Abstract
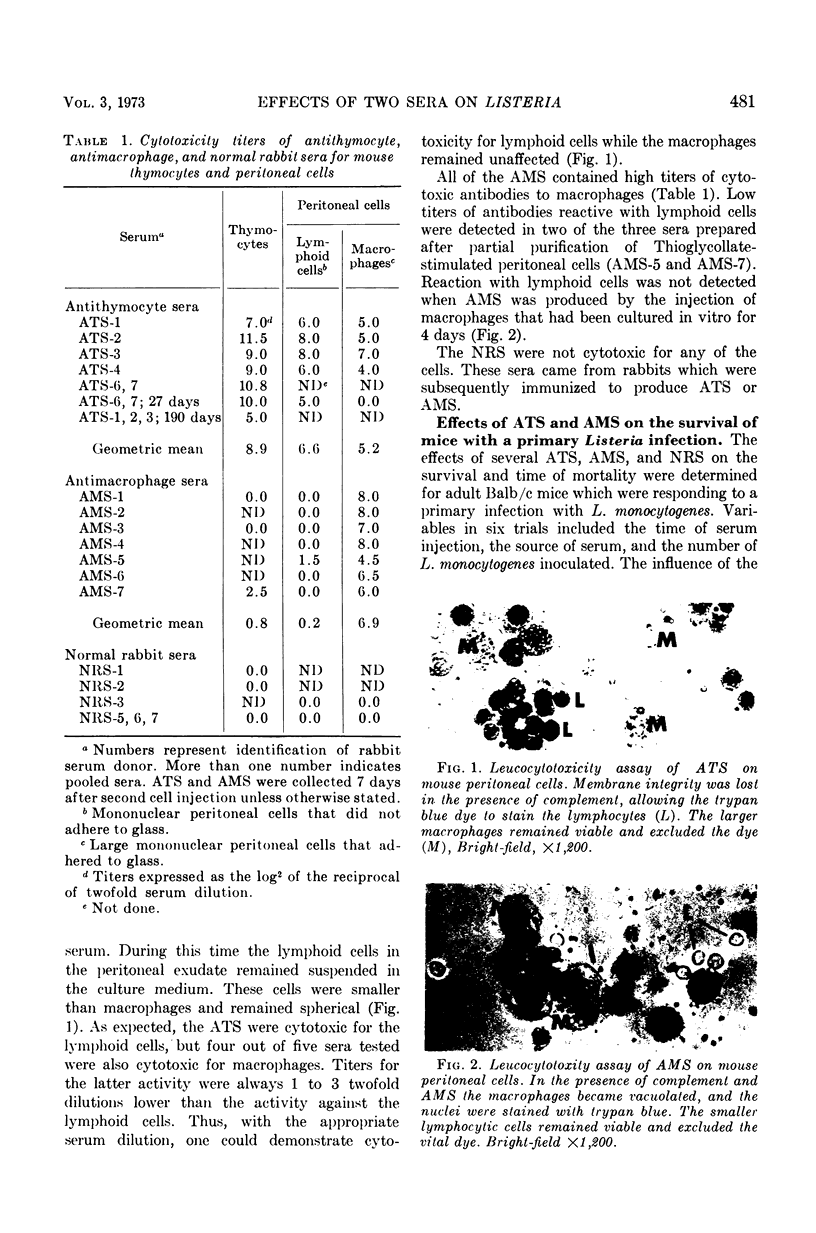
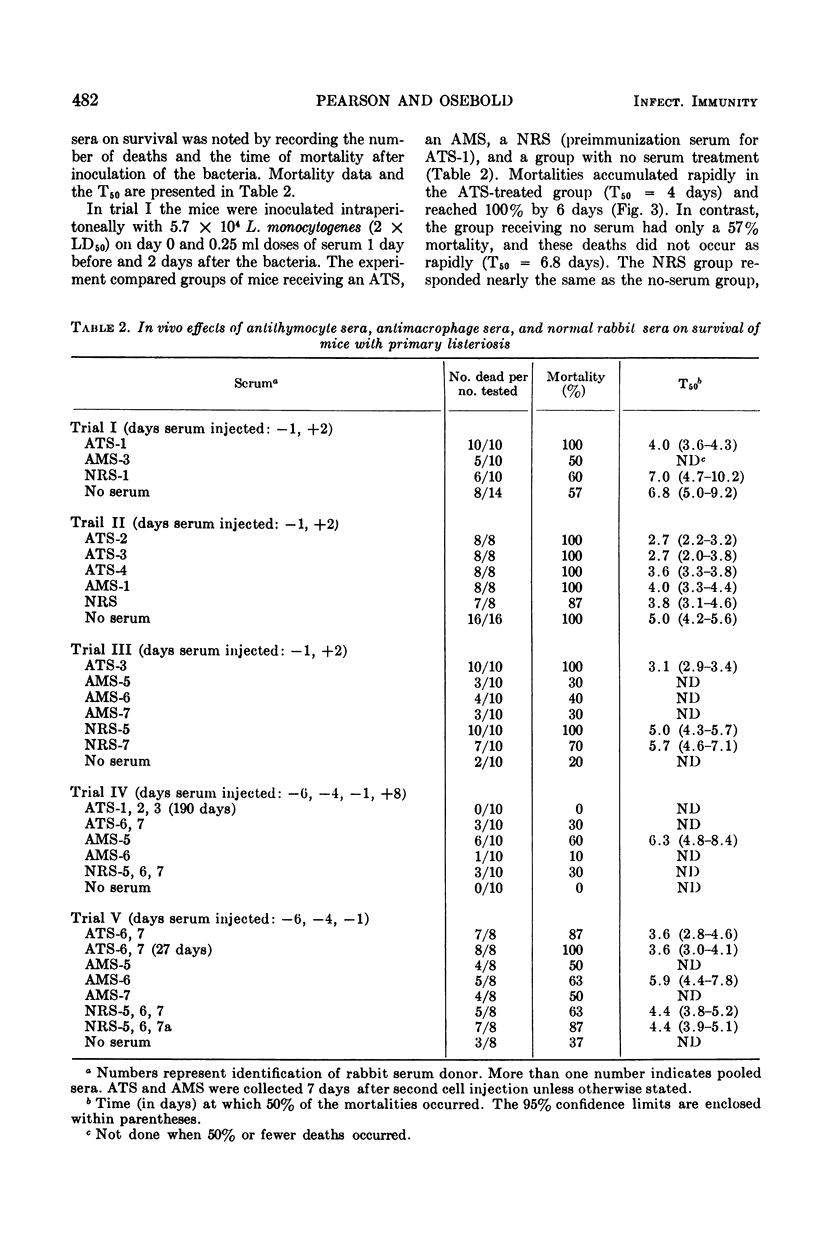
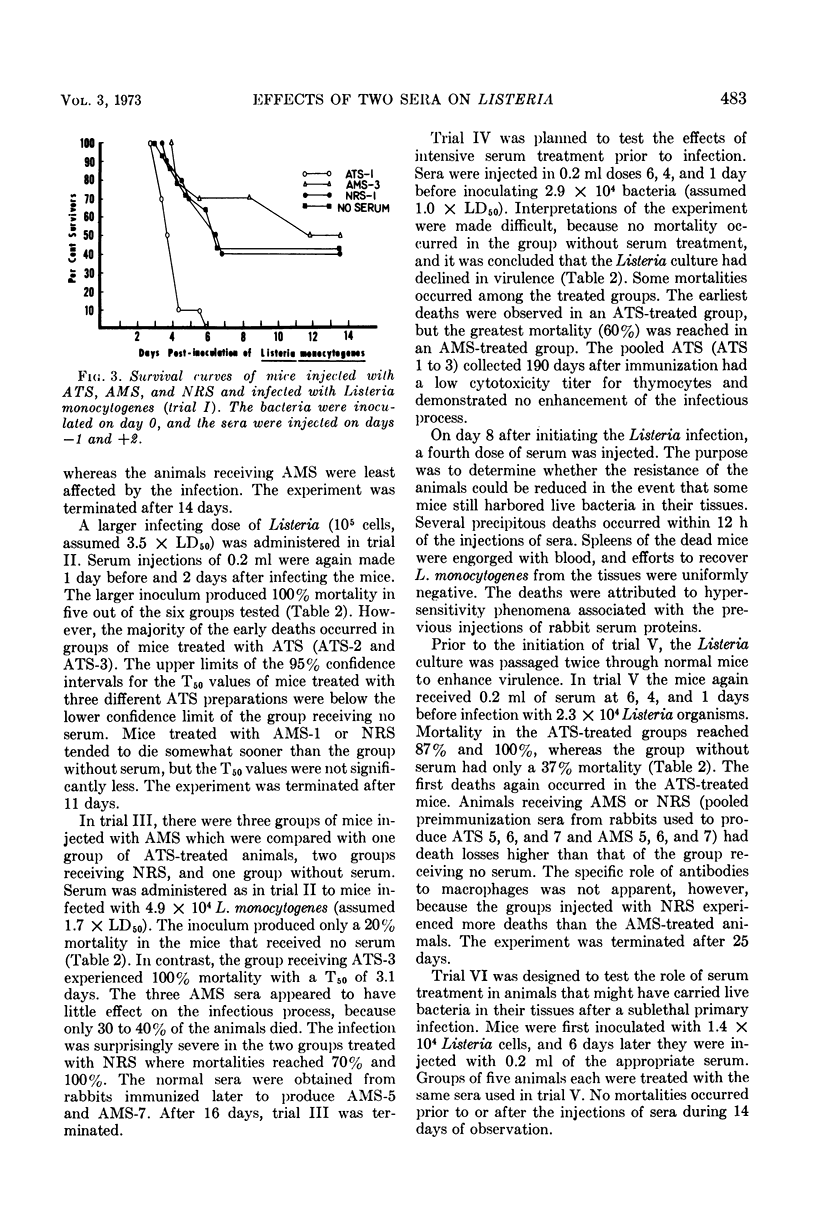
Antisera prepared in rabbits against purified mouse thymocytes (antithymocyte serum; ATS) and peritoneal macrophages (antimacrophage serum; AMS) were injected intraperitoneally into Balb/c mice infected with the bacterium Listeria monocytogenes. When administered near the initiation of infection, the ATS significantly decreased the survival time of the animals and increased the mortality rate. When ATS was administered 6 days after a sublethal dose of L. monocytogenes had been inoculated, an overt disease did not evolve. ATS that significantly potentiated primary listeriosis also had high cytotoxicity titers for thymocytes and lymphoid cells from the peritoneal cavity. Although cytotoxic activity against peritoneal macrophages could be demonstrated in lower dilutions of the ATS, this activity did not appear to correlate with the effects of the sera on listeriosis. The injection of AMS did not enhance the infectious process. In some trials more deaths occurred among mice receiving normal rabbit serum than those receiving AMS. All of the AMS had cytotoxic titers against peritoneal macrophages, and the sera were usually inactive against thymocytes and peritoneal lymphoid cells. Listeria was isolated from fatally infected mice with nearly equal success in all of the serum-treated groups, and the serum treatments did not appear to alter the pattern of gross lesions. The afferent limb of the immune response was markedly affected by the presence of antibodies to lymphocytes. However, antibodies reacting with macrophages did not demonstrably enhance the Listeria process, which depends upon cellular immunity as the principal means of acquired host defense.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson D. M., Cozad G. C. Effect of antilymphocyte serum on animals experimentally infected with Histoplasma capsulatum or Cryptococcus neoformans. J Bacteriol. 1969 Dec;100(3):1271–1276. doi: 10.1128/jb.100.3.1271-1276.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C. Effects of antilymphocytic serum on bacterial and viral infections and virus oncogenesis. Fed Proc. 1970 Jan-Feb;29(1):167–168. [PubMed] [Google Scholar]
