Abstract
The attachment function of tibial spurs and pretarsal claws in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae) during locomotion was examined in this study. First, we measured the angle, at which the beetles detached from substrates with different roughness. At a surface roughness of 12 μm and higher, intact animals were able to cling to a completely tilted platform (180°). Second, we estimated the forces the beetles could exert in walking on smooth and rough cylinders of different diameters, on a plane and also between two plates. To elucidate the role of the individual structures, we ablated them consecutively. We found tibial spurs not to be in use in walking on flat substrates. On some of the curved substrates, ablation of tibial spurs caused an effect. A clear effect of tibial spurs was revealed in walking between two plates. Thus, these structures are probably used for generating propulsion in narrowed spaces.
Insects are by far the most species-rich group of animals on our planet with about one million described species, which makes more than 60% of all described animals1. This implies that they inhabit very diverse environments which make different demands on the locomotion ability of the organisms. Insects encounter rather different substrates while walking and climbing in their natural habitat, being able to produce propulsive forces on unpredictable and changing terrain. There are many different functional solutions for attachment generation in insects2,3.
Structure and function of attachment devices of insects have been previously characterized in various insect groups. Among these are beetles4,5,6,7,8, locusts9,10, earwigs11, cockroaches12,13,14, flies15,16,17,18, stick insects19,20,21, and hymenopterans22,23,24. Other works lay the focus on the adhesive fluids in different insects9,19,23,25. The attachment ability of several insects has been investigated on different substrates to elucidate the substrate-dependent performance of these insects4,5,8,21,26,27,28. Most research has focused on the smooth or hairy attachment pads in the above mentioned groups. These pads are specialized for rather smooth substrates.
The attachment to rough surfaces seems to be much more trivial at first sight. Nearly all adult insects possess claws on the distal ends of their tarsi29, which are used for interlocking with surface asperities or for penetration of soft substrates. Although the function of the claw system is by far more obvious than that of the attachment pads, surprisingly only little literature can be found so far on the exact role of the claws in attachment. Previous authors described the role of the claws in the beetle Pachnoda marginata by measuring the forces of freely walking beetles on differently rough surfaces, and by quantifying the breaking stress of the claws26. Similar experiments have been done for the locust Locusta migratoria malinensis30. Here, additionally the role of the smooth tarsal pads in attachment was examined. A related study has also been done on cockroaches31. The authors examined the walking and climbing ability of several cockroach species on different substrates, removing parts of their tarsi consecutively. Other authors measured the walking ability of ladybird beetles (Coccinellidae) on the pitcher surfaces of carnivorous Nepenthes alata plants32. They examined intact beetles as well as beetles with ablated claws.
Attachment pads are absent in P. marginata beetles. However, the beetles possess passively movable spurs at the distal ends of their tibiae. The role of these spurs, which are also found in many other insects, such as cockroaches31 or representatives of Ensifera, Hemiptera, and Coleoptera33, is not yet clear. One might assume that they aid in interlocking on rough surfaces, since they are comparable in size to the claws. But no study so far has experimentally uncovered the exact function of these structures, especially in insects bearing no other attachment structures than the pretarsal claws, as it is the case in P. marginata. One study dealt with the function of tibial spurs of different arthropods in walking over a wire-mesh substrate34. They found that distributed spurs on the tarsi and tibiae facilitated locomotion on such a corrugated terrain, especially in spiders with a nearly vertical penetration of the mesh.
The claws of insects are controlled by a muscle (M. retractor unguis) situated in the tibia, and sometimes also in the femur (e.g. stick insects35), whose tendon leads through the tarsus to the pretarsus, where it inserts at the proximal part of the unguitractor. The distal part of the unguitractor is connected to the claws through two tendon-like ligaments. By contracting, the muscle moves the claws ventrally towards the ground where they interlock with surface asperities, wherever possible. Another effect of a contraction of this muscle is a stiffening of the tarsus, as a pull on the tendon tightens the joints of the tarsal segments. This provides stability for the tarsal chain, which is necessary for transmitting force to the ground during walking24. In a previous study, we already examined the activity of the claw retractor muscle in walking P. marginata beetles on smooth and rough substrates36. This work now completes our understanding of the locomotion and attachment system of these beetles.
P. marginata is a soil- and plant-inhabiting beetle. Hence, the claws and spurs are likely to be used for walking on rough ground and climbing up plants. To elucidate the role of the tibial spurs and their interaction with the pretarsal claws in clinging to substrates and in walking, we performed a series of ablation experiments. These experiments were accomplished with intact beetles and with beetles with removed spurs, claws or both types of structures. With this procedure, we aimed at understanding the role of the different structures in attachment and locomotion.
Results
Scanning electron microscopy
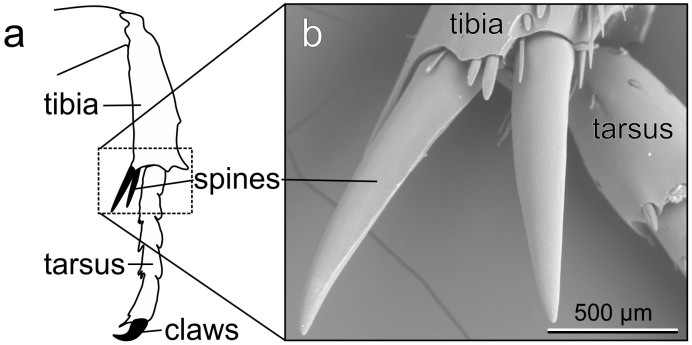
We examined the tibial spurs in front, middle and hind legs of P. marginata and measured the curvature of the spur tips. Both middle and hind legs bear a pair of spurs on the distal ends of their tibiae, while front legs possess only one spur per leg. The diameter of curvature of the spur tips from all legs amounted to 15.24 ± 0.93 μm (mean ± s.d., N = 3 animals, n = 18 measurements). We found no differences in diameter of curvature of spur tips between front, middle and hind legs after taking the mean of left and right leg measurements of each respective beetle segment (p = 0.259, F2,6 = 0.908, One Way ANOVA). The region of the tibio-tarsal joint of a female middle leg with the tibial spurs is shown in fig. 1 from a ventral view.
