Highlights
-
•
Both risk-taking and threat-reactivity increase in adolescence.
-
•
We propose a model of adolescent affective development to help reconcile this paradox.
-
•
We posit that puberty may be related to a greater capacity to experience fears as thrills.
-
•
Testosterone predicts more threat-activation longitudinally in both amygdala and NAc.
-
•
Interactive effects of amygdala and NAc predict sensation seeking and anxiety.
Keywords: Adolescence, Threat, Reward, Risk taking, Anxiety, Amygdala, Nucleus accumbens, Testosterone, Puberty
Abstract
Adolescent development encompasses an ostensible paradox in threat processing. Risk taking increases dramatically after the onset of puberty, contributing to a 200% increase in mortality. Yet, pubertal maturation is associated with increased reactivity in threat-avoidance systems. In the first part of this paper we propose a heuristic model of adolescent affective development that may help to reconcile aspects of this paradox, which focuses on hypothesized pubertal increases in the capacity to experience (some) fear-evoking experiences as an exciting thrill. In the second part of this paper, we test key features of this model by examining brain activation to threat cues in a longitudinal study that disentangled pubertal and age effects. Pubertal increases in testosterone predicted increased activation to threat cues, not only in regions associated with threat avoidance (i.e., amygdala), but also regions associated with reward pursuit (i.e., nucleus accumbens). These findings are consistent with our hypothesis that puberty is associated with a maturational shift toward more complex processing of threat cues—which may contribute to adolescent tendencies to explore and enjoy some types of risky experiences.
Strange to say, if you do not stamp yourself with the words exhilarated or terrified, those two things [can] feel exactly the same in a body.
Barbara Kingsolver
1. Introduction
Fear is a fundamentally aversive sensation—an affective state that not only increases vigilance to possible danger, but also initiates action tendencies to seek safety and a natural desire to ‘turn off’ the distressing alarm signal. Yet, a surge of fear can sometimes contribute to a desirable sensation, if experienced as exhilarating or thrilling. Understanding this capacity to experience some frightening situations—like a roller coaster, horror movie, or risky sex—as a source of enjoyable thrills, may provide important insights into a paradox in the development of threat processing during adolescence. That is, risk taking and dangerous behaviors increase dramatically during adolescence despite the fact that reactivity in threat-avoidance systems increases during pubertal maturation (e.g., Guyer et al., 2008, Moore et al., 2012, Quevedo et al., 2009).
Compared to children or adults, adolescents are more likely to binge drink, smoke cigarettes, have casual sex, engage in violent and other criminal behavior, and be involved in fatal or serious automobile crashes (largely attributable to risky driving/driving under the influence of alcohol, Steinberg, 2008). There is a growing consensus that the long-term consequences of these preventable risk behaviors represent a leading threat—to the immediate and long-term health—of youth in our nation (Ozer and Irwin, 2009).
Intense interest in the causes of these high-impact problems in youth has led to a number of neurobehavioral theories (e.g., Ernst et al., 2006, Steinberg, 2007) that have focused on the interaction of three systems (or a subset): (1) a motivational system involved in pursuing reward (sometimes termed approach/appetitive motivation), (2) a motivational system involved in escaping threat (sometimes termed avoidance/defensive motivation), and (3) a self-regulatory system involved in inhibiting inappropriate behavior (sometimes termed cognitive control). These theories generally posit a developmental increase in reward reactivity, a decrease in threat reactivity, and relatively ineffective or inconsistent self-regulation.
The proposed increase in reward reactivity during adolescence is thought to promote engagement in risky behavior, because adolescents tend to be more susceptible to the potential positive outcomes of these behaviors. Supporting this hypothesis, both behavioral and neuroscience research has demonstrated that reward processing increases during this maturational period (Galvan, 2010). For example, monetary reward in adolescence leads to enhanced anti-saccade performance (Hardin et al., 2009) and increased reactivity in brain regions commonly associated with reward (e.g., nucleus accumbens, Galvan et al., 2006).
Research focusing on the role of deficient self-regulation as a source of adolescent risk taking has yielded a more nuanced understanding of adolescent development of cognitive control (Crone and Dahl, 2012, Pfiefer and Allen, 2012). An extensive set of studies indicates that adolescent risk behavior is not attributable to: (a) cognitive deficits in decision making or (b) an adolescent ‘sense of invulnerability’. Decades of research have failed to demonstrate any substantive cognitive deficits in risk assessment in adolescents relative to adults (Reyna and Farley, 2006). For example, the ability to estimate the likelihood and severity of adverse outcomes is comparable to adults by the mid-teens—at precisely the age when real-life dangerous behaviors increase enormously. Similarly, the myth that adolescents believe they are personally invulnerable to these dangers has been strongly refuted by data showing that adolescents tend to over-estimate the chances that they will suffer dire consequences from risky behaviors (Reyna and Farley, 2006).
Finally, some theories aimed at understanding risk-taking increases in adolescence focused on possible changes in fear and threat processing, such as a decrease in reactivity of the threat-avoidance that might cause adolescents to ignore or undervalue the potential negative outcomes of their decisions had been considered (e.g., Ernst et al., 2006). Contrary to theory, however, both behavioral and neuroscience data show that adolescent development is not associated with fearlessness, but rather increased activity in threat-avoidance systems. For example, pubertal development has been associated with increased fear-potentiated startle (Quevedo et al., 2009) and adolescents demonstrate increased reactivity in brain regions commonly associated with threat (e.g., amygdala, Guyer et al., 2008, Moore et al., 2012). At first glance, evidence of increased activity in the threat-avoidance system leads to an apparent contradiction. If adolescents are more reactive to potential negative outcomes why do they engage in more risk taking?
