Abstract
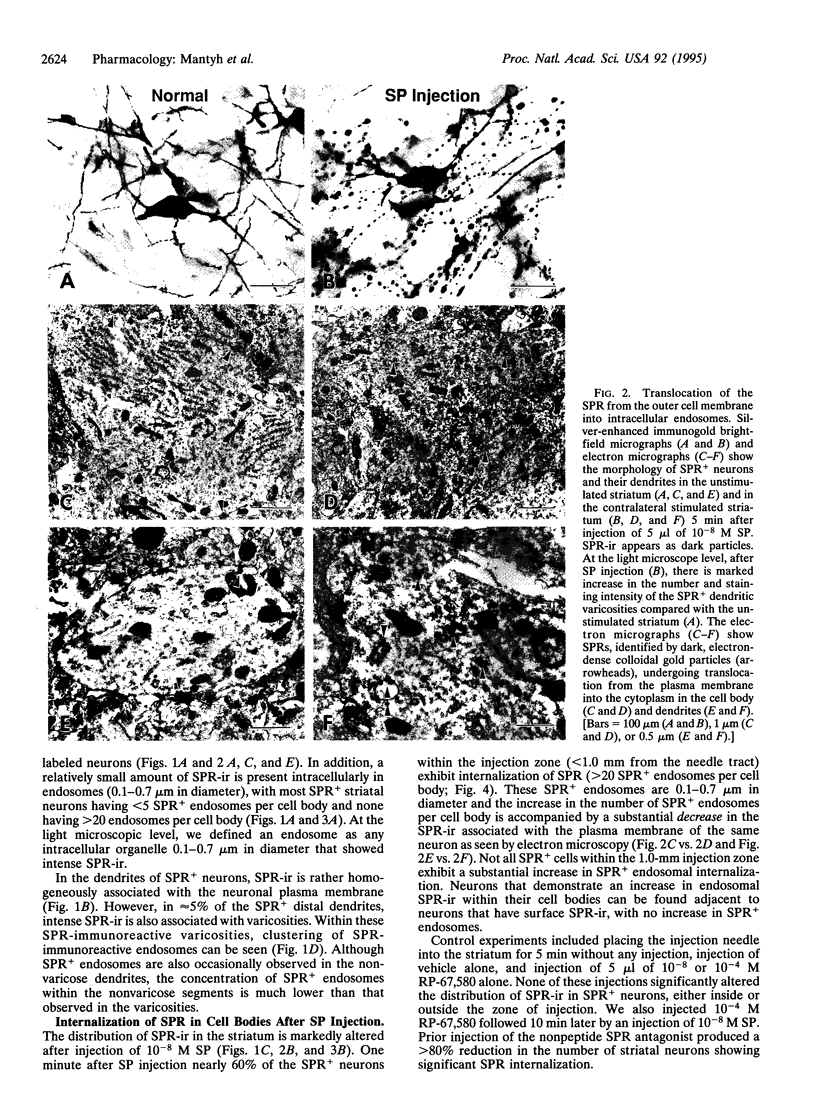
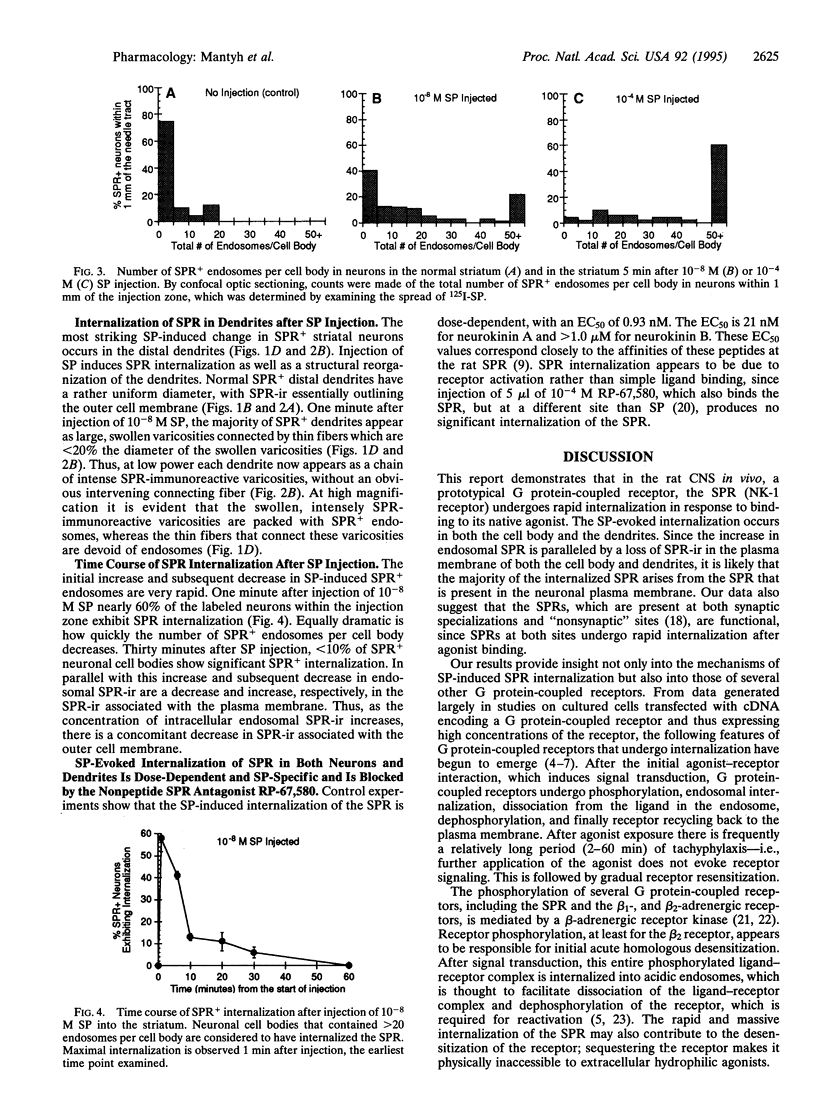
Studies on cultured cells have shown that agonists induce several types of G protein-coupled receptors to undergo internalization. We have investigated this phenomenon in rat striatum, using substance P (SP)-induced internalization of the SP receptor (SPR) as our model system. Within 1 min of a unilateral striatal injection of SP in the anesthetized rat, nearly 60% of the SPR-immunoreactive neurons within the injection zone display massive internalization of the SPR--i.e., 20-200 SPR+ endosomes per cell body. Within the dendrites the SPR undergoes a striking translocation from the plasma membrane to endosomes, and these dendrites also undergo a morphological reorganization, changing from a structure of rather uniform diameter to one characterized by large, swollen varicosities connected by thin fibers. In both cell bodies and dendrites the number of SPR+ endosomes returns to baseline within 60 min of SP injection. The number of neurons displaying substantial endosomal SPR internalization is dependent on the concentration of injected SP, and the SP-induced SPR internalization is inhibited by the nonpeptide neurokinin 1 receptor antagonist RP-67,580. These data demonstrate that in the central nervous system in vivo, SP induces a rapid and widespread SPR internalization in the cell bodies and dendrites and a structural reorganization of the dendrites. These results suggest that many of the observations that have been made on the internalization and recycling of G protein-coupled receptors in in vitro transfected cell systems are applicable to similar events that occur in the mammalian central nervous system in vivo.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci. 1991 Oct;14(10):458–461. doi: 10.1016/0166-2236(91)90045-v. [DOI] [PubMed] [Google Scholar]
- Bowden J. J., Garland A. M., Baluk P., Lefevre P., Grady E. F., Vigna S. R., Bunnett N. W., McDonald D. M. Direct observation of substance P-induced internalization of neurokinin 1 (NK1) receptors at sites of inflammation. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron M. G., Lefkowitz R. J. Catecholamine receptors: structure, function, and regulation. Recent Prog Horm Res. 1993;48:277–290. doi: 10.1016/b978-0-12-571148-7.50014-2. [DOI] [PubMed] [Google Scholar]
- Collins S., Caron M. G., Lefkowitz R. J. From ligand binding to gene expression: new insights into the regulation of G-protein-coupled receptors. Trends Biochem Sci. 1992 Jan;17(1):37–39. doi: 10.1016/0968-0004(92)90425-9. [DOI] [PubMed] [Google Scholar]