Figure 1. Interlocking structures of P. marginata.
(a) The drawing shows both the tibia and tarsus of the leg. The enlarged area, shown in the SEM image, is marked with a frame. (b) Scanning electron microscopy (SEM) image of the tibial spurs in a female middle leg, ventral view.
Sliding experiments
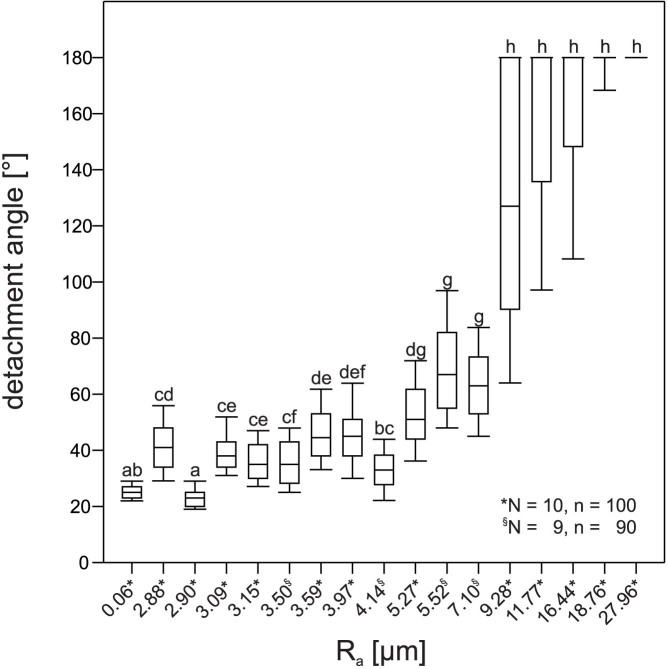
We measured the detachment angle of intact beetles on seventeen different substrates. The roughness (Ra values) of all substrate surfaces and the corresponding median detachment angles of beetles are shown in Fig. 2 (with a ranked abscissa). Low detachment angles were characteristic for surface roughness values up to about 4 μm, before an increase in detachment angle took place. The highest values were obtained at the surface roughness above 9 μm, which corresponds to the diameter of curvature of the claw tip26. At a surface roughness above 12 μm, at least half of the beetles were able to hold on to a completely inverted platform. The median detachment angles thus reached 180° on these surfaces. While the detachment angles on numerous smoother surfaces were significantly different (Kruskal-Wallis ANOVA on ranks; p < 0.001, H16 = 1404.420), they were not significantly different between the surfaces with the roughness above 9 μm. On all substrates, we observed no contact of the tibial spurs to the surface, especially when the tilting angle exceeded 90° and the body was pulled away from the substrate by gravity. In this situation, the attachment was due to interlocking of the substrate asperities between the claws of contralateral legs.
Figure 2. Dependence of the angles, at which beetles detached from the platform, of the surface roughness (Ra).
Detachment angles were measured on each surface with 9–10 individual beetles and with 10 runs per beetle. A detachment angle of 180° is equivalent to a successful attachment of the beetles to a completely inverted platform. The diagram has a ranked abscissa. Statistical analysis of the sliding experiments shows the boxes corresponding to the median (middle lines) and the 1st and 3rd quartiles. The whiskers denote the 10th and 90th percentiles. Different letters indicate significant differences. N is the number of animals, n is the total number of measurements including all animals on each respective substrate.
Force measurements of walking beetles
To elucidate the role of pretarsal claws and tibial spurs in locomotion and attachment to substrates with different shape and roughness, we performed experiments with beetles having manipulated tarsi. We tested beetles with (1) intact tarsi (control), (2) removed spurs, (3) removed claws, and (4) both spurs and claws removed. The tethered beetles pulled on the force transducer while walking on different substrates, and the force, exerted onto the surface by the animals, was recorded and then processed.
Experiment 1. Walking on rough sticks
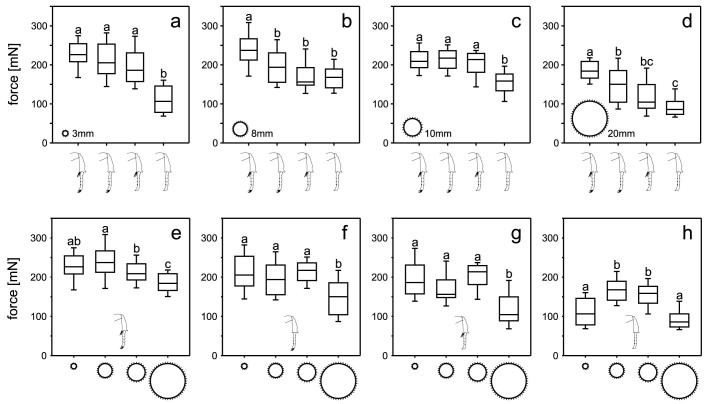
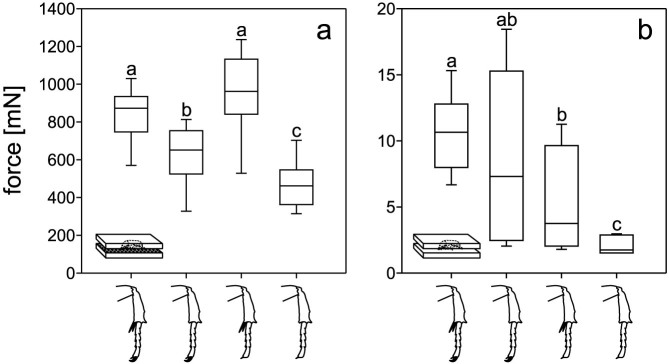
We tested the maximal force transmitted by the above groups of beetles to the surface during their walking on horizontally aligned rough sticks (Ra = 21.03 μm) of different diameters (3, 8, 10, and 20 mm). On all four sticks, a general trend towards lower forces was revealed from intact animals to animals with ablated spurs, animals with ablated claws, and finally animals with both structures removed (Fig. 3a–d). In particular, on the 3 mm stick, we found significantly lower forces of the beetles without both spurs and claws (106.4 mN) compared to the other three beetle groups (intact: 226.4 mN, without spurs: 205.4 mN, without claws: 186.2 mN; Fig. 3a; Kruskal-Wallis ANOVA on Ranks; p < 0.001, H3 = 89.030). On the 8 mm stick, intact animals showed significantly higher forces (237.3 mN) than all manipulated animals (without spurs: 193.9 mN, without claws: 156.2 mN, without spurs and claws: 167.9 mN; Fig. 3b; p < 0.001, H3 = 55.091). On the 10 mm stick, the same results were obtained as on the 3 mm stick, with significantly lower forces of animals with completely ablated tarsal structures (158.6 mN), if compared to the three other groups of animals (intact: 208.9 mN, without spurs: 217.3 mN, without claws: 213.6 mN; Fig. 3c; p < 0.001, H3 = 59.783). On the thickest stick of the 20 mm diameter, intact animals exhibited higher forces (184.1 mN) than the three other groups of animals (without spurs: 150.2 mN, without claws: 104.6 mN, without spurs and claws: 85.9 mN). In addition, on this diameter, beetles with ablated spurs generated higher forces than the ones with both spurs and claws removed (Fig. 3d; p < 0.001, H3 = 83.199).
