Abstract
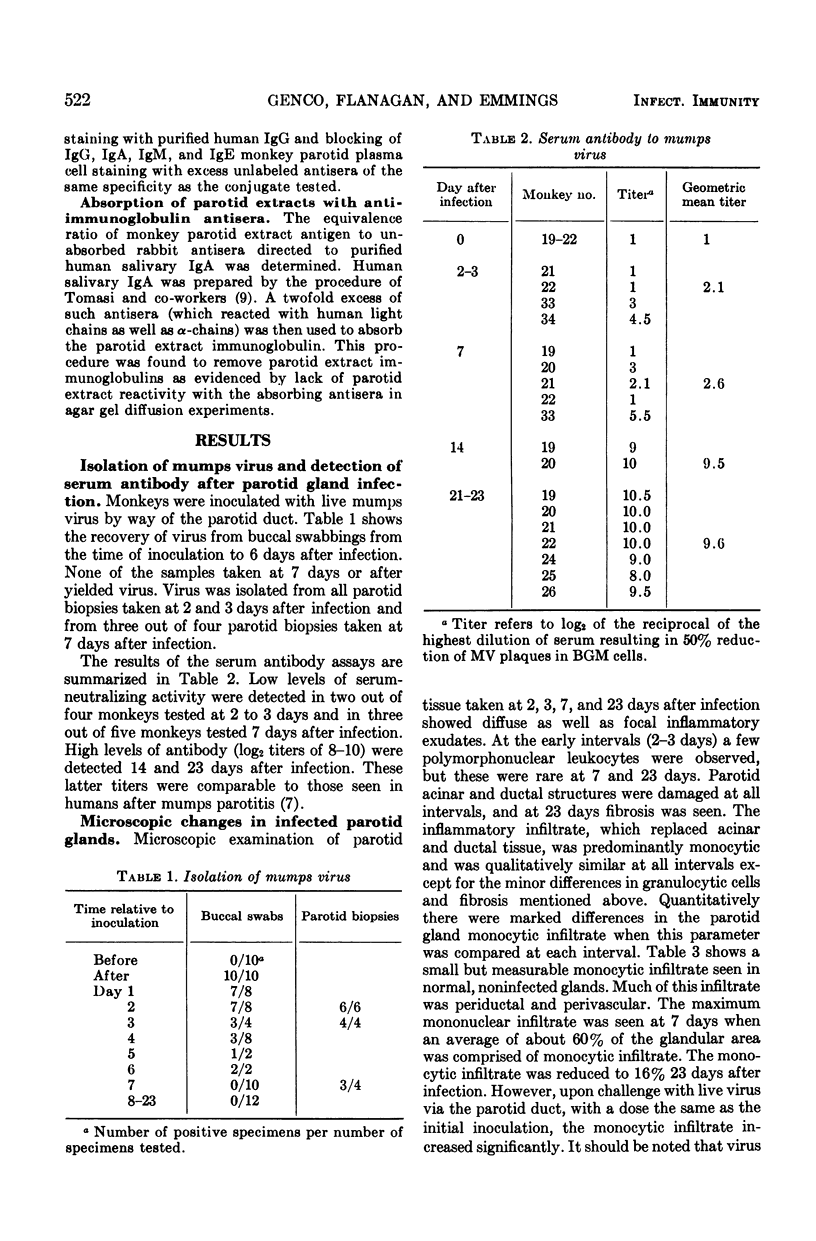
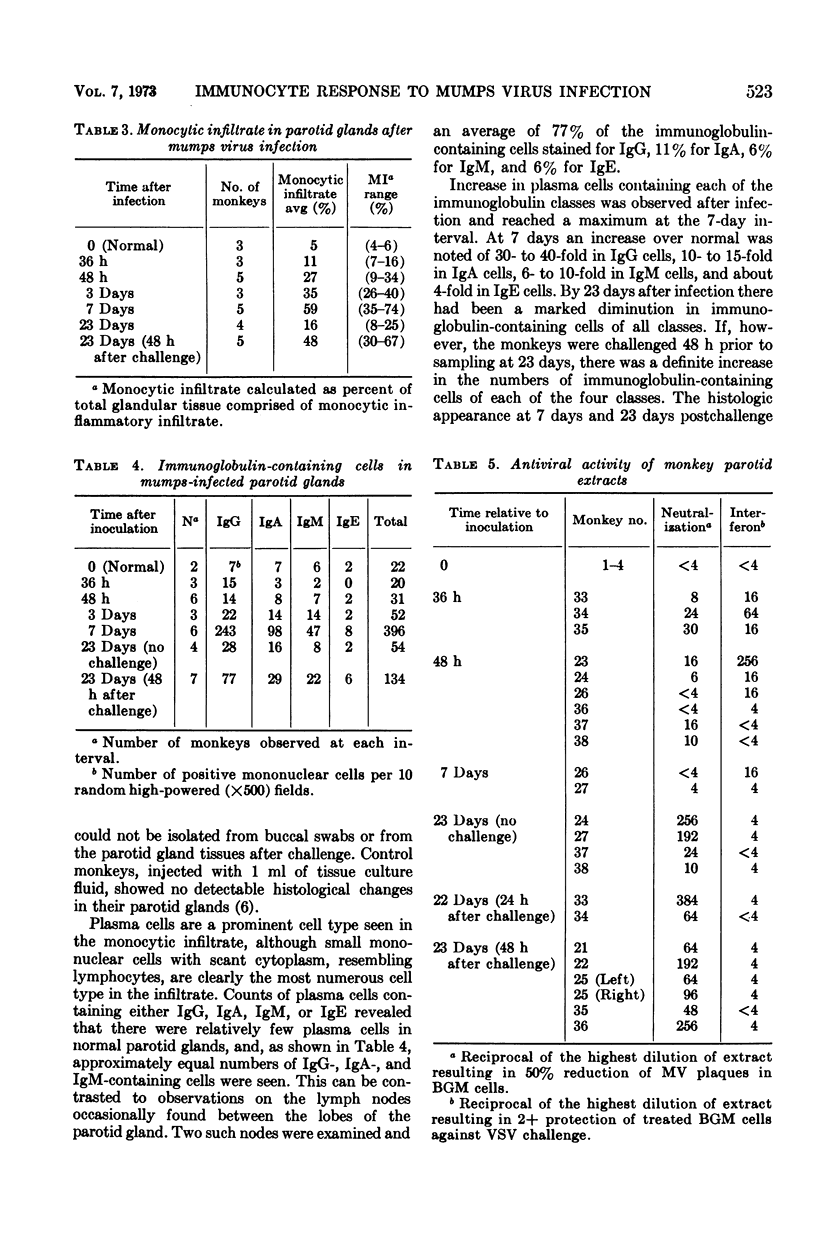
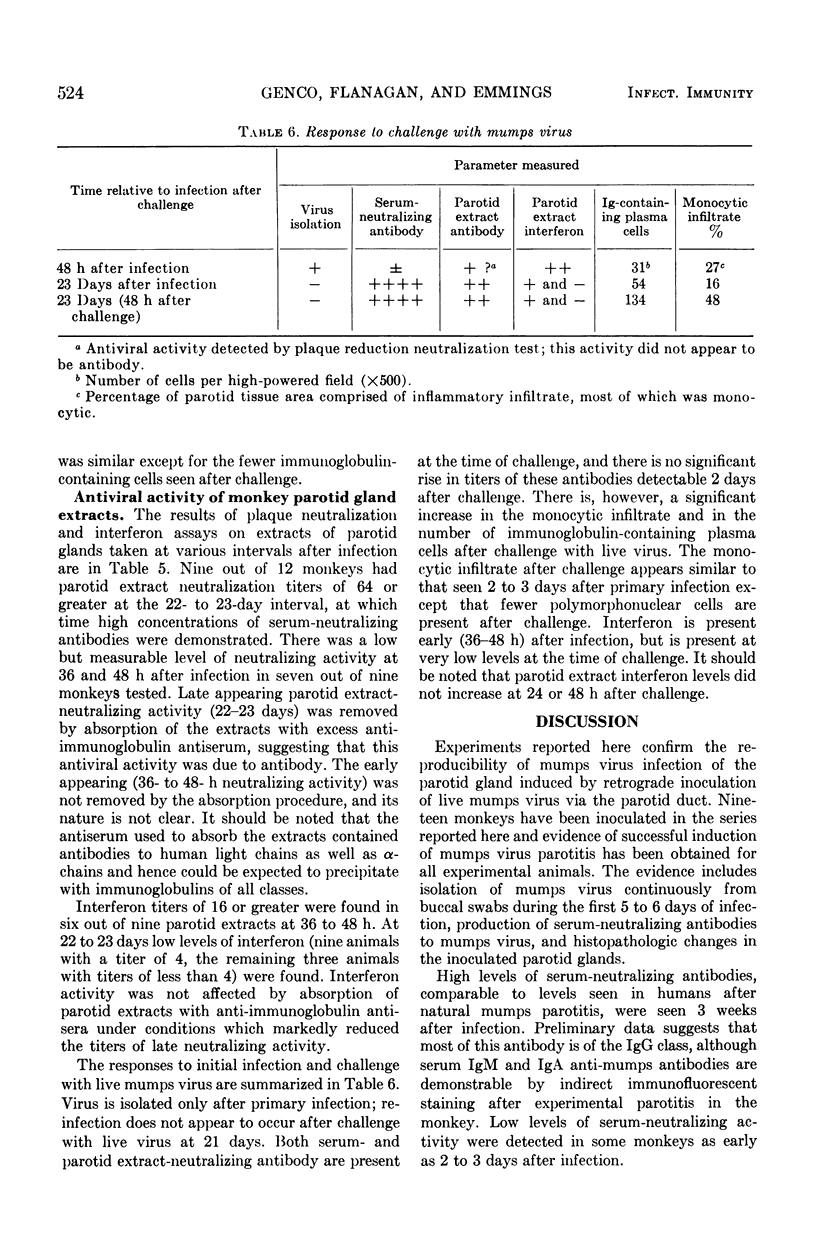
Nineteen rhesus monkeys were inoculated with mumps virus by retrograde ductal instillation into the parotid gland. Evidence of infection was obtained in all instances. Virus was isolated from buccal swabbings and parotid biopsies for 1 week after inoculation. A vigorous serum-neutralizing antibody response occurred within 3 weeks, and there was marked monocytic infiltration of the parotid stroma. The monocytic infiltrate comprised as much as 60% of the total gland volume 1 week after infection. The predominant inflammatory cells were non-immunoglobulin-containing mononuclear cells resembling lymphocytes. Plasma cells containing immunoglobulin G (IgG), IgA, IgM, and IgE increased in numbers in the gland after infection, the greatest increase occurring in IgG-containing cells. Neutralizing antibodies and interferon were found in extracts prepared from the infected glands. Neutralizing activity was highest in samples taken 3 weeks after infection but was detectable in samples taken as soon as 36 to 48 h after