1.1. A heuristic model
To help resolve this paradox, we propose a novel heuristic model of adolescent affective development that focuses, in part, on maturational shifts in the tendency to experience a potential threat in more complex ways—including an enhanced capacity to experience these affective signals of threat and arousal (activated in a frightening situation) as part of a more ambiguous and potentially exhilarating sensation. As mentioned above, the affective signal of fear is usually aversive; however, a frightening situation can sometimes create an affective signal that is experienced as a desirable feeling of thrill. Moreover, courageous behavior in the face of danger can occur amidst intense fear, and an increased capacity to experience some of these situations as thrilling could facilitate learning to act boldly despite threat signals indicating potential harm. Thus, a developmental shift in the capacity to experience (and to learn to experience) some high-intensity fears as enjoyable ‘thrills’ could enhance the ability to overcome fears and demonstrate brave behavior. This proposed maturational shift (toward more complex and ambiguous appraisal of threat as ‘thrills’) may contribute not only to healthy versions of learning to be brave (or even heroic) in some extremely frightening situations, but also could contribute to the developmental increases in dangerous and unhealthy versions of thrill seeking and ‘enjoyable’ risk taking observed in adolescence.
This capacity to enjoy high-intensity fears may be part of a broader developmental increase in sensation seeking. As we (Dahl, 2004, Forbes and Dahl, 2010) and others (Steinberg, 2008) have reviewed, the onset of adolescence is associated with an increased tendency to seek novel, high-arousal sensations, and this appears to be directly linked to pubertal maturation (though, see Vetter-O’Hagen and Spear, 2012, for evidence in rodents that novelty seeking is related to age not puberty). There is extensive evidence showing that pubertal increases in sensation seeking predict real-world risky behavior, such as smoking and sexual risk taking (Martin et al., 2002). More generally, we (Crone and Dahl, 2012) have hypothesized that pubertal increases in sensation seeking, along with a larger set of socio-affective changes, promote healthy exploration and learning in adolescence as well as increases in potentially dangerous behavior. Accordingly, we propose that pubertal increases in sensation seeking underpin an enhanced capacity to ‘like’ (and thus, approach) some types of high-arousal, novel, and uncertain situations—even when these situations entail some risk and fear. An increased tendency to ‘like’ a mixed state of fear/excitement (i.e., ‘thrills’) at the onset of puberty may have evolved, at least in part, because an enhanced capacity to learn to master fear and act courageously in some social contexts might have conferred adaptive advantages in dealing with the challenges that faced adolescents in the human ancestral environment (see Schlegel and Barry, 1991, Ellis et al., 2012).
In summary, we propose that pubertal changes in the capacity to process threat in a more ambiguous way, that includes mixed elements of fear and reward (and an increased capacity to experience these as exhilarating or thrilling), represents an important step in reconciling the apparent paradox in threat processing outlined in Section 1. We suggest that these increased tendencies to ‘like’ high intensity sensations, along with neuromaturational changes in the ability to process threats in a more complex way, leads to a greater capacity to experience exhilaration in ways that may facilitate adaptive learning processes important to adolescent development: learning to master fears in some social contexts.
Among the myriad of neurobiological changes occurring during puberty, we believe that the pubertal surge in testosterone is likely to play a particularly important role in driving the proposed affective changes. For example, neuroscience research has linked testosterone to adolescent maturational increases in motivational processing (Forbes et al., 2010, Op de Macks et al., 2011) and preliminary research suggests that testosterone is associated specifically with pubertal increases in sensation seeking (e.g., Kirillova et al., 2001, Martin et al., 2006). Thus, testosterone appears to be a particularly promising target for investigating the proposed pubertal shifts in threat processing.
2. Initial test of key features of the model in a longitudinal study
A crucial hypothesis stemming from our model is that, if pubertal surges in testosterone are often associated with increases in the tendency to experience threat cues as thrilling, then adolescents should both (1) react more strongly to cues signaling potential threat and (2) simultaneously be able to experience these cues as rewarding. In order to test this feature of the proposed model, we examined neural responses to threat cues in a longitudinal study designed to disentangle age and pubertal effects and using serum measures of testosterone and a well-established fMRI task of threat reactivity (Hariri et al., 2002). In the task, participants were presented with social cues that commonly signal the potential for threat (i.e., human faces exhibiting anger or fear).
Based on our heuristic model, we hypothesized that, if pubertal testosterone is associated with an increase in the threat-avoidance system, then concurrent amygdala activation in response to cues signaling threat should also increase, given strong evidence that amygdala is central to learning that stimuli are predictive of threat and responds strongly to such stimuli (for review, see Phelps, 2009). Similarly, if pubertal testosterone is associated with an increase in the tendency to experience potential threat as rewarding, then concurrent activation in nucleus accumbens (NAc) to threat stimuli should also increase, given strong evidence that NAc is central to learning that stimuli are predictive of reward and responds strongly to such stimuli (for review, see Haber, 2009).
Importantly, we tested these hypotheses longitudinally during pubertal maturation by collecting data at two time points (approximately 2 years apart) and examining intra-individual change over time. This provides a more definitive test of our hypotheses than a cross-sectional design (Kraemer et al., 2000). Additionally, given our heuristic model focusing on puberty-specific changes, we utilized a design (selecting youth in a narrow age range but varying in pubertal maturation at time 1), which provided a greater ability to disentangle age and pubertal influences on development.
A second set of hypotheses stemming from our model is that puberty-related increases in activity of the threat-avoidance system should be associated with increased risk taking and decreased anxiety only when stimuli predictive of threat are also experienced as rewarding. These hypotheses were tested by examining whether the relationship between the change in amygdala activation over time and sensation seeking/anxiety depended on the change in NAc activation over time. Specifically, we hypothesized that increased activity in threat-avoidance processing over time, which should be associated with increased amygdala activation, would be associated with higher sensation seeking and decreased anxiety only in those participants who came to experience threat as rewarding, which should be associated with increased NAc activation.
2.1. Methods
2.1.1. Participants
Participants were recruited from the community through advertisements, flyers, and phone lists. By design, participants were recruited within a relatively narrow age range near the onset of puberty (11–12 in girls; 12–13 in boys). Participants were free of current and lifetime psychiatric disorders, did not have braces, and had no history of head injury, serious medical illness, or psychotropic medication, alcohol, or illicit drug use. Parents/guardians provided informed consent, and participants provided assent.