Figure 3. Comparisons of the force measurements on rough cylindrical rods.
(a–d). Forces obtained for differently manipulated animals are compared separately on each rod diameter. (e–h). Forces measured on different rod diameters are compared for each type of animal manipulation. The drawings of the beetle legs indicate the manipulation status: intact (e), removed spurs (f), removed claws (g), and both structures removed (h). The boxes show the median (middle lines) and the 1st and 3rd quartiles. The whiskers denote the 10th and 90th percentiles. Different letters above the boxes indicate statistically significant differences between data samples. We tested ten intact beetles on each stick, ten beetles with removed spurs, five beetles with removed claws, and nine beetles with both spurs and claws removed. With each beetle we performed five runs.
Comparisons of the different diameters within one group of experimental animals showed in most cases a decrease in generated forces from smaller to larger substrate diameters, except for the completely ablated animals (Fig. 3e–h). For intact animals, we revealed the forces on the 20 mm stick to be significantly lower than on all three other stick diameters, and the force values on the 10 mm stick were also significantly lower than those on the 8 mm stick (Fig. 3e; p < 0.001, H3 = 50.372). Beetles with ablated tibial spurs showed significantly lower values on the thickest stick (20 mm) than on all three other ones (Fig. 3f; p < 0.001, H3 = 43.914). The same result was found for the beetles with removed claws (Fig. 3g; p < 0.001, H3 = 37.603). For the animals with completely ablated tarsal structures (without both spurs and claws), we found no difference between the forces generated on the 3 mm and 20 mm sticks. Forces here were significantly lower than those on the 8 mm and 10 mm sticks (Fig. 3h; p < 0.001, H3 = 88.106). We tested ten intact beetles on each stick, ten beetles with removed spurs, five beetles with removed claws, and nine beetles with both spurs and claws removed. With each beetle we performed five runs.
Experiment 2. Walking on smooth sticks
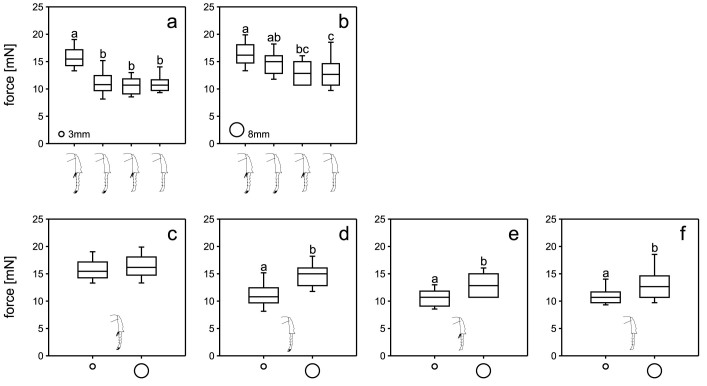
We also performed walking experiments on horizontally arranged smooth sticks with diameters of 3 mm and 8 mm. We did not use thicker smooth sticks, as the beetles were not able to hold on to smooth Perspex sticks with diameters larger than 8 mm. On the smooth 3 mm thick stick, beetles were not able to walk upright in most cases, but rather hung down from the sticks and moved forwards upside down, while on the thicker stick, they were able to keep an upright position. The force values generated by the intact animals (15.5 mN) on the 3 mm stick were significantly higher than those obtained for the other animal groups (without spurs: 10.8 mN, without claws: 10.7 mN, without spurs and claws: 10.7 mN; Fig. 4a; p < 0.001, H3 = 76.121), while animals in all three ablation conditions showed very similar forces. For the 8 mm stick, we found the forces of intact animals to be significantly higher (16.2 mN) than those of completely ablated animals (12.7 mN) and those without claws (12.8 mN), while the values of spur-ablated beetles (15.0 mN) were also significantly higher than those of beetles with both structures removed (Fig. 4b; p < 0.001, H3 = 36.509). Comparison of forces generated by intact animals showed no differences between the two stick diameters (Fig. 4c). The beetles with ablated tibial spurs showed significantly higher values on the 8 mm stick than on the 3 mm stick (Fig. 4d; Mann-Whitney Rank Sum Test; p < 0.001, U = 549.000). The same is true for the beetles with ablated pretarsal claws (Fig. 4e; p < 0.001, U = 498.500), as well as for the animals with both structures removed (Fig. 4f; p = 0.001, U = 1408.000). We tested ten intact beetles on each stick, ten beetles with removed spurs, five beetles with removed claws, and nine beetles with both spurs and claws removed. With each beetle we performed five runs.
