Abstract
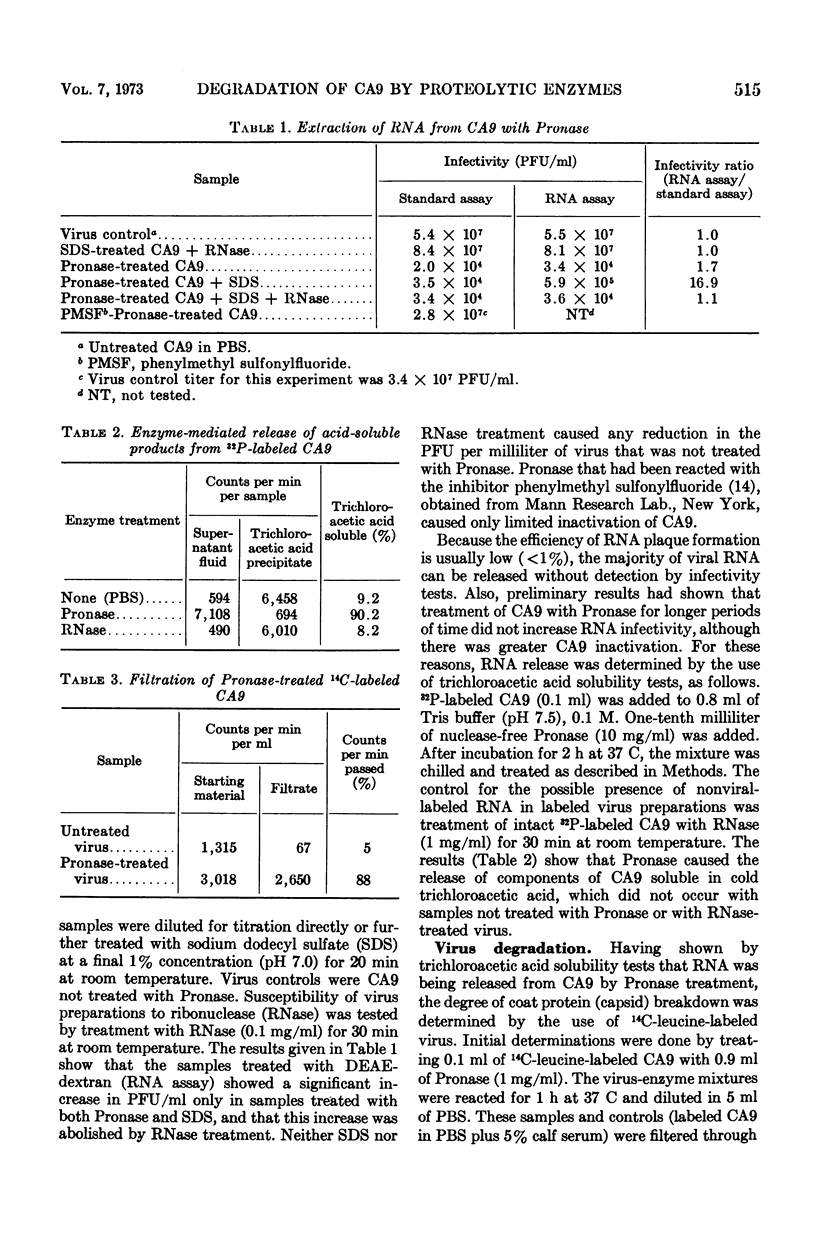
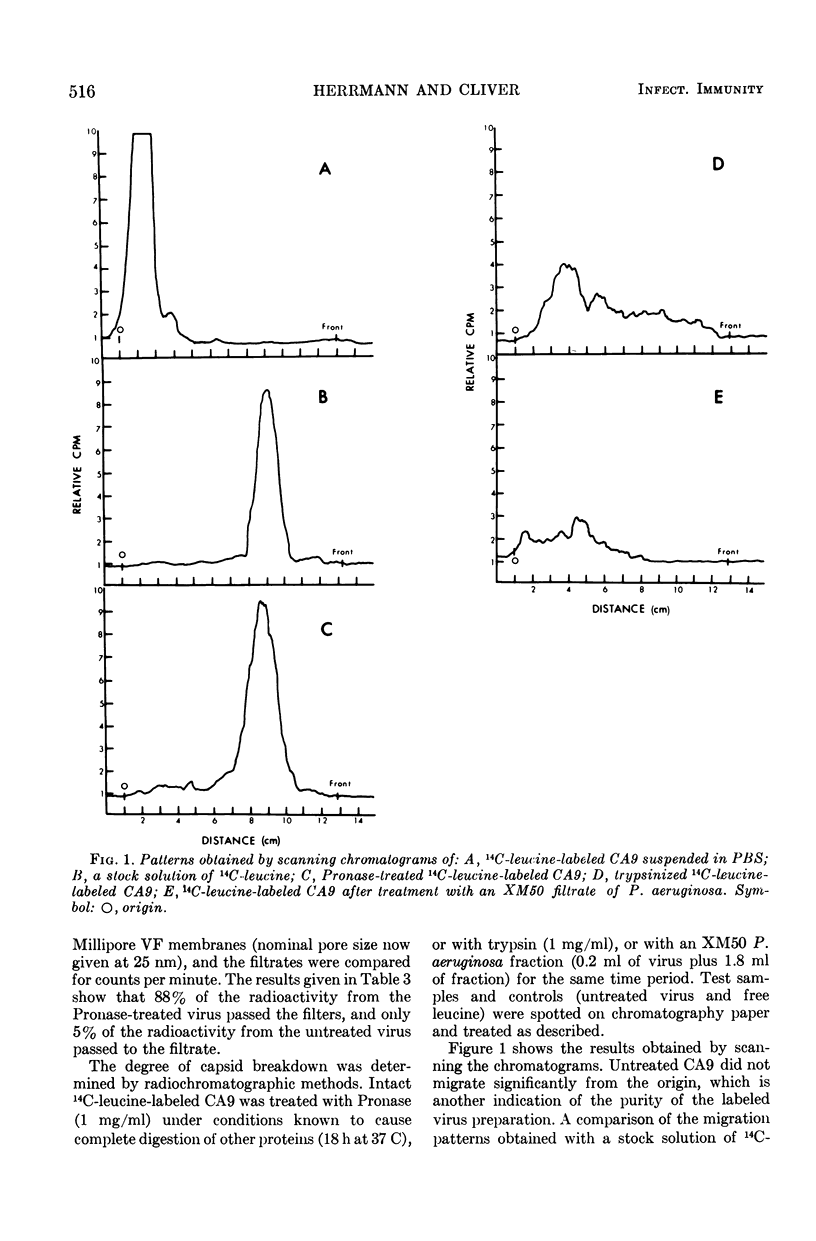
The means by which coxsackievirus type A9 (CA9) is inactivated by proteolytic enzymes was investigated. After reaction of 14C-leucine-labeled CA9 with Pronase, free leucine was liberated as measured by radiochromatography. Treatment of 14C-leucine-labeled CA9 with trypsin or proteolytic filtrates of Pseudomonas aeruginosa caused the release of a variety of labeled substances. The extent of viral ribonucleic acid (RNA) release after exposure of CA9 to Pronase was determined by RNA infectivity tests or trichloroacetic acid solubility tests. Infective viral RNA was found not to be consistently released by reaction of CA9 with Pronase, but further treatment with 1% sodium dodecyl sulfate at pH 7.0 promoted viral RNA release. Sodium dodecyl sulfate treatment of CA9 that had not been reacted with Pronase did not inactivate virus or cause viral RNA release. Reaction of Pronase with 32P-labeled CA9 resulted in the liberation of virus components soluble in cold trichloroacetic acid, whereas untreated CA9 or CA9 reacted with ribonuclease were precipitated by cold trichloroacetic acid. These results demonstrate that the primary means by which protease-sensitive enteroviruses are inactivated is by degradation of the virus capsid, with subsequent release of viral RNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breindl M. The structure of heated poliovirus particles. J Gen Virol. 1971 Jun;11(3):147–156. doi: 10.1099/0022-1317-11-3-147. [DOI] [PubMed] [Google Scholar]
- Cliver D. O., Herrmann R. M. Economical tissue culture technics. Health Lab Sci. 1969 Jan;6(1):5–17. [PubMed] [Google Scholar]