Function magnetic resonance imaging (fMRI) and testosterone data were collected from 61 participants at two time points, 2 years apart (mean = 2.1, standard deviation = .2). Data were not used if motion was ≥5 mm (n = 15), if there were outliers ≥3 SD in the extracted fMRI data (n = 2), or if fMRI data exhibited motion-related artifact (e.g., if the 1st-level β maps exhibited rings around the brain, Poldrack et al., 2011; n = 2). In the final sample (N = 38, 55% female), mean ages were 11.4 years (s.d. = .6) for females and 12.4 years (s.d. = .6) for males at time 1, and 13.5 years (s.d. = .7) for females and 14.4 years (s.d. = .6) for males at time 2. Females were purposefully sampled to be younger based on epidemiologic findings that girls in the United States undergo puberty earlier than boys (e.g., Wu et al., 2002). Seventy-nine percent of sample participants were Caucasian, 16% were African American, and 5% were biracial. Mean socioeconomic status was 41.6 (SD = 12). Importantly, ethnicity and socioeconomic status were not correlated with change in testosterone, sensation seeking, or change in anxiety symptoms (see below for descriptions of these measures), indicating that these factors did not drive observed effects.
2.1.2. Measures
Data for all measures (i.e., testosterone, fMRI, questionnaires, and reaction time) were examined for both univariate and multivariate outliers. Exclusion due to outliers is reported in the relevant section of the methods.
Circulating levels of testosterone were assessed in the morning through bloodspot sampling using a minimally invasive finger-stick procedure (Worthman and Stallings, 1997). Testosterone assays were a modification of a commercially available serum/plasma radioimmunoassay kit (Pantex, Santa Monica, CA). No participants were below the minimal detectable dose sensitivity criterion (14.2 ng/dL males, 14.0 ng/dL females). Sensitivity and inter-assay coefficients of variation were acceptable. To model testosterone increases over time, a change predictor was created by subtracting time 1 from time 2. To model overall levels of testosterone, a predictor was created by averaging levels at times 1 and 2. To remove gender-related differences in the mean and variance of these predictors, they were z-scored separately within each gender.
Participants completed the Sensation Seeking Scale for Children (SSSC, Russo et al., 1993). Due to a data collection error, only time 2 data were available. Anxiety symptoms were assessed using the DSM Anxiety scale of the Child Behavior Checklist (CBCL-DSM-Anxiety, Achenbach and Dumenci, 2001). To assess change in anxiety symptoms over time, a change predictor was created by subtracting time 1 from time 2. CBCL data were not available for 2 participants. Data from 1 participant were not used in CBCL analyses, because it was an extreme outlier (>3 SD).
2.1.3. Threat processing paradigm
The threat processing paradigm was a block-design, face-processing task used in dozens of studies of threat processes (e.g., Hariri et al., 2002). During the face task, participants viewed a trio of stimuli and indicated which of two stimuli (bottom) was identical to the target stimulus (top). The stimuli of interest were facial expressions with anger or fear expressions, and geometric shapes were used as a neutral control condition. For more detail, see Forbes et al. (2011).
In order to test whether increases in pubertal testosterone and amygdala/NAc activation were associated with greater approach behavior to threat (i.e., faster responding), mean reaction time (RT) was computed for each participant, for each timepoint, separately for the threat-face and neutral-control (geometric shape) conditions. RT for the neutral-control condition was subtracted from the threat-face condition to isolate variance related to responding to threat. To determine whether RT changed over time, a paired t-test was calculated. To model change over time, RT for time 1 was subtracted from time 2, and this variable was correlated with change in testosterone, brain activation, sensation seeking, and anxiety symptoms. Due to collection error, RT was not available for 4 participants at one timepoint, and 1 participant was excluded from these analyses due to RT > 3 SD.
2.1.4. fMRI data acquisition and processing
Details regarding fMRI acquisition can be found in Forbes et al. (2011). Functional data for each participant were motion corrected, temporally high-pass filtered (<.007 Hz), spatially smoothed (FWHM = 5 mm), slice-timing corrected, and intensity normalized in FSL's FEAT. To remove motion-related variance, independent component analysis was carried out for each data set using FSL's MELODIC (Beckmann and Smith, 2004). Components reflecting motion were removed.
Regression analyses were performed on the processed time series using FSL's FILM (Woolrich et al., 2001). Predictors corresponding to each stimulus type were included in the regression model. Each predictor was convolved with a gamma function to better approximate the temporal course of the BOLD hemodynamic response and yielded a per-voxel effect-size parameter estimate (β) map representing the magnitude of activation associated with that predictor. To create the comparison of interest, β values for the threat face condition were contrasted against β values for the geometric shape condition.
Amygdala and NAc were segmented in each structural scan using FSL's FIRST (Patenaude et al., 2011). Functional data were registered to the structural with Boundary Based Registration in FSL's FLIRT, and the inverse of this transform was applied to the segmented masks to convert them into functional space. The mean (across voxels) z-values for each mask were extracted for each participant, separately for time 1 and time 2.
Four repeated-measures GLMs (one each for left/right amygdala/NAc) were conducted to test the hypothesis that increased testosterone over time is associated with increased threat-related activation (across gender). Threat-related activation for each ROI was the dependent variable, with Time as the repeated factor. Testosterone Change over time and Mean Testosterone were continuous predictors, with the main test of interest being the Time × Testosterone Change interaction. To remove variance due to different task-condition counterbalancing orders at the two time points, a covariate of no interest was included that modeled consistency of counterbalancing. To remove variance due to whether data collection occurred on a weekday (vs. weekend) at both time points, a covariate of no interest was included that modeled consistency of data collection day. Importantly, these covariates were not correlated with change in testosterone, indicating that the inclusion of these covariates does not bias the analyses (Miller and Chapman, 2001). In addition, all results remain significant when these covariates were not included. Covariate results are not reported. To ensure that findings were not confounded by change in age, analyses were repeated with an additional covariate that modeled age change.
To test the hypotheses that increased activity in the threat-avoidance system is associated with increased sensation seeking and decreased anxiety only when stimuli predictive of threat are also experienced as rewarding, predictors modeling change in threat-related activation over time in left/right amygdala/NAc were created by subtracting activation in each ROI at time 1 from time 2. To model the interaction between amygdala and NAc, cross products were calculated. Finally, SSSC at time 2 was regressed on amygdala, NAc, and interaction predictors. Similar regressions were conducted with CBCL DSM anxiety symptom change over time as the dependent variable.