Figure 4. Comparisons of the force data obtained on smooth rods of two different diameters.
(a–b). Forces of different manipulations are compared for each diameter. (c–f). Forces on the different diameters are separately compared for each manipulation. The drawings of the beetle legs indicate the manipulation status: intact (c), removed spurs (d), removed claws (e), and both structures removed (f). We tested ten intact beetles on each stick, ten beetles with removed spurs, five beetles with removed claws, and nine beetles with both spurs and claws removed. With each beetle we performed five runs.
Experiment 3. Walking on the flat substrate
On the horizontally aligned flat, rough surface (Ra = 21.03 μm), we found high force values generated by intact beetles (164.3 mN) and by beetles with ablated spurs (172.8 mN). The other two remaining groups of beetles, however, showed very low forces: 11.3 mN for the beetles without claws, and 10.7 mN for the beetles without both spurs and claws (Fig. 5a). The force values of both intact and spur-ablated animals were significantly higher than those of beetles in the two remaining conditions (p < 0.001, H3 = 108.398).
Figure 5. Comparisons of the traction force data obtained on tethered beetles walking on the flat rough surface (a) and the flat smooth surface (b).
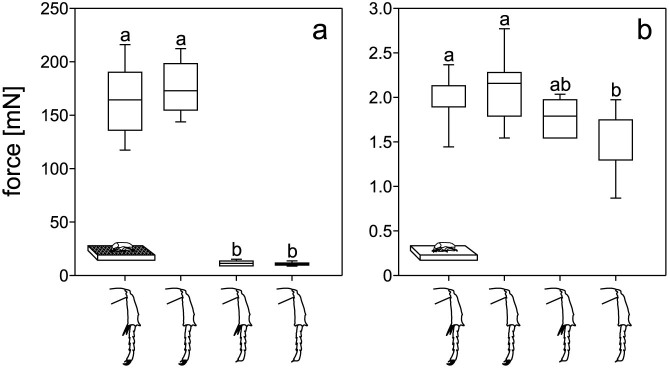
In both diagrams the forces are sorted by the manipulation status, which is indicated by the drawings of the beetle legs: intact, removed spurs, removed claws, and both structures removed. In the case of the intact animals in (b), the median is equivalent to the 1st quartile, in the case of completely ablated animals, it is equivalent to the 3rd quartile. On the rough surface, ten intact beetles were tested, five beetles without spurs, five without claws, and nine without both spurs and claws. On the smooth substrate, we tested four beetles in each ablation situation. With each beetle we performed five runs.
On the flat smooth surface (Ra = 0.07 μm), beetles were hardly able to walk at all. Most of the trials they failed in getting enough grip to produce a measurable propulsive force. Nevertheless, in four beetles we detected force values that could be distinguished from the noise. Forces of intact beetles (1.8 mN) and of those with removed spurs (2.1 mN) were significantly higher than those of the animals with both tarsal structures ablated (1.7 mN; p < 0.001, H3 = 25.282). Beetles with removed claws did not exhibit significantly different forces from beetles of all remaining experimental groups (1.7 mN) (Fig. 5b). On the rough surface, ten intact beetles were tested, five beetles without spurs, five without claws, and nine without both spurs and claws. On the smooth substrate, we tested four beetles in each ablation situation. With each beetle we performed five runs.
Experiment 4. Walking between two plates
Beetles were put onto the same flat, horizontal surface with a roughness of 21.03 μm as in the previous experiment. A smooth plate was used to clamp the beetles from above in between the two plates with a force of about 1 N, to simulate locomotion in a narrowed space (Fig. 6a). Here we found, that intact beetles exhibited similar forces (872.8 mN) as the ones without claws (961.8 mN), with even higher forces in the latter group, but this difference was not statistically significant. However, both groups of beetles showed significantly higher forces than beetles without spurs (651.2 mN) and those with both tarsal structures removed (461.3 mN). The forces generated by the two last ones were also significantly different (Fig. 7a; p < 0.001, H3 = 99.519).
Figure 6. Diagram of the traction force measurements of beetles walking in a narrowed space between two plates (A) and on cylindrical rods (B).
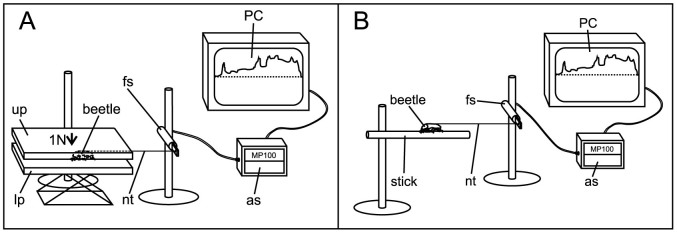
(A). A tethered beetle attached to a force sensor (fs) through a nylon thread (nt) was put onto the rough (Ra = 21.03 μm) lower platform (lp). The upper platform (up) was then lowered until the beetle was clamped between lower and upper platform with a force of 1 N. (B). A beetle was put on a cylindrical rod. The beetles pulled horizontally on the sensor (fs). The data acquisition system (as) connected to the PC continuously recorded the traction force generated by the beetle.
Figure 7. Comparisons of the traction force data obtained on tethered beetles walking between two plates with a rough lower substrate (a) and a smooth lower substrate (b).
In both diagrams the forces are sorted by the manipulation status, which is indicated by the drawings of the beetle legs: intact, removed spurs, removed claws, and both structures removed. On the rough surface, we tested ten intact beetles, ten beetles with removed spurs, ten with removed claws, and nine with both spurs and claws removed. On the smooth surface, ten intact beetles were tested, six with removed spurs, seven with removed claws, and four with both types of structures removed. With each beetle we performed five runs.
