Abstract
In non-apoptotic cells, Bak constitutively resides in the mitochondrial outer membrane. In contrast, Bax is in a dynamic equilibrium between the cytosol and mitochondria, and is commonly predominant in the cytosol. In response to an apoptotic stimulus, Bax and Bak change conformation, leading to Bax accumulation at mitochondria and Bak/Bax oligomerization to form a pore in the mitochondrial outer membrane that is responsible for cell death. Using blue native-PAGE to investigate how Bax oligomerizes in the mitochondrial outer membrane, we observed that, like Bak, a proportion of Bax that constitutively resides at mitochondria associates with voltage-dependent anion channel (VDAC)2 prior to an apoptotic stimulus. During apoptosis, Bax dissociates from VDAC2 and homo-oligomerizes to form high molecular weight oligomers. In cells that lack VDAC2, constitutive mitochondrial localization of Bax and Bak was impaired, suggesting that VDAC2 has a role in Bax and Bak import to, or stability at, the mitochondrial outer membrane. However, following an apoptotic stimulus, Bak and Bax retained the ability to accumulate at VDAC2-deficient mitochondria and to mediate cell death. Silencing of Bak in VDAC2-deficient cells indicated that Bax required either VDAC2 or Bak in order to translocate to and oligomerize at the mitochondrial outer membrane to efficiently mediate apoptosis. In contrast, efficient Bak homo-oligomerization at the mitochondrial outer membrane and its pro-apoptotic function required neither VDAC2 nor Bax. Even a C-terminal mutant of Bax (S184L) that localizes to mitochondria did not constitutively target mitochondria deficient in VDAC2, but was recruited to mitochondria following an apoptotic stimulus dependent on Bak or upon over-expression of Bcl-xL. Together, our data suggest that Bax localizes to the mitochondrial outer membrane via alternate mechanisms, either constitutively via an interaction with VDAC2 or after activation via interaction with Bcl-2 family proteins.
Bax and Bak are the key effectors of the intrinsic apoptotic pathway initiated in response to diverse stimuli including anoikis, DNA damage and growth factor withdrawal.1 Both proteins are normally dormant in healthy cells, but upon reception of an apoptotic stimulus, they undergo conformation change that allows their self-association to form pores in the mitochondrial outer membrane (MOM).2, 3, 4, 5, 6, 7 The consequence of disruption of the MOM is twofold; it impairs the ability of mitochondria to generate ATP by oxidative phosphorylation and it allows the release of intermembrane proteins including cytochrome c that agonizes caspases that dismantle the cell.
Bak and Bax share significant structural homology in their inactive states and have conserved mechanism of conformation change and oligomerization.3, 8, 9, 10 Further, genetic studies reveal that Bak and Bax perform at least partially overlapping function, with deficiency in both necessary to perturb apoptosis during embryonic development and in response to toxic insult.1, 11 However, whether Bak and Bax are regulated similarly is unclear. Whereas Bak is constitutively anchored in the MOM via its hydrophobic C-terminal transmembrane domain, Bax is predominantly cytosolic in the majority of non-apoptotic cells.12 Recent evidence indicates that Bax is in a dynamic equilibrium between cytosol and mitochondria and is constantly trafficked away from the MOM in non-apoptotic cells.13, 14 In response to apoptotic stress this ‘retrotranslocation' is disrupted causing Bax to accumulate at mitochondria; a hallmark of most apoptotic cells. The mechanism governing the dynamic distribution of Bax in healthy and apoptotic cells is unclear with interactions with pro-survival proteins debated.13, 14
Voltage-dependent anion channels (VDACs) are the major channels responsible for ion passage across the MOM. Studies have also implicated an additional role for the VDACs in the regulation of Bak or Bax apoptotic function or potentially even constituting a component of the Bak/Bax apoptotic pore.15, 16, 17, 18 However, these studies have provided contrasting findings relating to whether VDACs might positively or negatively regulate Bak/Bax apoptotic function.
We used blue native-PAGE (BN-PAGE) to investigate how Bax oligomerizes in the MOM during apoptosis. We observed that VDAC2 is a determinant of the constitutive association of both Bax and Bak with the MOM. The defect in Bax mitochondrial localization can be bypassed by Bak-dependent recruitment during apoptosis. Thus, our data suggest that mitochondrial localization of Bax occurs via distinct mechanisms in healthy and apoptotic cells and that either VDAC2 or Bak is required for the efficient translocation of Bax and hence for the oligomerization at the MOM and Bax apoptotic function.
