preparations are usually variable and the data from this study are expected, especially participants’ own in-house routine cultural viable count assay with different solid media and culturing methodologies were used. The CV in each participating laboratory also had a wide range from 7.6% to 46.2% (Table 1).
There were large differences in the distribution of the mean ATP (ng) content obtained from all participants as shown in Table 2. The expanded uncertainty (95% confidence) for this preparation is 1.67–47.71 ng/ampoule. The CV in each participating laboratory ranged from 16.6% to 37.7%. This high variability of the modified ATP results was similar to the previous study [10]. The dilution effect of samples gave inconsistent results leading to only the ATP contents from neat reconstituted samples being used in the estimation of the mean ATP content in this BCG preparation. The results of CFU and ATP content were compared directly. This collaborative study clearly demonstrated that the modified ATP assay was not an improved method in terms of providing more consistent estimation of the viability in a lyophilized BCG preparation when compared with the cultural viable count assay. Some of the participating laboratories have limited experience in performing this ATP assay and this may, in part, contribute to the high variability of the results. However, this assay remains a rapid method for estimating the viability of lyophilized BCG preparations and has been validated for quality control testing in one of the participating laboratories [6]. There was good agreement of results for the mPCR assay for identification of this BCG sub-strain. All participating laboratories of this assay returned the expected fingerprints of five PCR products for the BCG Moreau-RJ sub-strain analyzed in the study (Fig. 3). This demonstrates that this assay is an effective and robust method to confirm the identity of a BCG sub-strain.
The establishment of WHO Reference Reagent of BCG vaccine of Moreau-RJ sub-strain was approved by WHO ECBS in October 2012 with a content of 6.51 million CFU or 24.69 ng ATP per ampoule. This Reagent (NIBSC code: 10/272) is available and distributed by NIBSC-MHRA, UK. All the Reference Reagents of BCG vaccine are stored in a −20 °C facility with a trend monitoring system. The real-time stability of these Reference Reagents is monitored annually to ensure the viability of the content is within an acceptable range. The data collected in the first few years demonstrated that these Reference Reagents of BCG vaccine are very stable when stored at −20 °C. The intended uses of these Reference Reagents are as comparators (1) for viability assays (such as cultural viable count and modified ATP assays); (2) for in vivo assays (such as the absence of virulent mycobacteria, dermal reactivity and protection assays) in the evaluation of candidate TB vaccines in non-clinical models; (3) for identity assays using molecular biology techniques.
Acknowledgments
Special thanks are due to Fundação Ataulpho de Paiva for preparing and donating of ampoule-filled lyophilized preparation of BCG vaccine for the establishment of the WHO Reference Reagent for BCG vaccine of Moreau-RJ sub-strain.
Fundação Ataulpho de Paiva was supported by funds of Decit/SCTIE/MS-MCT-CNPq-FNDCT-CAPES to Brazilian National Institute of Science and Technology on Tuberculosis (INCT-TB) and would like to acknowledge financial support awarded by FAPERJ (Grant E-26/190.025/2011).
Appendix I. List of participants
Ms Ivana Vukmirica, Institute of Virology, Vaccines and Sera ‘Torlak’, 458 Vojvode Stepe Str., Belgrade, Serbia.
Dr Jens Henriksen, Quality Control, Statens Serum Institut, Artillerivej 5, DK-2300 Copenhagen, Denmark.
Dr Wieslawa Janaszek-Seydlitz, National Institute of Public Health – National Institute of Hygiene, Department of Sera and Vaccine Evaluation, 24 Chocimska Str., 00-791 Warsaw, Poland.
Prof Dr Plamen Nenkov, Bul Bio-NCIPD, 26 Yanko Sakazov Boulevard, 1504 Sofia, Bulgaria.
Dr Masaaki Seki, Japan BCG Laboratory, 3-1-5, Matsuyama, Kiyose City, Tokyo 204-0022, Japan.
Dr Luiz RR Castello-Branco, Fundação Ataulpho de Paiva, Av. Pedro II, 260. São Cristovão, Rio de Janeiro, Brazil.
Dr Zhang Lei and Ms Wang Jing, Quality Control Department, Chengdu Institute of Biological Products, 379 #, 3rd Jinhua Road, Jinjiang District, Chengdu 610023, PR China.
Dr Sunil Gairola, Serum Institute of India Limited, 212/2, Hadaspar, Pune 411028, India.
Prof Guozhi Wang and Miss Aihua Zhao, 1st Division of Bacterial Vaccines, National Institute for Food and Drug Control, No.2, Tiantan Xili, Beijing 100050, PR China.