- Argyris B. F. Role of macrophages in immunological maturation. J Exp Med. 1968 Sep 1;128(3):459–467. doi: 10.1084/jem.128.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYSE E. A., OLD L. J., THOMAS G. A report on some observations with a simplified cytotoxic test. Transplant Bull. 1962 Apr;29:63–67. [PubMed] [Google Scholar]
- CORDY D. R., OSEBOLD J. W. The neuropathogenesis of listeria encephalomyelitis in sheep and mice. J Infect Dis. 1959 Mar-Apr;104(2):164–173. doi: 10.1093/infdis/104.2.164. [DOI] [PubMed] [Google Scholar]
- Despont J. P., Cruchaud A. In vivo and in vitro effects of anti-macrophage serum. Nature. 1969 Aug 23;223(5208):838–839. doi: 10.1038/223838a0. [DOI] [PubMed] [Google Scholar]
- Gallily R., Gornostansky M. Specificity and nature of binding of antimacrophage serum. Immunology. 1972 Mar;22(3):431–439. [PMC free article] [PubMed] [Google Scholar]
- Gaugas J., Rees R. J. Enhancing effect of antilymphocytic serum on mycobacterial infections in mice. Nature. 1968 Jul 27;219(5152):408–409. doi: 10.1038/219408a0. [DOI] [PubMed] [Google Scholar]
- Grogan J. B. Effect of antilymphocyte serum on mortality of pseudomonas aeruginosa-infected rats. Arch Surg. 1969 Sep;99(3):382–384. doi: 10.1001/archsurg.1969.01340150090017. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S. Effects of antilymphocytic serum on host responses to infectious agents. Fed Proc. 1970 Jan-Feb;29(1):169–170. [PubMed] [Google Scholar]
- Hirsch M. S., Gary G. W., Jr, Murphy F. A. In vitro and in vivo properties of antimacrophage sera. J Immunol. 1969 Mar;102(3):656–661. [PubMed] [Google Scholar]
- Jennings J. F., Hughes L. A. Inhibition of phagocytosis by anti-macrophage antibodies. Nature. 1969 Jan 4;221(5175):79–80. doi: 10.1038/221079a0. [DOI] [PubMed] [Google Scholar]