Given that activation change in amygdala and NAc may be significantly correlated, it is possible that significant interaction tests may actually be due to quadratic variance in the two individual regions. In order to rule out this potential confound, analyses were repeated with relevant quadratic effects entered as covariates of no interest. In addition, correction for multiple comparisons was performed using the Holm–Bonferroni method (Holm, 1979).
2.2. Results
2.2.1. Testosterone change predicting threat-related activation over time
Testosterone significantly increased over time in both males (t16 = 16.3, p < .001) and females (t20 = 5.6, p < .001), and all participants individually demonstrated an increase in testosterone over time, except for 1 female who maintained the same level.
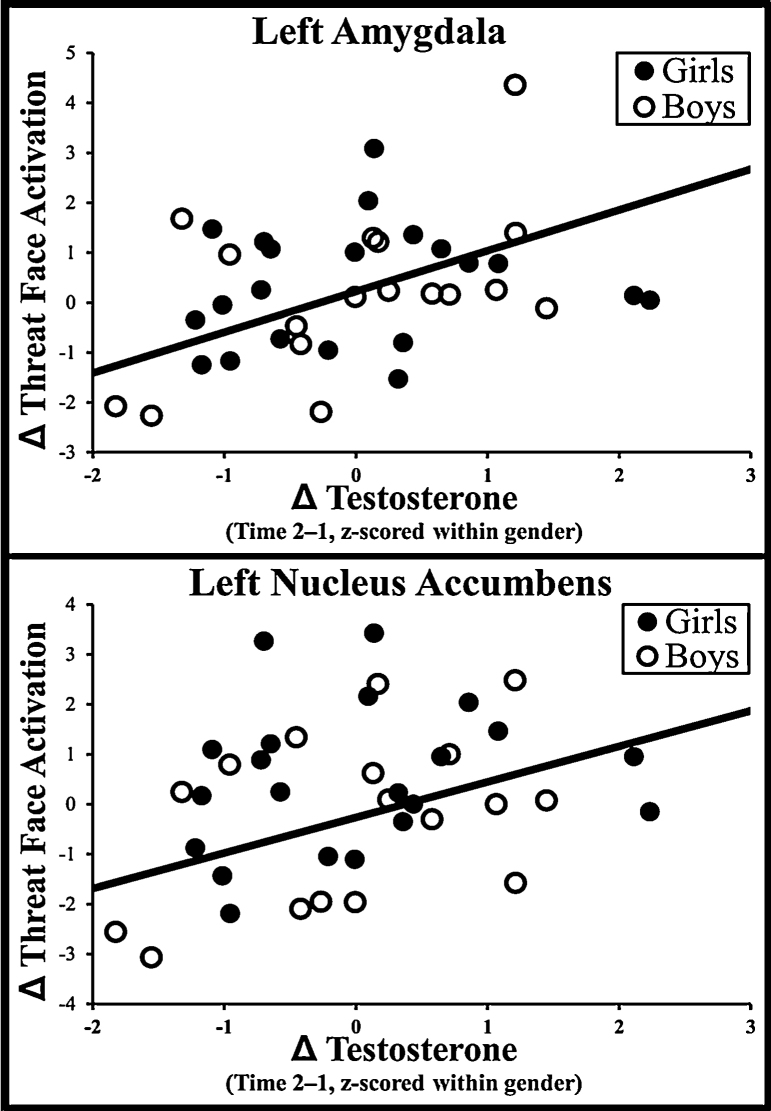
Simple effects of Time, Testosterone Change, and Mean Testosterone were not significant, with the exception of marginal effects of Testosterone Change (F(1,33) = 3.0, p = .09, partial η2 = .08) and Mean Testosterone (F(1,34) = 3.3, p = .08, partial η2 = .09) on right amygdala. As hypothesized, Time and Testosterone Change interacted significantly in left amygdala (F(1,33) = 5.0, p = .03, partial η2 = .13), right amygdala (F(1,33) = 5.7, p = .02, partial η2 = .15), and left nucleus accumbens (F(1,33) = 5.4, p = .03, partial η2 = .14), and marginally in right nucleus accumbens (F(1,33) = 3.3, p = .08, partial η2 = .09). Further examination indicated that increased testosterone predicted increased threat-related activation over time (see Fig. 1). Mean Testosterone did not interact with time in any region. All effects remained significant when age change was covaried out.
Fig. 1.
Relationship between change in testosterone and change in activation to threat faces. Note: ΔTestosterone = testosterone at time 1 subtracted from time 2, z-scored within gender. ΔThreat Face Activation = threat − shape for time 1 subtracted from time 2.
When examining effects within boys, Time and Testosterone Change interacted significantly in left amygdala (F(1,13) = 5.7, p = .03, partial η2 = .30), right amygdala (F(1,13) = 5.0, p = .04, partial η2 = .28) and marginally in left nucleus accumbens (F(1,13) = 4.3, p = .06, partial η2 = .25) and right nucleus accumbens (F(1,13) = 3.8, p = .07, partial η2 = .23). When examining effects within girls, Time and Testosterone Change did not interact significantly in any region, although all effects were in the expected direction and lack of significance may be due to reduced power.
Change over time in RT to threat faces (relative to neutral control) was negatively correlated with SSSC (r = −.35, p = .04) and change in activation to threat in left amygdala (r = −.43, p = .01), right amygdala (r = −.56, p < .01), left NAc (r = −.40, p = .02), and right NAc (r = −.42, p = .01). RT to threat faces (relative to neutral control) did not significantly change over time, no significant correlations were found with mean RT over time, and change over time in RT was not correlated with either change in testosterone or mean testosterone. RT (change or mean over time) was not related to testosterone (change or mean over time).