Results
Bax associates with a discrete high molecular weight complex requiring VDAC2 in non-apoptotic cells
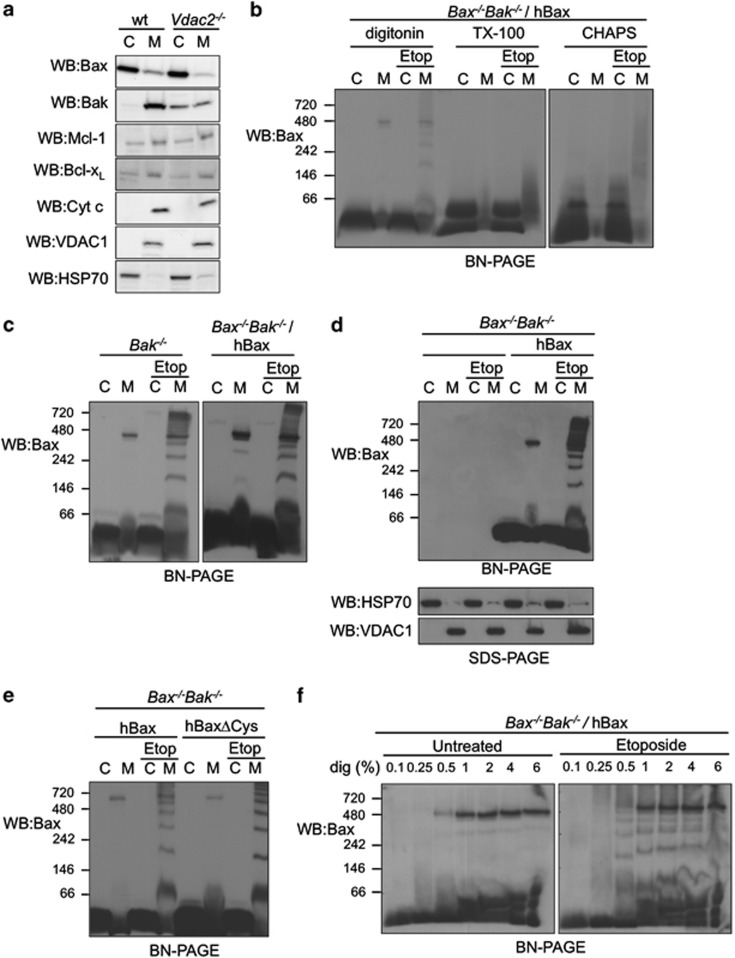
In healthy mouse embryonic fibroblasts (MEFs), Bax is predominantly cytosolic, but with a population that constitutively resides at mitochondria (Figure 1a).12, 14 Bak, on the other hand, is constitutively mitochondrial (Figure 1a). To examine the oligomeric state of Bax at each subcellular location, we analysed Bak−/−MEFs or Bak−/−Bax−/− MEFs stably expressing human Bax by BN-PAGE. To analyse Bax complexes, we used digitonin as it retains Bax in its native conformation5 and retains protein–protein interactions of Bax19 and Bak.20, 21 Although the zwitterionic detergent CHAPS is thought to maintain Bax in its inactive state,5, 12 it has also been reported to disrupt Bax interactions,19 and fails to efficiently solubilize certain MOM proteins such as VDAC1.22 When solubilized in digitonin, but not CHAPS or Triton X-100, distinct Bax complexes in subcellular fractions (cytosol and mitochondria-enriched heavy membrane) of non-apoptotic or apoptotic cells were observed (Figure 1b). Endogenous Bax in Bak−/− MEFs formed similar complexes to ectopically expressed hBax (Figure 1c) and none were detected in Bak−/−Bax−/− MEFs (Figure 1d) thereby confirming specificity.
Figure 1.
Bax forms discrete mitochondrial complexes detectable on BN-PAGE. (a) Bax and Bak exhibit altered subcellular localization in the absence of VDAC2. Wild-type (wt) and Vdac2−/− MEFs were permeabilized and cytosol (C) and mitochondria-enriched heavy membrane fractions (M) were immunoblotted for Bak, Bax, Bcl-xL, Mcl-1, cytochrome c, VDAC1 (mitochondrial marker) or HSP70 (cytosolic marker). (b) Bax mitochondrial complexes are stable in digitonin. Bak−/−Bax−/− MEFs stably expressing hBax were treated or not with etoposide in the presence of Q-VD.oph for 24 h. Cytosol (C) and membrane (M) fractions were solubilized in 1% (w/v) digitonin, Triton X-100 (TX-100) or CHAPS and analysed by BN-PAGE. (c) Endogenous mBax forms similar complexes to ectopically expressed hBax. Bak−/−MEFs or Bak−/−Bax−/− MEFs stably expressing hBax were treated or not with etoposide prior to fractionation of cytosol (C) and membrane (M) and analysis by BN-PAGE. (d) Bax complexes detected on BN-PAGE are specific. Cytosol and membrane fractions from Bak−/−Bax−/− MEFs or Bak−/−Bax−/− MEFs stably expressing hBax were solubilized in 1% (w/v) digitonin and analysed by BN-PAGE. (e) Bax complexes do not involve disulphide linkage. Cytosol and membrane fractions from Bak−/−Bax−/− MEFs stably expressing hBax or hBax in which both endogenous cysteines were mutated to serine (C14S/C166S, hBaxΔCys) were analysed on BN-PAGE. (f) Bax oligomers are integrated into the MOM, but monomers remain peripheral in apoptotic cells. Bak−/−Bax−/− MEFs were treated or not with etoposide. Membrane fractions were solubilized in the indicated concentration of digitonin (w/v) and analysed by BN-PAGE. All experiments are representative of at least three independent experiments
Prior to an apoptotic stimulus, Bax in the cytosol and mitochondria-enriched membrane fractions was detected in a similar low molecular weight form (Figures 1b and c). Bax has been previously shown to be monomeric in the cytosol of healthy cells,12, 23 suggesting that the mitochondrial population was likewise monomeric. However, a population of Bax was additionally resident in a high molecular weight (>400 kDa) complex in mitochondria of MEFs and a variety of cells, including primary and of different genetic backgrounds (Supplementary Figure 2). We confirmed that the complex was not an artefact of disulphide-linkage during solubilization as a Bax mutant that lacks both endogenous cysteines similarly localized to this complex (Figure 1e). A concentration of digitonin (1% w/v, 8 mM) significantly above its critical micelle concentration (0.5 mM) was required to efficiently solubilize the high molecular weight complex of Bax (Figure 1f) and likewise the membrane-integrated MOM protein VDAC1 (Supplementary Figure 2). In contrast, monomeric Bax could be solubilized from the MOM at a low concentration of digitonin (<0.1% w/v, 0.8 mM), consistent with monomeric Bax being peripherally attached to the MOM in healthy cells (Supplementary Figure 2). Increasing digitonin concentrations did not disrupt the high molecular weight form of Bax, but instead slightly retarded the migration of monomeric Bax on BN-PAGE (Figure 1f and Supplementary Figure 3, compare lanes 3 and 7), consistent with a previous report for other membrane-associated proteins.22
The localization of Bax to a high molecular weight complex in mitochondria of non-apoptotic MEFs was reminiscent of Bak complexing with VDAC2.20, 21 To assess whether Bax also associates with VDAC2, we analysed Vdac2−/− MEFs. As with Bak,20, 21 the high molecular weight Bax complex was undetectable in Vdac2−/− MEFs (Figure 2a) and also HCT116 cells where VDAC2 was disrupted using TALEN (ΔVDAC2, Figure 2b and Supplementary Figure 3). The VDAC2-Bax complex was not observed when VDAC2-proficient mitochondria were solubilized in the presence of cytosolic Bax confirming that the complex was not driven by digitonin (Supplementary Figure 4). Ectopic expression of a haemagglutinin (HA)-tagged VDAC2 in Vdac2−/− MEFs restored the large molecular weight Bax complex and also monomeric Bax in the MOM in healthy cells (Figure 2c, compare lanes 2 and 6 and Supplementary Figure 5). Antibody gel-shift assay confirmed that Bax and VDAC2 reside in the same complex (Supplementary Figure 6). Thus, endogenous Bax (Figure 2a), as well as Bak,15, 20 associates with a large molecular weight complex involving VDAC2 in mitochondria of healthy cells. This contrasts with other Bcl-2 family proteins, for example, neither Bcl-xL nor Bcl-2 associated with this large complex when imported into mitochondria (Supplementary Figure 7).