- Jooste S. V., Lance E. M., Levey R. H., Medawar P. B., Ruszkiewicz M., Sharman R., Taub R. N. Notes on the preparation and assay of anti-lymphocytic serum for use in mice. Immunology. 1968 Nov;15(5):697–705. [PMC free article] [PubMed] [Google Scholar]
- Kingdon G. C., Sword C. P. Cardiotoxic and Lethal Effects of Listeria monocytogenes Hemolysin. Infect Immun. 1970 Apr;1(4):373–379. doi: 10.1128/iai.1.4.373-379.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITCHFIELD J. T., Jr A method for rapid graphic solution of time-per cent effect curves. J Pharmacol Exp Ther. 1949 Dec;97(4):399-408, 3 tab. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewi G., Temple A., Nind A. P., Axelrad M. A study of the effects of anti-macrophage sera. Immunology. 1969 Jan;16(1):99–106. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKI K., MACKANESS G. B. THE PASSIVE TRANSFER OF ACQUIRED RESISTANCE TO LISTERIA MONOCYTOGENES. J Exp Med. 1964 Jul 1;120:93–103. doi: 10.1084/jem.120.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Hill W. C. The effect of anti-lymphocyte globulin on cell-mediated reistance to infection. J Exp Med. 1969 May 1;129(5):993–1012. doi: 10.1084/jem.129.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst F. A., Hill M. J., Glynn A. A. The effect of antilymphocyte serum on subcutaneous staphylococcal infections in normal, immune and complement-deficient mice. J Med Microbiol. 1969 May;2(2):147–159. doi: 10.1099/00222615-2-2-147. [DOI] [PubMed] [Google Scholar]
- Miner N. A., Koehler J., Greenaway L. Intraperitoneal injection of mice. Appl Microbiol. 1969 Feb;17(2):250–251. doi: 10.1128/am.17.2.250-251.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris P. J., Burke J. F. Antilymphocyte serum and staphylococcal infection. Nature. 1967 Jun 10;214(5093):1138–1139. doi: 10.1038/2141138a0. [DOI] [PubMed] [Google Scholar]
- OSEBOLD J. W., SAWYER M. T. Immunization studies on listeriosis in mice. J Immunol. 1957 Apr;78(4):262–268. [PubMed] [Google Scholar]