In the experiments between two plates with a smooth lower substrate, intact animals showed significantly higher forces (10.6 mN) than beetles with removed claws (3.7 mN) and beetles with both claws and spurs removed (1.7 mN). Beetles with removed spurs (7.3 mN) showed higher values than animals with completely amputated tarsal structures, but with a very large dispersion of force data (Fig. 7b; p < 0.001, H3 = 47.865). On the rough surface, we tested ten intact beetles, ten beetles with removed spurs, ten with removed claws, and nine with both spurs and claws removed. On the smooth surface, ten intact beetles were tested, six with removed spurs, seven with removed claws, and four with both types of structures removed. With each beetle we performed five runs.
Discussion
The main motivation for this work was to discover the role of tarsal and tibial structures in attachment and locomotion in an insect, which bears no specialized adhesive pads for attachment to smooth surfaces. The control experiments and observations on P. marginata on a flat smooth substrate (Ra = 0.07 μm) revealed that the beetles were almost not able to walk on these surface. At first glance, it might seem to be obvious that the two passively movable spurs on hind and middle legs (only one on front legs), located distally at the ventral side of the tibiae, are used for interlocking with rough substrates, probably in connection with pretarsal claws as it was previously shown for several insects walking on a flat, rough substrate33. To test whether this simple assumption is true, we performed a set of experiments with operated animals clinging to a turnable platform and walking on substrates with various shapes and roughness. We expected that proximally oriented claws and distally pointing spurs interact in the way, that they clamp the substrate and thus generate attachment on a rough surface, as it was previously proposed for Metrioptera bicolor (Ensifera), Himacerus apterus (Hemiptera), and Coccinella septempunctata (Coleoptera)33. Surprisingly, we found that the tibial spurs of P. marginata were not in contact with flat rough surfaces during normal locomotion. On rough substrates, where beetles were able to hold on to a completely inverted platform at an angle of 180° (Fig. 2), the attachment relied solely on the interlocking of the claws with the substrate asperities.
A previous study with Locusta migratoria28 showed that the locusts were able to cling to smoother surfaces much better than the P. marginata beetles in our study. This is easily explained by the absence of attachment structures specialized for smooth surfaces in P. marginata, while L. migratoria bears several tarsal adhesive pads: euplantulae on the tarsal segments and an arolium on the pretarsus. In another study the forces were measured, locusts could exert onto a flat, rough surface30. Experiments with locusts having ablated pretarsal claws demonstrated results consistent with ours: clawless animals exhibited significantly lower forces on the rough substrate than intact ones. The superior role of pretarsal claws in normal walking on rough terrain was thus confirmed by our study in consistence with studies on other insects.
However, the role of the tibial spurs remained unclear from the literature because only few studies dealt with related structures31,33,34. That is why we decided to test the walking ability and forces generated by intact and operated beetles on sticks with different diameters and different roughness. The removal of tibial spurs or tarsal claws revealed a significant effect on locomotion only on some combinations of thickness and surface texture of the sticks. Moreover, in those cases, the effect was independent of the kind of ablation: removing claws revealed the same effect as removing spurs. This result points towards a combined use of claws and spurs at least on some of the sticks, corresponding to the findings of Gladun and Gorb (2007) for different insects33. However, on the other sticks, such a putative cooperation of the two structures could not be shown. The results of Gladun and Gorb (2007) could thus only partially be confirmed for Pachnoda marginata. That is why we cannot make a general statement about the role of spurs in locomotion on stems covering a wide range of diameters and surface roughness.
In the experiments on the plane substrate, our results showed that claws are very important for the propulsion generation, as the claw amputation had a highly significant effect on beetle performance, which is in accordance with the results of Stork (1980), who measured the pulling forces of the beetle Chrysolina polita on cloth amongst other substrates4. In our study, ablation of claws decreased the maximum force beetles could exert onto the surface by a factor of more than 14, while ablation of spurs had no significant effect. Hence, the pretarsal claws are the essential structure for producing propulsive forces during walking on this substrate. This result is also supported by the fact that ablating both structures (claws and spurs) did not further decrease force generation, if compared to the situation with amputated claws. Dai et al. (2002) performed similar experiments with intact animals of the same species and obtained congruent results on corresponding substrate roughnesses26. Gladun and Gorb (2007) also revealed that insects with attachment devices on the tibia or proximal tarsomeres usually do not use these structures while walking on a plane, but only on stems33. In cockroaches, Roth and Willis (1952) found the claws to be essential structures in walking on rough flat substrates31. In addition, they stated, that cockroaches without tarsi use their tibial spurs on a rough ground. In contrast, in our experiments, P. marginata with ablated claws was not able to produce significant propulsive forces on a flat, rough surface by using just the tibial spurs.
Locomotion in a narrowed space, like burrowing into the soil or walking underground, is frequently observed in P. marginata. Such a behavioural situation was simulated in an experiment, where beetles were walking between two plates. Removal of the claws had no significant effect on forces. However, beetles without spurs showed significantly lower forces than intact animals, indicating an important function of the spurs for generating propulsion underground or below any obstacles, where the space for movements of the whole leg is limited. In such a situation, the use of pretarsal claws is also limited. We observed that beetles set the distal ends of their tibiae onto the substrate instead of the tarsi, and thus used the spurs as a substitution for the pretarsal claws, which probably also includes a switching of leg functions, because claws are normally used as pulling devices while spurs fulfill a pushing function. However, the significant difference between forces generated by beetles which (1) only had the claws and (2) were without any attachment devices, allows us to assume that the function of tibial spurs in locomotion in narrowed spaces can be partially compensated by the claws.
We have to notice that we found much higher forces in the situation of walking between two plates, compared to walking on a rough substrate without a second plate from above. The lowest forces in walking between two plates (both structures removed: 461.3 mN) were still much higher than the highest forces on a flat rough substrate (ablated spurs: 172.8 mN). This is probably due to the fact that we chose a rather high compressing force for the beetles to clearly work out the effect of a pressure from above. The situation of walking clamped between the two plates probably prevented the beetles from being pulled back by the strained thread and enabled them to rest without releasing the nylon thread before they pulled again on the force sensor. The forces thus added up to a much higher resulting force than in the “unclamped” situation without the second plate from above. This explains the rather high forces in these experiments. But also in the other walking situations we measured forces up to about 20 times the body weight of the beetles. This is in agreement with other studies4,26,30 where also pulling forces were recorded that were many times higher than the body weight of the animals. This represents the safety factor of the attachment system that enables the animals to cling to the substrate with only one leg or during strong disturbances on appropriate surfaces.