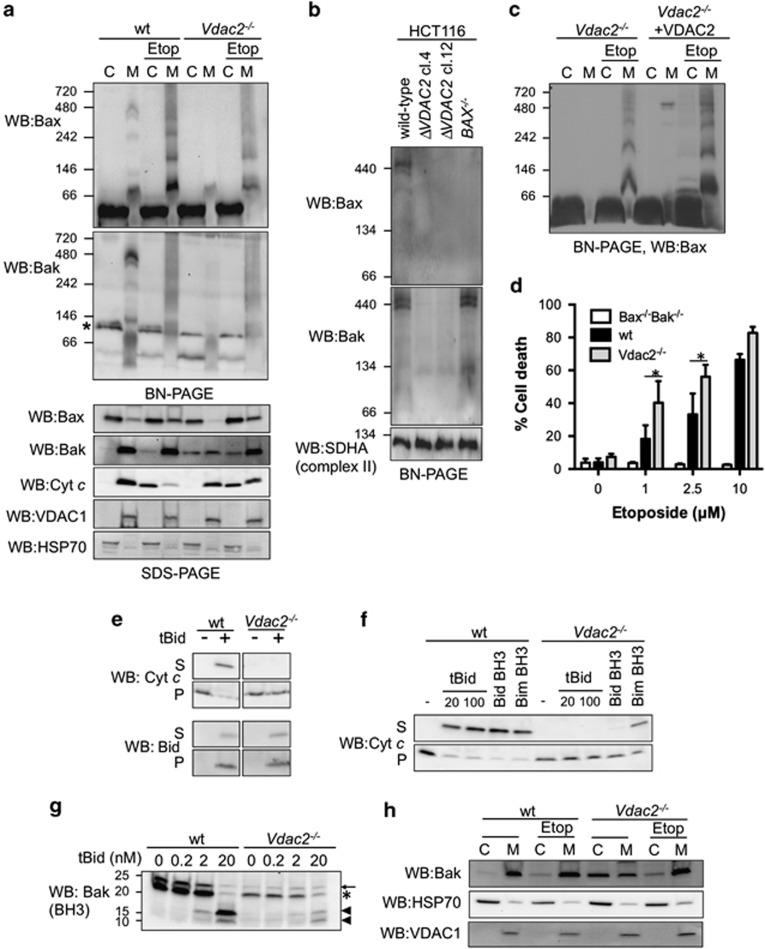
Figure 2.
Association with VDAC2 regulates Bax oligomerization. (a) Bax, but not Bak, oligomerization is reduced in cells lacking VDAC2. Wild-type (wt) and Vdac2−/− MEFs were permeabilized and cytosol (C) and membrane fractions (M) were analysed by BN-PAGE (upper panel) or by SDS-PAGE prior to immunoblotting with the indicated antibodies. * indicates a cross-reactive band detected by the anti-Bak antibody (Supplementary Figure 12). Data are representative of three independent experiments. (b) The large Bax and Bak complex is lost in VDAC2-deficient HCT116 cells. Membrane fractions from ΔVDAC2 HCT116 cells were assessed by BN-PAGE and immunoblotted for Bax, Bak or SDHA as a loading control. (c) Re-expression of VDAC2 rescues the association of Bax with the 400 kDa complex and enhances Bax oligomerization. Vdac2−/− MEFs or Vdac2−/− MEFs stably expressing HA-VDAC2 were treated or not with etoposide prior to analysis of cytosol (C) and membrane fractions (M) by BN-PAGE. Data are representative of two independent experiments. (d) Vdac2−/− MEFs are sensitized to etoposide. wt, Bak−/−Bax−/− or Vdac2−/− MEFs were treated with the indicated concentration of etoposide for 24 h prior to analysis of cell death by PI uptake. Note that because of the different genetic background of the Bak−/−Bax−/− cells, direct comparison with wt and Vdac2−/− MEFs is not appropriate, rather the Bak−/−Bax−/− are shown to confirm that the cell death induced by etoposide was by apoptosis. Mean±S.D. of three independent experiments. * represents P<0.05 based on Students unpaired t-test. (e) tBid can localize to VDAC2-deficient mitochondria. Membrane fractions from wt or Vdac2−/− MEFs were treated with recombinant tBid prior to fractionation into supernatant (S) and membrane (P) and immunoblotting. (f) Mitochondrial Bak in Vdac2−/− MEFs cannot mediate cytochrome c release. Membrane fractions from wt or Vdac2−/− MEFs were treated with recombinant tBid at the indicated concentrations (nM) or Bid BH3 or Bim BH3 peptide (10 μM) at 30 oC for 30 min prior to separation of supernatant (S) and pellet (P) and immunoblotting for cytochrome c. Data are representative of three independent experiments. (g) Bak in Vdac2−/− mitochondria is not hyperactive. Membrane fractions from wt or Vdac2−/− MEFs were treated with the indicated concentrations of recombinant tBid prior to limited proteolysis with proteinase K, SDS-PAGE and immunoblotting with an antibody targeting the Bak BH3 domain. Full-length Bak (arrow) is cleaved in its inactive form (*) and is further cleaved upon activation (arrowhead). Data are representative of two independent experiments. (h) Bak translocates from the cytosol to mitochondria during apoptosis of Vdac2−/− MEFs. wt or Vdac2−/− MEFs were treated or not with etoposide prior to fractionation into cytosol (C) and membrane (M) fractions and immunoblotting. Data are representative of four independent experiments
Bax dissociates from VDAC2 and appears in a range of oligomeric complexes during apoptosis
Upon reception of an apoptotic stimulus (etoposide, staurosporine or actinomycin D) a ladder of Bax complexes from 60 to 400 kDa was detected (Figures 1d, e and , Supplementary Figure 8).5 Although changes in oligomerization state during apoptosis were observed in CHAPS, this detergent did not resolve discrete complexes (Figure 1b), consistent with a previous report.24 In apoptotic cells, Bax oligomers could only be extracted with >0.25% digitonin, consistent with activated Bax being membrane-integrated. As in healthy cells, monomeric Bax in apoptotic cells was peripheral as it could be extracted when membranes were resuspended in low concentrations of digitonin (0.1% w/v) (Figure 1f) or even in the absence of digitonin (Supplementary Figure 9). This suggests that during apoptosis, Bax integration and oligomerization occur concurrently, or that Bax integration precedes oligomerization as suggested by previous studies.24, 25