- De Koninck Y., Henry J. L. Substance P-mediated slow excitatory postsynaptic potential elicited in dorsal horn neurons in vivo by noxious stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11344–11348. doi: 10.1073/pnas.88.24.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland A. M., Grady E. F., Payan D. G., Vigna S. R., Bunnett N. W. Agonist-induced internalization of the substance P (NK1) receptor expressed in epithelial cells. Biochem J. 1994 Oct 1;303(Pt 1):177–186. doi: 10.1042/bj3030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese J., Luttrell L. M., Iñiguez-Lluhi J. A., Touhara K., Koch W. J., Lefkowitz R. J. Functionally active targeting domain of the beta-adrenergic receptor kinase: an inhibitor of G beta gamma-mediated stimulation of type II adenylyl cyclase. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3637–3641. doi: 10.1073/pnas.91.9.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch J., Wolters I., Triller A., Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993 Dec 23;366(6457):745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- Kwatra M. M., Schwinn D. A., Schreurs J., Blank J. L., Kim C. M., Benovic J. L., Krause J. E., Caron M. G., Lefkowitz R. J. The substance P receptor, which couples to Gq/11, is a substrate of beta-adrenergic receptor kinase 1 and 2. J Biol Chem. 1993 May 5;268(13):9161–9164. [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. The adrenergic receptors. Adv Second Messenger Phosphoprotein Res. 1990;24:1–8. [PubMed] [Google Scholar]
- Lefkowitz R. J., Cotecchia S., Kjelsberg M. A., Pitcher J., Koch W. J., Inglese J., Caron M. G. Adrenergic receptors: recent insights into their mechanism of activation and desensitization. Adv Second Messenger Phosphoprotein Res. 1993;28:1–9. [PubMed] [Google Scholar]
- Liu H., Brown J. L., Jasmin L., Maggio J. E., Vigna S. R., Mantyh P. W., Basbaum A. I. Synaptic relationship between substance P and the substance P receptor: light and electron microscopic characterization of the mismatch between neuropeptides and their receptors. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1009–1013. doi: 10.1073/pnas.91.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio J. E. Tachykinins. Annu Rev Neurosci. 1988;11:13–28. doi: 10.1146/annurev.ne.11.030188.000305. [DOI] [PubMed] [Google Scholar]
- Mantyh P. W., Gates T., Mantyh C. R., Maggio J. E. Autoradiographic localization and characterization of tachykinin receptor binding sites in the rat brain and peripheral tissues. J Neurosci. 1989 Jan;9(1):258–279. doi: 10.1523/JNEUROSCI.09-01-00258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh P. W., Pinnock R. D., Downes C. P., Goedert M., Hunt S. P. Correlation between inositol phospholipid hydrolysis and substance P receptors in rat CNS. 1984 Jun 28-Jul 4Nature. 309(5971):795–797. doi: 10.1038/309795a0. [DOI] [PubMed] [Google Scholar]