- Crowell R. L., Philipson L. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J Virol. 1971 Oct;8(4):509–515. doi: 10.1128/jvi.8.4.509-515.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRON D. J., HELIMAN A. PURIFICATION OF POLIOVIRUS BY DEAE 'SEPHADEX A-25'. Nature. 1964 Oct 17;204:263–264. doi: 10.1038/204263a0. [DOI] [PubMed] [Google Scholar]
- Keller R. Studies on the mechanism of the enzymatic reactivation of antibody-neutralized poliovirus. J Immunol. 1968 May;100(5):1071–1099. [PubMed] [Google Scholar]
- Lerner A. M., Miranda Q. R. Cellular interactions of several enteroviruses and a reovirus after treatment with sodium borohydride or carbohydrases. Virology. 1968 Oct;36(2):277–285. doi: 10.1016/0042-6822(68)90145-1. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Hsieh H. C. Inhibition of protease production of various bacteria by ammonium salts: its effect on toxin production and virulence. J Bacteriol. 1969 Aug;99(2):406–413. doi: 10.1128/jb.99.2.406-413.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel B. Characterization of type 1 poliovirus by electrophoretic analysis. Virology. 1971 Jun;44(3):554–568. doi: 10.1016/0042-6822(71)90369-2. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., McCutchan J. H., Vaheri A. Factors influencing the enhancement of the infectivity of poliovirus ribonucleic acid by diethylaminoethyl-dextran. J Virol. 1967 Oct;1(5):891–897. doi: 10.1128/jvi.1.5.891-897.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trop M., Birk Y. The specificity of proteinases from Streptomyces griseus (pronase). Biochem J. 1970 Jan;116(1):19–25. doi: 10.1042/bj1160019. [DOI] [PMC free article] [PubMed] [Google Scholar]