The apoptotic Bax oligomers were not stabilized by disulphide linkage as they were similarly detected with a BaxΔCys variant (Figure 1e). The pattern of oligomeric Bax contrasts with oligomeric Bak, which under native (and reducing) conditions resolves predominantly as a homodimer.21 This may suggest that Bax and Bak high molecular weight oligomeric pores form by different mechanisms. However, as Bax and Bak can associate to form higher order oligomers, because of their conserved mechanism of symmetrical homodimerization, distinct mechanisms of pore formation appear unlikely.5, 21, 26, 27 A more likely explanation is that the higher order Bax homo-oligomers are more stable than Bak homo-oligomers upon removal from the MOM and BN-PAGE.5
Bax can associate with VDAC2-deficient mitochondria after an apoptotic stimulus
As our data indicated that Bax as well as Bak interacts with VDAC2 in healthy cells, we investigated the functional relevance of the Bax–VDAC2 interaction with respect to Bax subcellular localization, oligomerization and function. In the absence of VDAC2, Bax localization to the MOM in healthy cells was reduced (Figure 2a, compare lanes 2 and 6). Furthermore, Bax apoptotic oligomers were reduced in the absence of VDAC2 (Figure 2a).
Bak also exhibited reduced mitochondrial localization in untreated Vdac2−/− MEFs, and was readily detectable in the cytosol (Figure 2a). However, Bak retained an ability to localize to mitochondria and efficiently oligomerize during apoptosis to participate in cytochrome c release (Figure 2a). This indicates that VDAC2 facilitates the constitutive targeting of both Bax and Bak to the MOM, but is not absolutely necessary for either Bax or Bak to target mitochondria following an apoptotic stimulus.
Impaired localization of Bak and Bax to Vdac2−/− mitochondria was not because of a general defect in mitochondrial import or mitochondrial form, as other mitochondrial proteins Bcl-xL and Mcl-1, VDAC1 and cytochrome c localized to wild-type and Vdac2−/− mitochondria equally (Figure 1a) and Vdac2−/− cells exhibited no gross defect in mitochondrial morphology (Supplementary Figure 10).
Despite reduced mitochondrial Bak and Bax, Vdac2−/− cells were sensitized to etoposide-induced cell death compared with wild-type MEFs (Figure 2d), although the difference between Vdac2−/− and wild-type MEFs was only statistically significant at lower doses (1 and 2.5 μM). This heightened response of Vdac2−/− cells to apoptotic stimuli is consistent with the proposed role of VDAC2 as a negative regulator of Bak apoptotic function.15 To test whether mitochondrial Bak was hyperactive and could efficiently mediate cytochrome c release in the absence of VDAC2 as proposed15 or whether recruitment of cytosolic Bak or Bax was necessary, we isolated membrane fractions from wild-type or Vdac2−/− MEFs. Although recombinant tBid could still target Vdac2−/− mitochondria (Figure 2e), the resident Bak could not mediate cytochrome c release (Figure 2e) even in response to high doses of tBid (Figure 2f). Nor could resident Bak mediate cytochrome c release induced by high dose (10 μM) of Bid BH3 peptide or heat (Figure 2f and Supplementary Figure 11). Bim BH3 peptide induced some release from Vdac2−/− mitochondria, but still less than with wild-type mitochondria (Figure 2f), likely reflecting the high affinity of Bim for pro-survival proteins.28
To test whether Bak in Vdac2−/− mitochondria was more prone to undergo conformation change, we performed limited proteolysis. Bak in Vdac2−/− MEFs was activated by a similar concentration of tBid (20 nM) as Bak in wild-type mitochondria (Figure 2g). Together our data indicate that the mitochondrial pool of Bak in Vdac2−/− cells is not hyperactive and is actually insufficient to efficiently mediate MOM permeabilization. Thus, in order for Vdac2−/− MEFs to die efficiently (Figure 2d), the recruitment of cytosolic Bak (Figures 2a and h) and/or Bax (Figure 2a) is necessary.