2.2.2. Interaction of threat and reward systems predicting sensation seeking
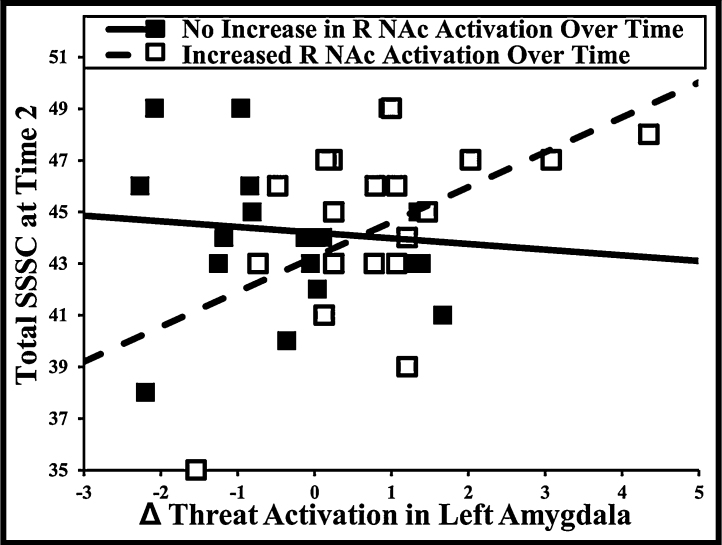
Simple effects of amygdala/NAc were not significant. Supporting our hypotheses, the interaction between left amygdala and right NAc (β = .33, p = .04, R2 = .10) significantly predicted SSSC, and the interaction between right amygdala and right NAc (β = .30, p = .06, R2 = .09) was marginal. As shown in Fig. 2, activation change in left amygdala had a stronger relationship with SSSC in individuals with increased right NAc activation. The left amygdala/right NAc interaction remained significant when quadratic effects of each ROI were covaried out, indicating that the interaction is not being spuriously driven by variance shared with individual quadratic effects. The interaction between left amygdala and right NAc did not remain significant when controlling for multiple comparisons.
Fig. 2.
Relationship between change in amygdala activation and sensation seeking at in individuals with increased or no change in nucleus accumbens activation over time. Note: Change = Time 2 − Time 1. SSSC = Sensation Seeking Scale for Children, R NAc = right nucleus accumbens. The increased and no change groups were determined by median split of right nucleus accumbens activation.
When examining effects within gender, no interactions were significant. However, effects were in the expected direction in all cases, and effect sizes (R2) were similar (i.e., .07–.10), indicating that lack of significance is most likely due to reduced power.
2.2.3. Interaction of threat and reward systems predicting CBCL DSM anxiety symptoms
Simple effects of amygdala were not significant. The simple effect of right NAc was significant (β = −.36, p = .03, R2 = .13) and left NAc was marginal (β = −.31, p = .07, R2 = .10).
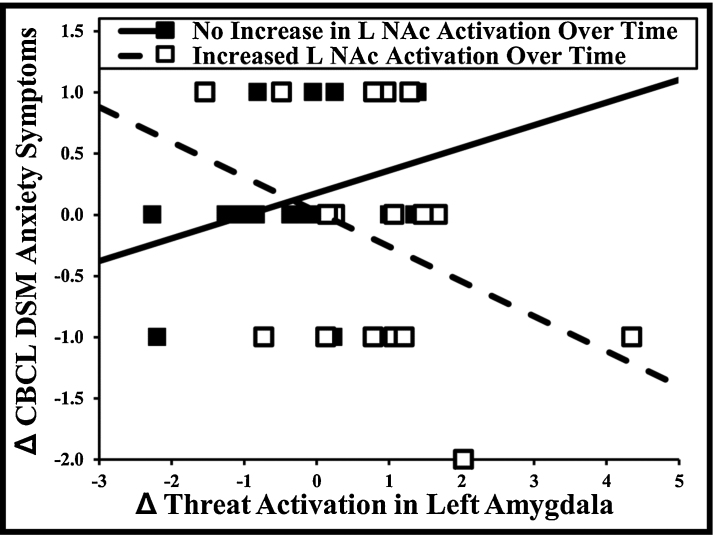
Supporting our hypotheses, the interactions between left amygdala and left NAc (β = −.42, p = .01, R2 = .17), left amygdala and right NAc (β = −.50, p < .01, R2 = .25), right amygdala and left NAc (β = −.34, p = .04, R2 = .11), and right amygdala and right NAc (β = −.41, p = .01, R2 = .17) significantly predicted change in CBCL DSM anxiety symptoms. Amygdala activation change had a positive relationship with anxiety symptoms in individuals with no change in NAc activation over time and a negative relationship in individuals with increased NAc activation (Fig. 3, other interactions exhibited similar patterns). All interactions remained significant when using the Holm–Bonferroni correction for multiple comparisons. All interactions remained significant when quadratic effects were covaried out, except for the right amygdala/left NAc interaction (which maintained the same pattern of effects).
Fig. 3.
Relationship between change in amygdala activation and CBCL DSM anxiety symptoms in individuals with increased or no change in nucleus accumbens activation over time. Note: Change = Time 2 − Time 1. CBCL = Child Behavior Checklist, L NAc = left nucleus accumbens. The increased and no change groups were determined by median split of left nucleus accumbens activation.
When examining effects within boys, the interactions between left amygdala and left NAc (β = −.59, p = .04, R2 = .34) and left amygdala and right NAc (β = −.59, p = .03, R2 = .34) significantly predicted change in CBCL DSM anxiety symptoms. The interactions between right amygdala and left NAc (β = −.53, p = .07, R2 = .27) and right amygdala and right NAc (β = −.51, p = .08, R2 = .24) were marginally significant.
When examining effects within girls, the interaction between left amygdala and right NAc (β = −.43, p = .06, R2 = .15) marginally predicted change in CBCL DSM anxiety symptoms. The other interactions were not significant, although all were in the expected direction.