- Mantyh P. W., Rogers S. D., Allen C. J., Catton M. D., Ghilardi J. R., Levin L. A., Maggio J. E., Vigna S. R. Beta 2-adrenergic receptors are expressed by glia in vivo in the normal and injured central nervous system in the rat, rabbit, and human. J Neurosci. 1995 Jan;15(1 Pt 1):152–164. doi: 10.1523/JNEUROSCI.15-01-00152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988 Nov;1(9):761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- Moussaoui S. M., Montier F., Carruette A., Blanchard J. C., Laduron P. M., Garret C. A non-peptide NK1-receptor antagonist, RP 67580, inhibits neurogenic inflammation postsynaptically. Br J Pharmacol. 1993 May;109(1):259–264. doi: 10.1111/j.1476-5381.1993.tb13562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naas E., Zilles K., Gnahn H., Betz H., Becker C. M., Schröder H. Glycine receptor immunoreactivity in rat and human cerebral cortex. Brain Res. 1991 Oct 4;561(1):139–146. doi: 10.1016/0006-8993(91)90758-n. [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Wenthold R. J. Light and electron immunocytochemical localization of AMPA-selective glutamate receptors in the rat brain. J Comp Neurol. 1992 Apr 15;318(3):329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Yokotani N., Wenthold R. J. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994 Feb;14(2):667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubi J. C., Kvols L., Krenning E., Lamberts S. W. Distribution of somatostatin receptors in normal and tumor tissue. Metabolism. 1990 Sep;39(9 Suppl 2):78–81. doi: 10.1016/0026-0495(90)90217-z. [DOI] [PubMed] [Google Scholar]
- Senogles S. E., Spiegel A. M., Padrell E., Iyengar R., Caron M. G. Specificity of receptor-G protein interactions. Discrimination of Gi subtypes by the D2 dopamine receptor in a reconstituted system. J Biol Chem. 1990 Mar 15;265(8):4507–4514. [PubMed] [Google Scholar]
- Sesack S. R., Aoki C., Pickel V. M. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994 Jan;14(1):88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin L., Brodin E., Nilsson G., Conlon T. P. Interaction of substance P with dispersed pancreatic acinar cells from the guinea pig. Binding of radioiodinated peptide. Acta Physiol Scand. 1980 May;109(1):97–105. doi: 10.1111/j.1748-1716.1980.tb06570.x. [DOI] [PubMed] [Google Scholar]
- Vigna S. R., Bowden J. J., McDonald D. M., Fisher J., Okamoto A., McVey D. C., Payan D. G., Bunnett N. W. Characterization of antibodies to the rat substance P (NK-1) receptor and to a chimeric substance P receptor expressed in mammalian cells. J Neurosci. 1994 Feb;14(2):834–845. doi: 10.1523/JNEUROSCI.14-02-00834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C. J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983 Dec 15;306(5944):686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Yu S. S., Lefkowitz R. J., Hausdorff W. P. Beta-adrenergic receptor sequestration. A potential mechanism of receptor resensitization. J Biol Chem. 1993 Jan 5;268(1):337–341. [PubMed] [Google Scholar]
- von Zastrow M., Link R., Daunt D., Barsh G., Kobilka B. Subtype-specific differences in the intracellular sorting of G protein-coupled receptors. J Biol Chem. 1993 Jan 15;268(2):763–766. [PubMed] [Google Scholar]