Deficiency in both VDAC2 and Bak inhibits Bax mitochondrial association, oligomerization and function during apoptosis
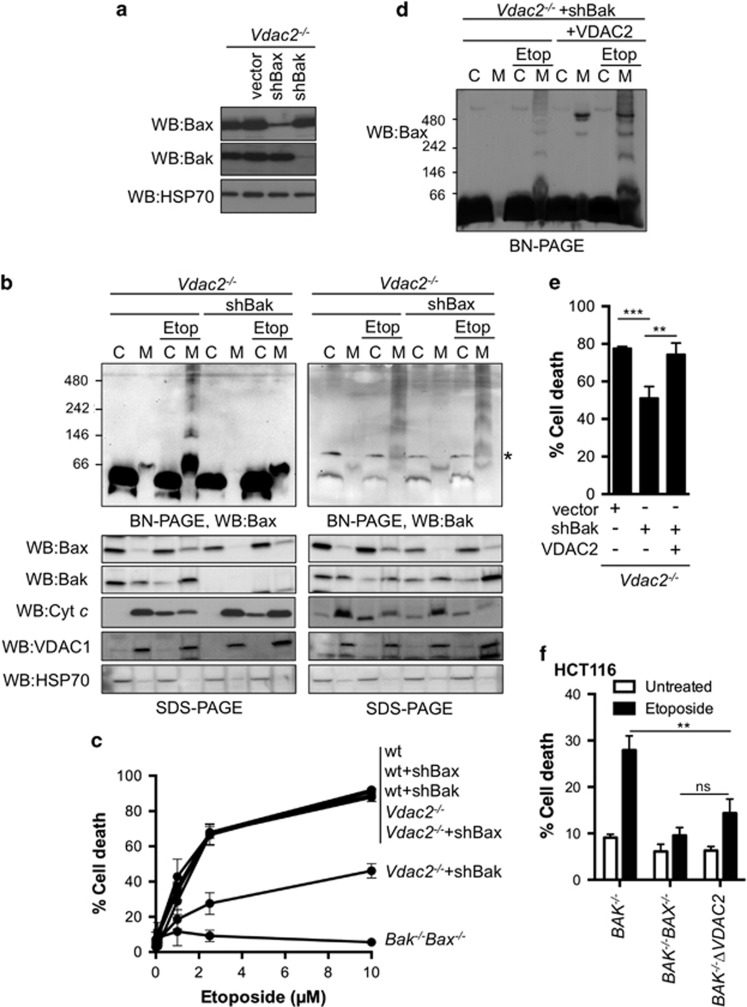
As Bax and Bak can hetero-oligomerize downstream of a death stimulus,5 we hypothesized that following an apoptotic stimulus, activated Bak at mitochondria may recruit cytosolic Bax and so bypass the need for VDAC2. Therefore, we tested the effect of silencing Bak expression in Vdac2−/− MEFs on Bax recruitment, oligomerization and function. Immunoblotting confirmed the efficient knockdown of Bax and Bak (Figure 3a). In the absence of VDAC2 and with depleted Bak, Bax oligomerization at the MOM and cytochrome c release was reduced (Figure 3b). In contrast, in Vdac2−/− MEFs with reduced Bax expression, Bak retained an ability to oligomerize at the MOM and mediate cytochrome c release (Figure 3b).
Figure 3.
Bax requires either VDAC2 or Bak to efficiently oligomerize in mitochondria and to kill cells. (a) Efficient silencing of endogenous Bak or Bax in Vdac2−/− MEFs. Vdac2−/− MEFs were stably infected with empty vector or plasmid containing shRNA targeting mBax or mBak prior to analysis of whole cell lysates by SDS-PAGE and immunoblotting. (b) VDAC2 and Bak are necessary for Bax to efficiently oligomerize at the MOM. Vdac2−/− MEFs with stable knockdown of Bak or Bax were treated or not with etoposide and cytosol (C) and membrane (M) fractions were analysed by BN-PAGE (upper panel) or SDS-PAGE (lower panels). * indicates a cross-reactive band detected by the anti-Bak antibody. Data are representative of three independent experiments. (c) Cells deficient in VDAC2 and Bak but not VDAC2 and Bax are less sensitive to etoposide. The indicated MEFs lines were treated with the indicated concentration of etoposide for 24 h prior to analysis of cell death by PI uptake. Mean±S.D. of three independent experiments. (d) Re-expression of VDAC2 restores ability of Bax to homo-oligomerize during apoptosis. Vdac2−/− MEFs re-expressing VDAC2 with or without stable knockdown of Bak were treated or not with etoposide and cytosol (C) and membrane (M) fractions were analysed by BN-PAGE. (e) Re-expression of VDAC2 restores apoptotic response to MEFs deficient in VDAC2 and Bak. The indicated MEFs were treated with etoposide (10 μM) for 24 h prior to analysis of cell death by PI uptake. Mean±S.D. of four independent experiments. ***P=0.001, **P<0.005 based on Students unpaired t-test. (f) Bax apoptotic function is reduced in the combined absence of Bak and VDAC2. BAK−/−, BAK−/−BAX−/− and BAK−/−ΔVDAC2 HCT116 cells were treated with etoposide (10 μM) for 48 h and assessed for cell death by PI uptake. Data are mean±S.D. of three independent experiments. *P<0.01 based on Students unpaired t-test
To understand the relative contributions of Bax and Bak to the cell death observed in Vdac2−/− cells, we tested the effect of silencing Bak or Bax expression. Consistent with the phenotypes of Bak−/− and Bax−/− MEFs,1 silencing of neither Bak nor Bax had significant effect on etoposide-induced death in wild-type MEFs (Figure 3c). However, consistent with the failure of Bax to efficiently oligomerize in the MOM in Vdac2−/−/shBak MEFs (Figure 3b), these cells exhibited significantly reduced cell death in response to etoposide (Figure 3c). In contrast, Vdac2−/− MEFs with silenced Bax exhibited no reduction in cell death (Figure 3c). Re-expression of VDAC2 was sufficient to rescue the ability of Bax to oligomerize in the MOM (Figure 3d) and cell death in response to etoposide (Figure 3e). Thus, the increased death observed in Vdac2−/− MEFs is largely attributable to Bak. To test whether the requirement for Bak and/or VDAC2 for Bax apoptotic function was a general phenomenon, we generated BAK−/− ΔVDAC2 HCT116 cells (Supplementary Figure 3). Like MEFs, these double knockout cells exhibited significantly reduced Bax-mediated apoptosis in response to etoposide compared with BAK−/− HCT116 cells (Figure 3f). Together, our data indicate that VDAC2 or Bak are necessary in order for Bax to efficiently mediate cell death.