In this study, we quantitatively demonstrated for the first time the function of tibial spurs in P. marginata in producing propulsive forces in tight spaces. Our initial hypothesis that the spurs might be used together with (or similar to) the pretarsal claws, i.e. interlocking with rough substrates in horizontal walking and vertical climbing, was rejected after analyzing the results of our experiments. The spurs were neither involved in clinging to rough substrates, nor in walking on flat surfaces.
We cannot exclude that the spurs have additional functions which could not be revealed with out experimental setup. This might be locomotion over a terrain with large gaps, as it was shown by Spagna et al. (2007)34. Tibial spurs of different arthropods allowed effective walking on a wire mesh substrate, while animals with removed spurs had difficulties on such surfaces. However, it can be questioned, whether the spurs of P. marginata are able to fulfill a similar task, as they are located only ventrally on the distal end of the tibia and oriented in a clearly distal direction, and not distributed across the whole leg like for example in the spider Hololena adnexa34.
As we performed numerous locomotion experiments, covering most walking situations in the natural habitat of the beetles, like walking on twigs, rough ground or burrowing in the soil, we are confident that the main function of tibial spurs in P. marginata and similar structures of other insects is propulsion generation in the soil or generally in narrowed substrates.
Methods
Animals
We used male and female Pachnoda marginata Kolbe beetles (Coleoptera, Scarabaeidae) for our experiments. The beetles were taken from our laboratory colony, where they were kept at 22–26°C and fed with various fruit. The beetles had a body weight of 11.3 mN ± 1.8 mN (mean ± s.d., N = 18). No differences in weight were found between males and females (p = 0.507, unpaired t-test). Before amputations of pretarsal claws and tibial spurs, the animals were put into the fridge for 15–30 min at 4°C for anesthetization and immobilization. The manipulations were then carefully accomplished with microscissors and a scalpel so that virtually no stumps of the corresponding structures were left which could be potentially used for locomotion. After ablation, the beetles recovered over night before they were used for experiments.
Scanning electron microscopy
We investigated the tibial and tarsal structures of P. marginata using a Hitachi TM-3000 scanning electron microscope with an accelerating voltage of 15 kV (Hitachi Ltd. Corporation, Tokyo, Japan). The tarsi were cut off dead beetles, washed with distilled water, rinsed with 70% ethanol and air-dried for several days. The samples were then sputter-coated with a gold-palladium layer of 20 nm thickness. For the estimation of the diameter of curvature at the spur tips we fitted ellipses to the tips in the SEM pictures at high magnification with ImageJ software (Version 1.45, National Institutes of Health, Bethesda, USA).
Surface preparation for sliding experiments
We made epoxy resin surfaces as positive replicas of 17 different grades of sandpaper, ranging from 0.06 μm to 29.96 μm, (MATADOR GmbH, Remscheid, Germany). For this purpose, we first made molds of the sandpaper with dental wax (Coltène PRESIDENT light body, Coltène/Whaledent AG, Switzerland). These negatives served as templates for the final plates, which were made of Spurr epoxy resin37. The fluid epoxy resin was poured in the dental wax molds and polymerized at 70°C for 20 h. Subsequently, the surface roughness was measured with the white light interferometer Zygo NewView 5000 (Zygo Corporation, Middlefield, CT). The roughness values are shown in Fig. 2.
Sliding experiments
For the sliding experiments we used a motorized platform that could be tilted from 0° up to 180°. The platform was equipped successively with the epoxy resin surfaces with different roughness (Fig. 8). At the beginning of each sliding experiment, a beetle was put onto the epoxy resin substrate at the 0° position. The platform was then tilted with an angular velocity of 8.3°/s. The motor was stopped, when the beetle started to slide off the substrate or fell off the platform. The detachment angle was then read off the angular scale of the platform. We tested the clinging ability of 9–10 intact beetles on seventeen different surfaces (Fig. 2), with ten trials on each surface.
Figure 8. Diagram of the sliding experiments.
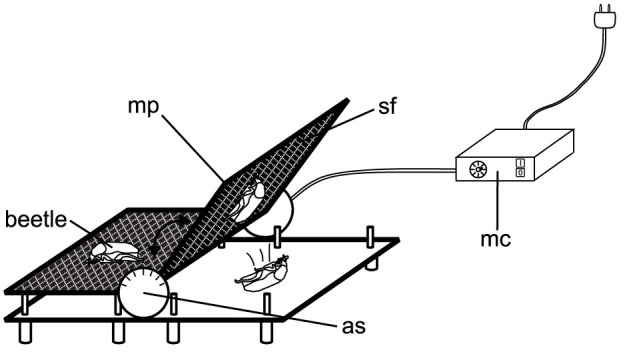
Beetles were put onto the surface (sf) attached to the motorized platform (mp) being in a horizontal position. Then the platform was started to tilt using the motor control unit (mc), and the angle, at which the beetle detached from the surface, was read from the angular scale (as).