3. Discussion
Among the myriad of changes that occur during puberty, is there a developmental increase in the capacity to experience some threat signals as exciting and thrilling? Although preliminary, our findings in this longitudinal within-subject study, suggests that (a) there is a shift to a more complex processing of the experience of threat during pubertal maturation and (b) this shift is specifically associated with testosterone. Specifically, we found that increases in testosterone over a 2-year period of pubertal maturation predicted increases over time in brain activation to stimuli typically associated with threat. As predicted, this increase was observed in both a brain region typically associated with threat avoidance (amygdala) and a region typically associated with reward pursuit (NAc). Moreover, increased activation in both amygdala and NAc was related to greater approach behavior (shorter RT to threat faces). These findings are consistent with two aspects of our proposed heuristic model. First, they indicate that pubertal maturation is associated with increased reactivity in brain systems active in threat-avoidance. Second, we found evidence suggesting that pubertal development is associated with a greater capacity to experience threat cues in a more complex, ambiguous way (which may be consistent with our hypothesized maturational enhancement in the capacity to experience some fears as exhilarating).
3.1. Individual differences in threat experience
We found that increased threat reactivity over time in amygdala was associated with decreased anxiety and higher sensation seeking only in adolescents who also showed increased NAc reactivity to threat, which is consistent with our proposal that some adolescents are experiencing threat as rewarding. It should be noted that the analysis with sensation seeking did not survive correction for multiple comparisons. It is possible that the use of only time 2 sensation-seeking data reduced power, because this measure mixed variance related to mean levels of sensation seeking with the variance of interest (change over time). Even so, caution should be used when interpreting this finding. However, given that this is only a preliminary test of the model, and the robustness of the findings for anxiety (for which longitudinal data were available), we believe that the sensation seeking finding is worthy of discussion.
Present findings suggest that individual differences in the degree to which some threats come to be experienced as more exciting/thrilling (i.e., rewarding) may contribute to the differential development of sensation seeking vs. anxiety. These findings may provide insight into the apparent conundrum that adolescent development is associated with increases in both risk taking and rates of pathological anxiety (Paus et al., 2008). Specifically, along with prior research (e.g., Quevedo et al., 2009) data from the present study are consistent with the hypothesis that puberty leads to an increase in threat reactivity. But whether this contributes to (a) a greater avoidance of threat (and increased problems with anxiety in some adolescents), (b) a healthy balance of increased fear and greater exploration and experimenting with some risk taking, or (c) excessive/reckless risk taking, may depend largely on individual differences (in sensation-seeking/anxious tendencies and/or individual experiences in social and affective learning). That is, these pubertal changes in affective processing may create a developmental inflection point for learning different ways to navigate complex situations involving high-stakes fear and rewards, which also interact with individual differences (genetic and/or early experiences that shape tendencies in sensation seeking/anxiety).
Another implication of present findings is that simply alerting adolescents to the potential dangers associated with risky or reckless behavior may fail to decrease risk taking, at least in part because the threatening aspects of the behavior may not be experienced as aversive. In fact, in the extreme, calling attention to the dangerous aspects of a choice may lead to increased risk taking in those adolescents who find exhilaration highly rewarding. The tendency to experience threat as exciting/thrilling may explain why some education programs to reduce risk taking in adolescents (e.g., D.A.R.E.) are often found to be ineffective (Steinberg, 2007).
3.2. Implications of the proposed model
On one hand, an ambiguous response to threat cues (e.g., our hypothesized simultaneous increase in threat avoidance and reward pursuit) could be considered maladaptive. On the other hand, this more complex version of affective processing might also confer an advantage during adolescent development when youth are learning to navigate the complex social challenges that are both frightening and potentially rewarding. That is, for young children, threat signals should (adaptively) activate action tendencies that promote safety-seeking behavior. However, adolescent development brings a greater likelihood of encountering more ambiguous situations, where adaptive responses to threat can sometimes necessitate brave behavior—moving toward a valued goal despite potential dangers and strong feelings of fear. Examples include, not only evolutionary considerations such as learning adult skills (e.g., hunting dangerous animals with a spear), but also the terrifying/thrilling social experiences of early teens in modern life—such as exploration of early romantic and sexual experiences.
More broadly, the combination of increased sensation seeking at puberty (greater tendency to ‘like’ high-intensity experiences) and a pattern of affective responses to threat that includes activation of both threat avoidance and reward pursuit, may facilitate youth tendencies to explore some novel and risky environments. Pubertal maturation may enhance a youth's capacity to enjoy these types of risky experiences despite high levels of threat-related arousal in ways that serve adaptive tendencies in adolescent development (e.g., learning to persist in pursuing highly valued goals despite fear, learning to master fears); however, the same maturational changes could also contribute to vulnerabilities to negative trajectories (e.g., increased likelihood of dangerous risk taking, and increased alcohol/substance use).
3.3. Strengths and limitations
The study benefits from a number of strengths, including the longitudinal design, which allows for more powerful and accurate tests and remains uncommon in the developmental neuroscience literature. Additionally, the design focused on puberty-specific effects and included blood-spot measures of testosterone longitudinally.
There also are limitations that must be considered when interpreting findings. First, participants in the present study were relatively healthy, and may not fully represent the extremes of risk taking and anxiety that are of particular interest as targets of intervention. Second, the purpose of the data portion of the present study was to test our proposed model in particular, rather than decide between competing theories. Future research should design experiments specifically to target those processes that differentiate between alternative models.
Third, although the design of the present study was excellent for disentangling the impact of puberty from those changes that occur simply due to age, general pubertal development remains somewhat confounded with individual differences in pubertal development (e.g., onset timing). To disentangle general development from the effect of differences in onset, a longitudinal study would need to collect data from at least 3 timepoints. For example, a study could collect data when (1) all participants are pre-pubertal, (2) earlier onset participants are mid-pubertal but later onset remain pre-pubertal, and (3) both early and late onset are mid/late pubertal (the present study collected #’s 2 and 3). This would allow for comparisons of pre vs. mid/late pubertal in both early and late developers, averaging across differences in timing, and moderation by timing could be assessed by the interaction of onset and change across time.