BaxS184L mutant fails to target mitochondria devoid of VDAC2
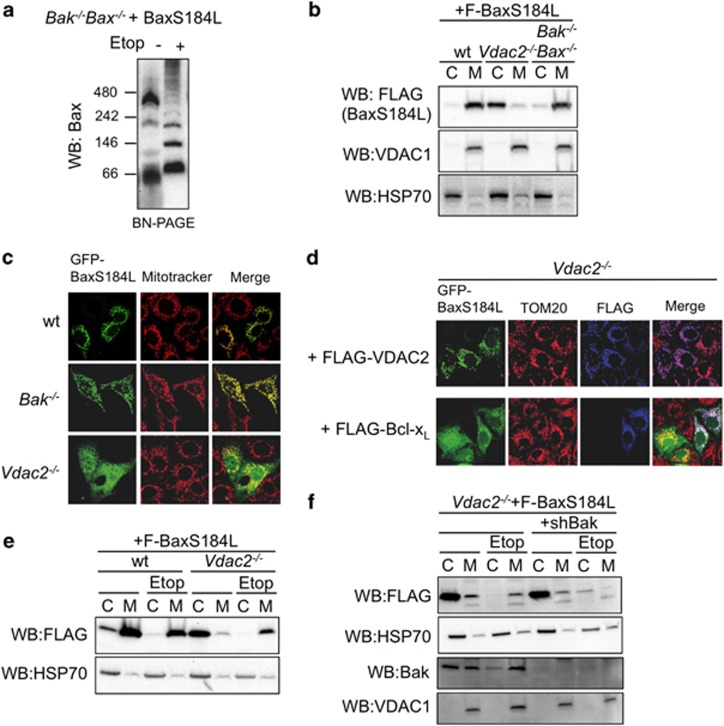
To test whether VDAC2 plays a role in the formation of the apoptotic pore as well as regulating Bax recruitment to mitochondria, we tested whether targeting Bax to mitochondria by mutagenesis of its C-terminal transmembrane domain was sufficient to bypass the requirement for VDAC2 and Bak and rescue the apoptotic defect in cells lacking both VDAC2 and Bak. Mutagenesis of S184 in the Bax C-terminus to a hydrophobic residue is sufficient to constitutively target Bax to mitochondria.4, 29 Like wild-type Bax, BaxS184L associated with the large molecular weight VDAC2 complex and oligomerized following an apoptotic stimulus (Figure 4a) as previously observed.30 Surprisingly, in contrast to when expressed in wild-type or Bak−/− MEFs, BaxS184L failed to target mitochondria when stably expressed in Vdac2−/− MEFs (Figures 4b and c). Re-expression of VDAC2 was sufficient to rescue the ability of BaxS184L to constitutively target mitochondria (Figure 4d). This suggests that the mutation of S184 to a more hydrophobic residue is not sufficient to constitutively target Bax to mitochondria, but rather that the mutation improves the affinity or stability of the association of Bax with VDAC2 resulting in a shift in the equilibrium of Bax from the cytosol to mitochondria. These data further support VDAC2 as an important mediator of Bax mitochondrial localization.
Figure 4.
Bax targets mitochondria via distinct mechanisms in healthy and apoptotic cells. (a) BaxS184L associates with VDAC2 in mitochondria. Bak−/−Bax−/− stably expressing the constitutively mitochondrial Bax mutant S184L were fractionated into cytosol (C) and membrane (M) and analysed by BN-PAGE. Data are representative of three independent experiments. (b) BaxS184L cannot target mitochondria that lack VDAC2. FLAG-BaxS184L was stably expressed in wt, Vdac2−/− or Bak−/−Bax−/− MEFs and cytosol (C) and membrane (M) fractions were immunoblotted with the indicated antibodies. Data are representative of three independent experiments. (c) BaxS184L cannot target mitochondria that lack VDAC2. wt, Vdac2−/− or Bak−/− MEFs were transiently transfected with GFP-BaxS184L, treated with MitoTracker Red CMXRos and analysed by confocal microscopy. (d) Ectopic expression of VDAC2 or Bcl-xL rescues the ability of BaxS184L to target mitochondria. Vdac2−/− MEFs stably expressing FLAG-VDAC2 or FLAG-Bcl-xL were transiently transfected with GFP-BaxS184L prior to immunstaining for TOM20, and FLAG and confocal analysis. (e) BaxS184L can target mitochondria that lack VDAC2 during apoptosis. wt and Vdac2−/− MEFs stably expressing FLAG-S184L were treated or not with etoposide prior to fractionation into cytosol (C) and membrane (M) fractions and analysis on SDS-PAGE. Data is representative of two independent experiments. (f) BaxS184L requires Bak to target mitochondria during apoptosis. Vdac2−/− MEFs stably expressing FLAG-S184L with or without stable knockdown of Bak were treated or not with etoposide prior to fractionation into cytosol (C) and membrane (M) fractions and analysis on SDS-PAGE. Data are representative of two independent experiments
Pro-survival Bcl-2 proteins such as Bcl-xL have also been implicated as important regulators of Bax subcellular localization by shuttling Bax either towards14 or away from13 mitochondria. Ectopic expression of Bcl-xL rescued the constitutive ability of BaxS184L to target mitochondria in Vdac2−/− MEFs evidenced by confocal microscopy (Figure 4d). This suggests that mitochondrial Bcl-xL allows Bax to accumulate at the MOM rather than actively traffic it to the cytosol.14
To test whether the defect in BaxS184L mitochondrial targeting due to VDAC2-deficiency could be bypassed by Bax activation, we assessed BaxS184L localization in Vdac2−/− MEFs after an apoptotic stimulus. After etoposide treatment, although overall levels of BaxS184L were reduced, there was a shift of BaxS184L to the membrane fraction (Figure 4e), suggesting that activated BaxS184L does not require VDAC2 to target the MOM, consistent with activated Bax-binding Bcl-xL at mitochondria.31 To test whether BaxS184L was recruited by the endogenous mitochondrial pool of Bak in these cells, we silenced Bak expression with shRNA. In the absence of both VDAC2 and Bak, activated BaxS184L failed to target mitochondria following an apoptotic stimulus (Figure 4f).