- Osebold J. W., Aalund O. Interpretation of serum agglutinating antibodies to Listeria monocytogenes by immunoglobulin differentiation. J Infect Dis. 1968 Apr;118(2):139–148. doi: 10.1093/infdis/118.2.139. [DOI] [PubMed] [Google Scholar]
- POMALES-LEBRON A., FERNANDEZ C. A method for estimating the number of bacteria in liquids and tissues. J Bacteriol. 1952 Dec;64(6):837–845. doi: 10.1128/jb.64.6.837-839.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panijel J., Cayeux P. Immunosuppressive effects of macrophage antiserum. Immunology. 1968 Jun;14(6):769–780. [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Porwit-Bòbr Z., Chlap Z. Transformation of hamster macrophages into giant cells with antimacrophage serum. Nature. 1970 Feb 14;225(5233):655–657. doi: 10.1038/225655a0. [DOI] [PubMed] [Google Scholar]
- Réthy L., Padányi M. Suppression of the primary immune response by antilymphocyte serum. Experientia. 1969;25(7):747–747. doi: 10.1007/BF01897602. [DOI] [PubMed] [Google Scholar]
- Schoenholz W. K. Studies on Bedsonia latency. II. Effect of immune lymphocytes and of rabbit-anti-lymphocyte globulin (RAMLG) on infected macrophages exposed to increased incubation temperature in vitro. Z Immunitatsforsch Allerg Klin Immunol. 1970 May;139(4):359–371. [PubMed] [Google Scholar]
- Smolin G., Okumoto M. Antilymphocyte serum potentiation of candida keratitis. Am J Ophthalmol. 1968 Nov;66(5):804–812. doi: 10.1016/0002-9394(68)92794-3. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Properties and some uses of anti-macrophage antibodies. Nature. 1968 Apr 6;218(5136):36–38. doi: 10.1038/218036a0. [DOI] [PubMed] [Google Scholar]
- Weiser R. S. Antitissue versus antimicrobial cellular immunity: a perspective. J Reticuloendothel Soc. 1971 Jul;10(1):17–27. [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Recent studies on acquired immunity in tuberculosis. Curr Top Microbiol Immunol. 1969;48:129–178. doi: 10.1007/978-3-642-46163-7_6. [DOI] [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]