Fourth, although the present study tested hypotheses about threat and reward processing by examining activation in amygdala and NAc, respectively, it is clear that the roles of these structures are more complex than a simple one-to-one mapping. For example, research has supported a role for amygdala in pleasantly-valenced emotions and reward learning (e.g., Baxter and Murray, 2002). It is not appropriate to use reverse inferences to suggest that activity in a particular brain region should be interpreted, de facto, as indicating that an experience is ‘rewarding’ or ‘threatening’. Importantly, in the present study we engage in forward inference, because we hypothesize about psychological processes that, if occurring, should be associated with brain activation. Therefore, it is appropriate to conclude that the observed activation patterns are consistent with our model, but not that they prove our model to be true.
These results provide preliminary support of key features of our heuristic model. Noting the limitations and cautions about making reverse inferences we believe there are additional reasons to consider (and further test) features of the model. For example, the possibility that amygdala activation to threat cues in the present study reflects reward processing is undercut by previous findings of pubertal increases in fear-potentiated startle (Quevedo et al., 2009), which is intimately linked to threat-specific processing in amygdala (Miserendino et al., 1990). Furthermore, reward-related amygdala processing would not be inconsistent with our proposal that puberty is associated with a shift toward experiencing potential threat as more rewarding.
With regard to NAc, although it is possible that processing in this structure is related to threat avoidance rather than reward pursuit, numerous studies indicate that NAc activity decreases rather that increases in response the threat (e.g., Cooper and Knutson, 2008, Delgado et al., 2000). Moreover, although some research has found increased activation to aversive stimuli in the ventral striatum (e.g., Levita et al., 2009, Levita et al., 2012, Seymour et al., 2007), there is reason to believe that the region of striatum which responds to aversive stimuli is located posterior and/or superior to nucleus accumbens. For example, Seymour and colleagues (2007) found that reward prediction error correlated with activation in NAc, whereas aversive prediction error correlated with activation in globus pallidus. This anatomical distinction between striatal regions associated with appetitive and aversive stimuli was confirmed by a recent meta-analysis of studies of subjective value which found threat/punishment related activation to be reliably superior/posterior to NAc (whereas reward related activation was located in NAc; Bartra et al., 2013). Importantly, activation in NAc was reliably greater for appetitive than aversive stimuli. In addition, punishment related activation superior/posterior to NAc also emerged when performing the same meta-analysis with Neurosynth software, indicating the robustness of this finding. Given our careful within-participant segmentation of NAc ROIs, we feel confident that our findings reflect processing in NAc, rather than surrounding structures. Therefore, although we cannot infer that the increased NAc activation found in the present study reflects greater reward processing, it does appear unlikely that the NAc findings are driven by greater threat-avoidance/less reward-pursuit processing. This is consistent with our finding that amygdala reactivity was negatively associated with anxiety in those participants with a large increase in NAc activity, which would not be expected if NAc activation reflected threat-avoidance processing.
Rather than reflecting threat or reward per se, it is also possible that amygdala and NAc activation reflects salience/surprise and/or social processing. Further research is needed to disentangle these possibilities. One piece of data supportive of our model is the finding that increased anxiety was related to increased amygdala and decreased NAc, given that there is no obvious reason why greater salience, for example, should lead to this opposing pattern of neural activation in individuals with anxiety. Specifically, if activation in NAc reflects salience per se, we would expect anxiety to be associated with greater activation over time.
A further caution is that the present theory (and tests of that theory) rests on the conceptualization of the neural, behavioral, and subjective state (i.e., feelings) responses to threat as being part of a larger construct of fear (e.g., Kozak and Miller, 1982). It is important to note that other views are extant in the literature, including recent calls to reserve the term “fear” for subjective states (e.g., LeDoux, 2013). Therefore, the implications of present findings for the understanding of fear should be treated with caution. Additionally, the degree to which participants experienced the threat cues as arousing is unclear. Future research using other measures reflecting arousal (e.g., galvanic skin response) would be useful in determining arousal level.
Given these limitations, further studies will be needed to replicate and extend these findings and to deepen our understanding of affective changes in threat processing during pubertal maturation. For example, future research could examine pubertal development in amygdala–NAc coupling and how maturation in this coupling may contribute to the development of risk taking and anxiety. Nonetheless, the present study reports novel findings that are consistent with our heuristic model. Specifically, we demonstrated that increases in testosterone over two years predicted increased activation to threat cues in both (1) a region typically associated with threat avoidance and (2) a region typically associated with reward pursuit. These results are consistent with our hypothesis that puberty is associated with a maturational shift toward experiencing (some) fears as exciting thrills in ways that may help to reconcile the seemingly paradoxical finding that both risk taking and threat reactivity/anxiety increase during adolescence.
Conflict of interest statement
None.
Acknowledgements
We gratefully acknowledge the support of the National Institute of Health (NIH grant R01 DA018910) for this work. We would also like to acknowledge Jill A. Tarr for her extensive work and leadership in recruiting and retaining the sample and Laura Trubnick and the laboratory staff who did a superb job running the youth through this longitudinal study. We also would like to thank the youth and their families who participated in this study.