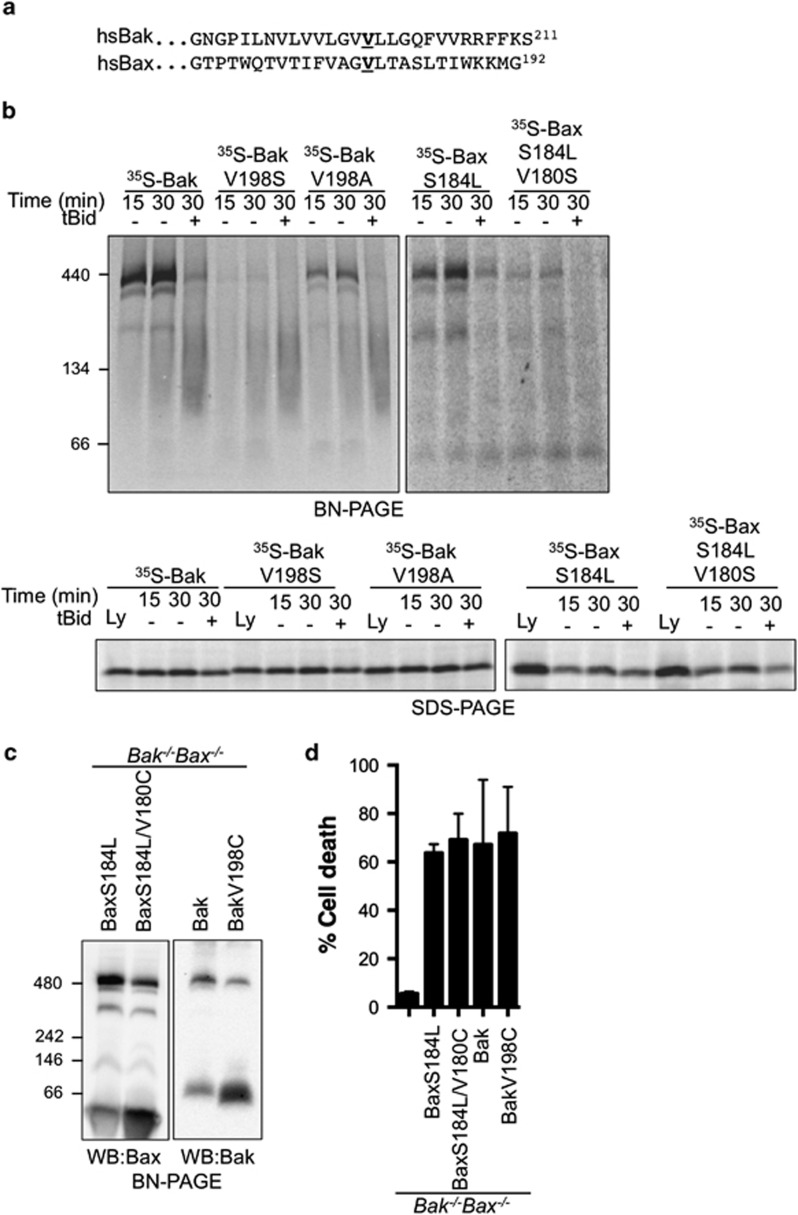
As the BaxS184L required VDAC2 to target mitochondria, it suggested that the C-terminus of Bax was involved in its interaction with VDAC2, as reported for Bak.20 Mutation of a conserved valine in the C-termini of hBak (V198) and hBax (V180) (Figure 5a) impaired association with the VDAC2 complex (Figure 5b, upper panel), but the mutants still targeted to mitochondria (Figure 5b, lower panel). Furthermore, mutation of these C-terminal residues to cysteine also decreased VDAC2 association when these functional mutants were stably expressed in Bak−/−Bax−/− MEFs (Figure 5c and d). These data suggest that the Bax and Bak C-termini are important for interaction with VDAC2.
Figure 5.
The Bax and Bak C-terminal membrane anchors are important for interaction with VDAC2. (a) Amino acid alignment of human Bak and human Bax. Conserved valine that was mutated to alanine, serine or cysteine is indicated (bold, underlined). (b) Mutation of the Bak and Bax C-termini impairs association with VDAC2 upon import to mitochondria. Mitochondria isolated from wild-type MEFs were incubated with 35S-radiolabelled Bak, BakV198S, BakV198A, BaxS184L or BaxS184L/V180C for the indicated times. Following import, mitochondria were incubated with or without tBid where indicated prior to BN-PAGE (upper panel) or SDS-PAGE (lower panel) and autoradiography. Lysates (Ly) are translated 35 S-labelled protein, 20% input. (c) Mutation of the Bak and Bax C-termini disrupts association with VDAC2 in healthy cells. Membrane fractions from Bak−/−Bax−/− MEFs stably expressing the indicated Bax or Bak variants were solubilized with 1% digitonin and analysed by BN-PAGE. (d) Bak and Bax C-termini mutants retained apoptotic function. MEFs described in (c) were treated with etposide (10 mM, 24 h) prior to assessment of cell death by PI uptake. Data are mean±S.D. of three experiments
Discussion
Understanding how Bax and Bak are regulated is paramount for therapeutic targeting of their apoptotic function in disease. Recent evidence has suggested that Bax exists in a dynamic equilibrium between the cytosol and membrane and that altering this equilibrium can alter sensitivity to apoptotic stimuli.13, 14 However, the molecular mechanisms governing Bax localization and Bax accumulation at the MOM during apoptosis remain controversial.13, 14 We now propose that Bax employs distinct mechanisms to target mitochondria before and after an apoptotic stimulus. In healthy cells, endogenous Bax that is resident at mitochondria associates with a large molecular weight complex containing VDAC2, as does Bak.20, 21 Whether Bax and VDAC2 interact directly and whether their complex involves additional proteins is unknown. However, Bak is not an intermediary in the Bax–VDAC2 interaction as the complex is observed in Bak−/− cells.5
In an earlier study of Bax complexes by BN-PAGE, no discrete Bax complexes were observed either before or after an apoptotic stimulus when membranes were solubilized with CHAPS.24 Additionally, Bax could co-immunoprecipitate with VDAC1 in neurons solubilized in digitonin, whereas other detergents, including CHAPS, disrupted this interaction.19 Our findings are consistent with these studies as supramolecular complexes involving Bax in healthy and apoptotic cells were only resolved when mitochondria were solubilized in digitonin, supporting digitonin as the preferred detergent to examine protein–protein interactions of the Bcl-2 family.5, 19, 21 As Bax interacts with VDAC1 in neurons,19 it is possible that Bax is regulated by different VDAC isoforms in different cell types. However, because the authors of that study note that the VDAC1 antibody used also immunoreacted with VDAC2 and VDAC3,19 it is possible that the observed interaction was with VDAC2. This would be consistent with our data showing that high molecular weight complexes involving Bax are undetectable in Vdac2−/− cells that retain VDAC1 expression.21
Our studies support VDAC2 as an important determinant of the constitutive subcellular distribution of Bax and Bak in healthy cells.16, 32 This role for VDAC2 is seemingly specific for Bak and Bax as the mitochondrial targeting of other Bcl-2 family members including Bcl-xL, Mcl-1 and tBid was not affected by the absence of VDAC2. Recently, Bcl-xL was shown to interact with VDAC1,33 but not VDAC2,34 suggesting that other VDAC isoforms may play a role in mitochondrial targeting of these Bcl-2 family proteins.