Force measurements on sticks
For the force measurements of walking beetles on sticks, we used acrylic glass sticks with diameters of 3, 8, 10 and 20 mm. One set of sticks covering each diameter was wrapped with sandpaper (Ra = 21.03 μm). A second set remained as a control in its smooth condition. The sticks were horizontally mounted on a vertically adjustable stand. The force was measured with the MP100 force measuring system and analyzed with the software Acqknowledge 3.7.3 (both from Biopac Systems, Inc., Goleta, CA, USA). The force sensor was adjusted in height to the vertical position of the stick, so that it was ensured that the beetles pulled in the horizontal direction on the force sensor (Fig. 6b). A nylon thread was glued to the beetles' scutellum with a wax/colophony mixture, and the other side of the thread was tied to the force sensor. If the beetles did not start to pull on the force sensor spontaneously, walking behaviour was elicited by a gentle touch of the abdomen with a brush. In this and the following experimental setups we recorded force-time curves and used the maximal force the beetles could exert onto the respective surface for further analysis to test for influences of the single attachment systems on the maximum traction force level of the beetles. We tested ten intact beetles on each stick, ten beetles with removed spurs, five beetles with removed claws, and nine beetles with both spurs and claws removed. With each beetle we performed five runs on the four rough sticks and on the 3 mm and 8 mm smooth sticks. As the beetles were not able to hold on to the 10 mm and 20 mm smooth sticks, these two diameters were omitted in the smooth surface condition.
Force measurements on flat surfaces
The setup for the force measurements of walking beetles on a flat surface was nearly the same as for the measurements on sticks, except that we used a flat surface instead of the sticks. Beetles and forces sensor were again aligned at the same height to ensure horizontal force transmission. We used an epoxy resin cast of the sandpaper which was used for the experiments on sticks (Ra = 21.03 μm) and a smooth cast of a glass plate (Ra = 0.07 μm). On the rough surface, ten intact beetles were tested, five beetles without spurs, five without claws, and nine without both spurs and claws. On the smooth substrate, we tested four beetles in each ablation situation, as we found that the beetles were hardly able to move forwards on this surface. With each beetle we performed five runs.
Force measurements of beetles walking between two plates
This experimental setup was the same as for the force measurements on a flat surface, except for a second (smooth) plate above the first one. Between these two plates the beetles were clamped with a force of 1 N (Fig. 6a). We chose this force to simulate movement of the beetles in a narrowed space. We used the same substrates for beetle locomotion as in the previous experiment: rough (Ra = 21.03 μm) and smooth (Ra = 0.07 μm) epoxy resin plates. On the rough surface, we tested ten intact beetles, ten beetles with removed spurs, ten with removed claws, and nine with both spurs and claws removed. On the smooth surface, ten intact beetles were tested, six with removed spurs, seven with removed claws, and four with both types of structures removed. With each beetle we performed five runs.
Statistics
Statistical analysis was done with SigmaPlot for Windows (Version 10.0, Systat Software, San José, CA, USA). As data were always at least partly non-normally distributed, we compared the groups with a Kruskal-Wallis-ANOVA on ranks, and further pairwise comparisons were performed with Dunn's method, if the sample size of the compared groups was different. In case of equal sample size, the Tukey Test was used. Two groups with non-normally distributed data were compared with the Mann-Whitney Rank Sum Test. The only normally distributed data were the data on the spur tip diameter. Thus, there we used a One Way ANOVA for comparison. Post-hoc tests were performed only, when the corresponding global tests revealed a significant difference. Results are shown as boxplots. In the graphic representation, the boxes always show the median line and the first and third quartile. Whiskers denote the 10%- and 90%-percentiles. Statistically significant differences are indicated by different letters above the boxes.
Author Contributions
P.B. and S.G. wrote the manuscript text, P.B. prepared all figures. P.B. performed the sliding experiments, D.K. helped in conducting the ablation and force measurement experiments. Statistical analysis was done by P.B. All authors reviewed the manuscript.
Acknowledgments
Olav Bernecker helped with the sliding experiments. This study was supported by the German Science Foundation (DFG) Initiative ‘Bionik’ (DFG grant no. GO995/7-1 to SG).
References
- Grimaldi D. & Engel M. S. Evolution Of The Insects (Cambridge University Press, New York, 2005). [Google Scholar]
- Beutel R. G. & Gorb S. N. Ultrastructure of attachment specializations of hexapods (Arthropoda): evolutionary patterns inferred from a revised ordinal phylogeny. J. Zool. Syst. Evol. Res. 39, 177–207 (2001). [Google Scholar]
- Gorb S. N. & Beutel R. G. Evolution of locomotory attachment pads of hexapods. Naturwissenschaften 88, 530–534 (2001). [DOI] [PubMed] [Google Scholar]
- Stork N. E. Experimental analysis of adhesion of Chrysolina polita (Chrysomelidae: Coleoptera) on a variety of surfaces. J. Exp. Biol. 88, 91–107 (1980). [Google Scholar]
- Gorb E. V. & Gorb S. N. Attachment ability of the beetle Chrysolina fastuosa on various plant surfaces. Entomol. Exp. Appl. 105, 13–28 (2002). [Google Scholar]
- Voigt D., Schuppert J. M., Dattinger S. & Gorb S. N. Sexual dimorphism in the attachment ability of the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) to rough substrates. J. Insect Physiol. 54, 765–776 (2008). [DOI] [PubMed] [Google Scholar]
- Bullock J. M. R. & Federle W. Why have more than one pad per leg? Determining the mechanical and adhesive properties of hairy attachment pads in beetles. Comp. Biochem. Physiol. 153A, 130 (2009). [Google Scholar]