Dr Eduardo C Leal, National Institut for Quality control in Health, Oswaldo Cruz Foundation, Ministry of Health, Av Brasil 4036, s/915 & 916, Rio de Janeiro 21041-210, Brazil.
Dr Keigo Shibayama, Department of Bacteriology II, National Institute of Infectious Diseases (NIID), 4-7-1 Gakuen, Musashimurayama, Tokyo 208-0011, Japan.
Dr Syamsudin, Apt, M.Si, Dr Sri Wahyuningsih and Dr Siam Subagyo, National Quality Control Laboratory of Drug and Food, National Agency of Drug and Food Control, Jl. Percetakan Negara, No. 23, Jakarta Pusat 10560, Indonesia.
Dr Mei M Ho and Ms Belinda Dagg, Bacteriology Division, NIBSC, Blanche Lane, South Mimms, Potters Bar, Herts., EN6 3QG, U.K.
Dr Stephane Maisonneuve and Dr Murielle Andre, Agence Française de Sécurité Sanitaire des Produits de Santé, Site de Lyon, 321 Avenue Jean Jaurès, 69007 Lyon, France.
Dr Diana Levi, BCG and Tuberculin Department, Tarassevich Institute, 41 Sivtsev Vrazec, Moscow, Russia.
Dr Jeewon Joung, National Center for Lot Release, National Institute of Food and Drug Safety Evaluation, Korea Food and Drug Administration, 194 Tongil-ro Eupyung-gu, Seoul, Republic of Korea.
References
- 1.Corbel M.J., Fruth U., Griffiths E., Knezevic I. Report on a WHO consultation on the characterisation of BCG strains, Imperial College, London 15–16 December 2003. Vaccine. 2004;22:2675–2680. doi: 10.1016/j.vaccine.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 2.Ho M.M., Corbel M.J., Knezevic I., Roumiantzeff M. Report on a WHO consultation on the characterisation of BCG vaccines, WHO, Geneva, Switzerland 8–9 December 2004. Vaccine. 2005;23:5700–5704. [Google Scholar]
- 3.Knezevic I., Corbel M.J. WHO discussion on the improvement of the quality control of BCG vaccines. Pasteur Institute, Paris, France, 7 June 2005. Vaccine. 2006;24:3874–3877. doi: 10.1016/j.vaccine.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 4.WHO Expert Committee on Biological Standardization . 2011. Recommendations to assure the quality, safety and efficacy of BCG vaccines. World Health Organisation Technical Report Series; No. 979: Annex 3; pp. 137–185. [Google Scholar]
- 5.Directorate for the Quality of Medicines of the Council of Europe (EDQM); Strasbourg, Cedex, France: 2012. European pharmacopoeia; pp. 819–820. BCG vaccine, freeze-dried. 01/2012: 0163. [Google Scholar]
- 6.Jensen S.E., Hubrechts P., Klein B.M., Haslov K.R. Development and validation of an ATP method for rapid estimation of viable units in lyophilised BCG Danish 1331 vaccine. Biologicals. 2008;36:308–314. doi: 10.1016/j.biologicals.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Ho M.M., Markey K., Rigsby P., Jensen S.E., Gairola S., Seki S. Report of an international collaborative study to establish the suitability of using modified ATP assay for viable count of BCG vaccine. Vaccine. 2008;26:4754–4757. doi: 10.1016/j.vaccine.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 8.Bedwell J., Kairo S.K., Behr M.A., Bygraves J.A. Identification of substrains of BCG vaccine using multiplex PCR. Vaccine. 2001;19:2146–2151. doi: 10.1016/s0264-410x(00)00369-8. [DOI] [PubMed] [Google Scholar]
- 9.Markey K., Ho M.M., Choudhury B., Seki M., Liu J., Castello-Branco L.R.R. Report of an international collaborative study to evaluate the suitability of multiplex PCR as an identity assay for different sub-strains of BCG vaccine. Vaccine. 2010;28:6964–6969. doi: 10.1016/j.vaccine.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 10.Ho M.M., Markey K., Rigsby P., Hockley J., Corbel M.J. Report of an International collaborative study to establish the first WHO reference reagents for BCG vaccines of three different sub-strains. Vaccine. 2011;29:512–518. doi: 10.1016/j.vaccine.2010.10.066. [DOI] [PubMed] [Google Scholar]
- 11.Gaines Das R.E., Rice L.R. SCAN, an exploratory program for preliminary analysis of bioassay and immunoassay data. Comput Methods Programs Biomed. 1985;21:25–33. doi: 10.1016/0169-2607(85)90059-8. [DOI] [PubMed] [Google Scholar]
- 12.Grubbs F. Procedures for detecting outlying observations in samples. Technometrics. 1969;11:1–21. [Google Scholar]