References
- Achenbach T.M., Dumenci L. Advances in empirically based assessment: revised cross-informant syndromes and new DSM-oriented scales for the CBCL, YSR, and TRF: comment on Lengua, Sadowski, Friedrich, & Fisher (2001) J. Consult. Clin. Psychol. 2001;69:699–702. [PubMed] [Google Scholar]
- Bartra O., McGuire J.T., Kable J.W. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M.G., Murray E.A. The amygdala and reward. Nat. Rev. Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Cooper J.C., Knutson B. Valence and salience contribute to nucleus accumbens activation. NeuroImage. 2008;39:538–547. doi: 10.1016/j.neuroimage.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone E.A., Dahl E.R. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dahl R.E. Adolescent brain development: a period of vulnerabilities and opportunities. Ann. N.Y. Acad. Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Nystrom L.E., Fissell C., Noll D.C., Fiez J.A. Tracking the hemodynamic responses to reward and punishment in the striatum. J. Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Ellis B.J., Del Giudice M., Dishion T.J., Figueredo A.J., Gray P., Griskevicius V., Hawley P.H., Jacobs W.J., James J., Volk A.A., Wilson D.S. The evolutionary basis of risky adolescent behavior: implications for science, policy, and practice. Dev. Psychol. 2012;48:598–623. doi: 10.1037/a0026220. [DOI] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Dahl R.E. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Ryan N.D., Phillips M.L., Manuck S.B., Worthman C.M., Moyles D.L., Tarr J.A., Sciarrillo B.A., Dahl R.E. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:162–172. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Phillips M.L., Silk J.S., Ryan N.D., Dahl R.E. Neural systems of threat processing in adolescents: role of pubertal maturation and relation to measures of negative affect. Dev. Neuropsychol. 2011;36:429–452. doi: 10.1080/87565641.2010.550178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Front. Hum. Neurosci. 2010;4:1–9. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Monk C.S., McClure-Tone E.B., Nelson E., Roberson-Nay R., Adler A.D., Fromm S.J., Leibenluft E., Pine D.S., Ernst M. A developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20:1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N. Anatomy and connectivity of the reward circuit. In: Dreher J.C., Tremblay L., editors. Handbook of Reward and Decision Making. Academic Press; London: 2009. pp. 3–27. [Google Scholar]
- Hardin M.G., Mandell D., Mueller S.C., Dahl R.E., Pine D.S., Ernst M. Inhibitory control in anxious and healthy adolescents is modulated by incentive and incidental affective stimuli. J. Child Psychol. Psychiatry. 2009;50:1550–1558. doi: 10.1111/j.1469-7610.2009.02121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., Kolachana B., Fera F., Goldman D., Egan M.F., Weinberger D.R. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Kirillova G.P., Vanyukov M.M., Gavaler J.S., Pajer K., Tarter R.E. Substance abuse in parents and their adolescent offspring: the role of sexual maturation and sensation seeking. J. Child Adolesc. Subst. Abuse. 2001;10:77–89. [Google Scholar]
- Kozak M.J., Miller G.A. Hypothetical constructs versus intervening variables: a re-appraisal of the three-systems model of anxiety assessment. Behav. Assess. 1982;4:347–358. [Google Scholar]
- Kraemer H.C., Yesavage J.A., Taylor J.L., Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am. J. Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- LeDoux J.E. The slippery slope of fear. Trends Cogn. Sci. 2013;17:155–156. doi: 10.1016/j.tics.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Levita L., Hare T.A., Voss H.U., Glover G., Ballon D.J., Casey B.J. The bivalent side of the nucleus accumbens. NeuroImage. 2009;44:1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L., Hoskin R., Champi S. Avoidance of harm and anxiety: a role for the nucleus accumbens. NeuroImage. 2012;62:189–198. doi: 10.1016/j.neuroimage.2012.04.059. [DOI] [PubMed] [Google Scholar]
- Martin C.A., Guenthner G., Bingcang C., Smith W.J., Curry T., Omar H.A., Raynes M.K., Kelly T.H. A pilot study: Attention Deficit Hyperactivity Disorder, sensation seeking, and pubertal changes. ScientificWorldJournal. 2006;6:637–642. doi: 10.1100/tsw.2006.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C.A., Kelly T.H., Rayens M.K., Brogli B.R., Brenzel A., Smith W.J., Omar H.A. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Miller G.A., Chapman J.P. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miserendino M.J.D., Sananes C.B., Melia K.R., Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Moore W.E., III, Pfeifer J.H., Masten C.L., Mazziotta J.C., Iacoboni M., Dapretto M. Facing puberty: associations between pubertal development and neural responses to affective facial displays. Soc. Cogn. Affect. Neurosci. 2012;7:35–43. doi: 10.1093/scan/nsr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks Z., Moor B.G., Overgaauw S., Guroglu B., Dahl R.E., Crone E.A. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev. Cogn. Neurosci. 2011;1:506–516. doi: 10.1016/j.dcn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer E.M., Irwin C.E. Adolescents and young adult health: From basic health to clinical interventions. In: Lerner R.M., Steinberg L., editors. Handbook of Adolescent Psychology. John Wiley & Sons; Hoboken, NJ: 2009. pp. 618–641. [Google Scholar]
- Patenaude B., Smith S., Kennedy D., Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T., Keshavan M., Giedd J.N. Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfiefer J.H., Allen N.B. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn. Sci. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A. The human amygdala and the control of fear. In: Whalen P.J., Phelps E.A., editors. The Human Amygdala. Guilford Press; New York, NY: 2009. pp. 204–219. [Google Scholar]
- Poldrack R.A., Mumford J.A., Nichols T.E. Cambridge University Press; New York: 2011. Handbook of functional MRI data analysis. [Google Scholar]
- Quevedo K.M., Benning S.D., Gunnar M.R., Dahl R.E. The onset of puberty: effects on the psychophysiology of defensive and appetitive motivation. Dev. Psychopathol. 2009;21:27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna V.F., Farley F. Risk and rationality in adolescent decision making: implications for theory, practice, and public policy. Psychol. Sci. Public Interest. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Russo M.F., Stokes G.S., Lahey B.B., Christ M.A.G., McBurnett K., Loeber R., Stouthamer-Loeber M., Green S.M. A sensation seeking scale for children: further refinement and psychometric development. J. Psychopathol. Behav. Assess. 1993;15:69–86. [Google Scholar]
- Schlegel A., Barry H. Free Press; New York: 1991. Adolescence: An Anthropological Inquiry. [Google Scholar]
- Seymour B., Daw N., Dayan P., Singer T., Dolan R. Differential encoding of losses and gains in the human striatum. J. Neurosci. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: new perspectives from brain and behavioral science. Curr. Dir. Psychol. Sci. 2007;16:55–59. [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C.S., Spear L.P. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev. Psychobiol. 2012;54:523–535. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worthman C.M., Stallings J.F. Hormone measures in finger-prick blood spot samples: new field methods for reproductive endocrinology. Am. J. Phys. Anthropol. 1997;104:1–21. doi: 10.1002/(SICI)1096-8644(199709)104:1<1::AID-AJPA1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Wu T., Mendola P., Buck G.M. Ethnic differences in the presence of secondary sex characteristics and menarche among US girls: the third national health and nutrition examination survey, 1988–1994. Pediatrics. 2002;110:752–757. doi: 10.1542/peds.110.4.752. [DOI] [PubMed] [Google Scholar]