infection. Interferon activity was detected in significant amounts 36 to 48 h after infection. Challenge of previously infected animals resulted in an increase in the monocytic infiltrate as well as an increase in numbers of immunoglobulin-containing plasma cells. However, reinfection did not occur as evidenced by the inability to culture shed virus after challenge. This model should be useful for in vivo study of biochemical mediators which evoke inflammatory cell infiltration and which may be significant both in protection and in tissue damage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A. L., Olshevsky C., Cohen M. M. Characteristics of the BGM line of cells from African green monkey kidney. Brief report. Arch Gesamte Virusforsch. 1970;32(4):389–392. doi: 10.1007/BF01250067. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Fjellanger I., Gjeruldsen S. T. Localization of immunoglobulins in human nasal mucosa. Immunochemistry. 1967 Jan;4(1):57–60. doi: 10.1016/0019-2791(67)90197-8. [DOI] [PubMed] [Google Scholar]
- Eidelman S., Berschauer J. A. A method for immunocytochemical study of human gastrointestinal suction biopsies. Stain Technol. 1969 Jan;44(1):43–44. [PubMed] [Google Scholar]
- Flanagan T. D., Andrada J. A., Barron A. L., Witebsky E. Response to experimental infection with mumps virus in rhesus monkeys. Infect Immun. 1971 May;3(5):642–647. doi: 10.1128/iai.3.5.642-647.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan T. D., Barron A. L. Plaque formation by mumps virus and inhibition by antiserum. Appl Microbiol. 1970 Feb;19(2):360–366. doi: 10.1128/am.19.2.360-366.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASI T. B., Jr, TAN E. M., SOLOMON A., PRENDERGAST R. A. CHARACTERISTICS OF AN IMMUNE SYSTEM COMMON TO CERTAIN EXTERNAL SECRETIONS. J Exp Med. 1965 Jan 1;121:101–124. doi: 10.1084/jem.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTZ J. P., SZWED C. F. Mumps III. Comparison of methods for detection of viruria. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:841–844. doi: 10.3181/00379727-110-27666. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Cohen S., Flanagan T. D. Leukotactic factors elaborated by virus-infected tissues. J Exp Med. 1972 May 1;135(5):1095–1103. doi: 10.1084/jem.135.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]