- Bullock J. M. R. & Federle W. The effect of surface roughness on claw and adhesive hair performance in the dock beetle Gastrophysa viridula. Insect Sci. 18, 298–304 (2011). [Google Scholar]
- Vötsch W. et al. Chemical composition of the attachment pad secretion of the locust Locusta migratoria. Insect Biochem. Mol. Biol. 32, 1605–1613 (2002). [DOI] [PubMed] [Google Scholar]
- Perez Goodwyn P., Peressadko A., Schwarz H., Kastner V. & Gorb S. N. Material structure, stiffness, and adhesion: why attachment pads of the grasshopper (Tettigonia viridissima) adhere more strongly than those of the locust (Locusta migratoria) (Insecta: Orthoptera). J. Comp. Physiol. 192, 1233–1243 (2006). [DOI] [PubMed] [Google Scholar]
- Haas F. & Gorb S. Evolution of locomotory attachment pads in the Dermaptera (Insecta). Arthropod. Struct. Dev. 33, 45–66 (2004). [DOI] [PubMed] [Google Scholar]
- Arnold J. W. Adaptive features on the tarsi of cockroaches (Insecta: Dictyoptera). Int. J. Insect Morphol. Embryol. 3, 317–334 (1974). [Google Scholar]
- Clemente C. J. & Federle W. Pushing versus pulling: division of labour between tarsal attachment pads in cockroaches. Proc. R. Soc. B 275, 1329–1336 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente C. J., Dirks J., Barbero D. R., Steiner U. & Federle W. Friction ridges in cockroach climbing pads: anisotropy of shear stress measured on transparent, microstructured substrates. J. Comp. Physiol. A 195, 805–814 (2009). [DOI] [PubMed] [Google Scholar]
- Bauchhenß E. Die Pulvillen von Calliphora erythrocephala (Diptera, Brachycera) als Adhäsionsorgane. Zoomorphology 93, 99–123 (1979). [Google Scholar]
- Gorb S. N. The design of the fly adhesive pad: distal tenent setae are adapted to the delivery of an adhesive secretion. Proc. R. Soc. B 265, 747–752 (1998). [Google Scholar]
- Niederegger S., Gorb S. & Jiao Y. Contact behaviour of tenent setae in attachment pads of the blowfly Calliphora vicina (Diptera, Calliphoridae). J. Comp. Physiol. A 187, 961–970 (2002). [DOI] [PubMed] [Google Scholar]
- Langer M. G., Ruppersberg J. P. & Gorb S. Adhesion forces measured at the level of a terminal plate of the fly's seta. Proc. R. Soc. B 271, 2209–2215 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsler P. & Federle W. Biomechanics of smooth adhesive pads in insects: influence of tarsal secretion on attachment performance. J. Comp. Physiol. A 192, 1213–1222 (2006). [DOI] [PubMed] [Google Scholar]
- Bullock J. M. R., Drechsler P. & Federle W. Comparison of smooth and hairy attachment pads in insects: friction, adhesion and mechanisms for direction dependence. J. Exp. Biol. 211, 3333–3343 (2008). [DOI] [PubMed] [Google Scholar]
- Bußhardt P., Wolf H. & Gorb S. N. Adhesive and frictional properties of tarsal attachment pads in two species of stick insects (Phasmatodea) with smooth and nubby euplantulae. Zoology 115, 135–141 (2012). [DOI] [PubMed] [Google Scholar]
- Federle W., Brainerd E. L., McMahon T. A. & Hölldobler B. Biomechanics of the movable pretarsal adhesive organ in ant and bees. Proc. Natl. Acad. Sci. USA 98, 6215–6220 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federle W., Riehle M., Curtis A. S. G. & Full R. J. An integrative study of insect adhesion: mechanics and wet adhesion of pretarsal pads in ants. Integr. Comp. Biol. 42, 1100–1106 (2002). [DOI] [PubMed] [Google Scholar]
- Frantsevich L. & Gorb S. N. Structure and mechanics of the tarsal chain in the hornet, Vespa crabro (Hymenoptera: Vespidae): implications on the attachment mechanism. Arthropod Struct. Dev. 33, 77–89 (2004). [DOI] [PubMed] [Google Scholar]
- Peisker H. & Gorb S. N. Evaporation dynamics of tarsal liquid footprints in flies (Calliphora vicina) and beetles (Coccinella septempunctata). J. Exp. Biol. 215, 1266–1271 (2012). [DOI] [PubMed] [Google Scholar]
- Dai Z., Gorb S. N. & Schwarz U. Roughness-dependent friction force of the tarsal claw system in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae). J. Exp. Biol. 205, 2479–2488 (2002). [DOI] [PubMed] [Google Scholar]
- Endlein T. & Federle W. Walking on smooth or rough ground: passive control of pretarsal attachment in ants. J. Comp. Physiol. A 194, 49–60 (2008). [DOI] [PubMed] [Google Scholar]
- Han L., Wang Z., Ji A. & Dai Z. Grip and detachment of locusts on inverted sandpaper substrates. Bioinspir. Biomim. 6, 046005 (2011). [DOI] [PubMed] [Google Scholar]
- Chapman R. F. [Legs and locomotion]. The Insects: Structure And Function [Chapman, R. F. (ed)] [151–152] (Cambridge Univ. Press, Cambridge, 1998). [Google Scholar]
- Wang L., Zhou Q. & Xu S. Role of locust Locusta migratoria manilensis claws and pads in attaching to substrates. Chin. Sci. Bull. 56, 789–795 (2011). [Google Scholar]
- Roth L. M. & Willis E. R. Tarsal structure and climbing ability of cockroaches. J. Exp. Zool. 119, 483–517 (1952). [Google Scholar]
- Gorb E. V. & Gorb S. N. The effect of surface anisotropy in the slippery zone of Nepenthes alata pitchers on beetle attachment. Beilstein J. Nanotechnol. 2, 302–310 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladun D. & Gorb S. N. Insect walking techniques on thin stems. Arthropod-Plant Interact. 1, 77–91 (2007). [Google Scholar]
- Spagna J. C., Goldman D. I., Lin P.-C., Koditschek D. E. & Full R. J. Distributed mechanical feedback in arthropods and robots simplifies control of rapid running on challenging terrain. Bioinspir. Biomim. 2, 9–18 (2007). [DOI] [PubMed] [Google Scholar]
- Radnikow G. & Bässler U. Function of a muscle whose apodeme travels through a joint moved by other muscles: why the retractor unguis muscle in stick insects is tripartite and has no antagonist. J. Exp. Biol. 157, 87–99 (1991). [Google Scholar]
- Bußhardt P. & Gorb S. N. Walking on smooth and rough ground: activity and timing of the claw retractor muscle in the beetle Pachnoda marginata peregrina (Coleoptera, Scarabaeidae). J. Exp. Biol. 216, 319–328 (2013). [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43 (1969). [DOI] [PubMed] [Google Scholar]