Although constitutive targeting of both Bax and Bak to Vdac2−/− mitochondria was impaired, a population of Bak retained an ability to associate with the MOM. This may implicate a role for alternative mitochondrial receptors for Bak such as VDAC isoforms 1 and 3 or alternatively Bak may integrate spontaneously into the MOM by a process that does not require a membrane receptor. During apoptosis, cytosolic Bax can circumvent VDAC2 to associate with activated mitochondrial Bak and participate in MOM permeabilization. An interaction of activated Bak and Bax involves a BH3–groove interaction,5 and so it is noteworthy that the mutation of residues in the Bax BH3 domain can abrogate mitochondrial targeting.35 The ability of Bcl-xL to rescue Bax targeting to mitochondria in the absence of VDAC2 presumably also involves an interaction of the Bax BH3 domain with the prosurvival groove. However, the Bax C-terminal tail has also been implicated in its interaction with Bcl-xL.36
The predominantly cytosolic locale of Bax is facilitated by the sequestration of the C-terminal transmembrane domain in its hydrophobic groove. Substitution of S184 in the Bax C-terminus for a hydrophobic residue disrupts interaction between the tail and groove and constitutively targets Bax to mitochondria.4, 29 As the mitochondrial localization of BaxS184L was completely abrogated in Vdac2−/− MEFs, our data now show that hydrophobic substitution at S184 is not sufficient for mitochondrial localization indicating that the mutation does not simply increase the interaction of the transmembrane domain with the lipids of MOM. Rather the mutation likely increases the affinity and/or stability of the association of the Bax C-terminus with VDAC2. BaxS184L associates with mitochondria at a similar rate to wild-type Bax, but its dissociation rate is lower,14 again suggesting that altering the tail improves the stability of Bax's association with mitochondria likely mediated by VDAC2. This is consistent with the role for the C-terminal tail of Bak in mediating its interaction with VDAC2,20 and the observed preponderance of the large complex with VDAC2 observed with BaxS184L (Figure 4a). Valentijn et al.37 have previously reported that the Bax C-terminus is sufficient to target GFP to mitochondria, with the oligomeric state of this fusion protein suggesting that this was probably because of interaction with other mitochondrial proteins. We propose that this mitochondrial protein is VDAC2.
TOM22 has been implicated as a Bax receptor in the MOM.38 As mitochondrial import of TOM22 is not impaired in Vdac2−/− cells,20 TOM22 is not sufficient to mediate mitochondrial targeting of Bax in the absence of VDAC2 (and Bak). However, TOM22 and VDAC2 may cooperate in Bax mitochondrial targeting.
Mounting evidence suggests that VDAC2 has an important role in regulating Bak and Bax apoptotic function. Studies have implicated a role for VDAC2 in negatively regulating Bak apoptotic function,15, 20 with cells lacking VDAC2 sensitized to apoptotic stimuli as Bak was proposed to be hyperactive.15 We likewise observed a sensitization to etoposide treatment of Vdac2−/− MEFs compared with wild-type, despite the significant re-distribution of Bak to the cytosol. However, our data indicate that the mitochondrial pool of Bak in Vdac2−/− MEFs is not in fact hyperactive and is not sufficient to efficiently mediate MOM permeabilization in response to apoptotic stimuli (tBid, Bid BH3, Bim BH3 and heat). Rather, in order for Vdac2−/− cells to die, recruitment of the cytosolic pool of Bak (and Bax) is necessary. That sensitization of Vdac2−/− MEFs was most evident at low doses of etoposide is consistent with previous observations of sensitization only at early time points of etoposide treatment,39 and may reflect that etoposide-induced death in wild-type MEFs can be efficiently mediated by Bax.40 Thus, any inhibitory effect of VDAC2 on Bak in wild-type MEFs may be obscured at higher doses or later time points.
We propose that downstream of an apoptotic stimulus, cytosolic Bak and Bax in Vdac2−/− MEFs can be recruited to the MOM via interaction with activated mitochondrial Bak, and thus bypass a potentially rate-limiting interaction with VDAC2, to participate in MOM permeabilization and thereby cell death. In contrast, in wild-type MEFs, a significant proportion of Bak and Bax must first dissociate from VDAC2 before it can participate in membrane damage. Thus, our findings reconcile the observation that Vdac2−/− cells are sensitized to Bak-mediated cell death,15, 20 whilst constitutive recruitment of Bak to the MOM is reduced in the absence of VDAC2.16, 39
Deletion of Bak rescues the apoptosis defect of Vdac2−/− thymocytes41 supporting a negative regulatory axis for VDAC2 and Bak.15 As our data indicate that Bax is unable to efficiently mediate cell death in the absence of both VDAC2 and Bak, one may predict that thymocytes deficient for both VDAC2 and Bak would phenocopy Bak−/−Bax−/− thymocytes.42 That they do not may indicate that in certain cells, Bax can target mitochondria sufficiently to mediate cell death even in the absence of Bak and VDAC2, perhaps facilitated by interaction with pro-survival Bcl-2 proteins or VDAC1.13, 19
In summary, VDAC2 facilitates the mitochondrial association and apoptotic function of Bax. Thus, elucidating the molecular mechanism involved in the Bax–VDAC2 association may reveal a novel target to perturb Bax-mediated apoptosis upstream of mitochondrial damage. Some cell types, such as cerebellar granule neurons,43 are reliant upon Bax for their apoptotic response, thus intervention may reveal novel opportunities to protect neuronal cells following stroke.
Materials and Methods
Cell lines, cell culture and induction of apoptosis
MEFs derived from wild-type or Bak−/−Bax−/− C57BL/6 mice, or wild-type or Vdac2−/− mice on a mixed C57BL/6;129/SvEv background were transformed with SV40 large T and cultured in Dulbecco's Modified Eagles medium supplemented with 10% foetal calf serum, 250 μM L-asparagine and 55 μM 2-mercaptoethanol as described.6 HeLa and DU145 stably expressing hBax were maintained in Dulbecco's Modified Eagles medium supplemented with 10% foetal calf serum. Foetal livers were isolated from wt C57BL/6 mice at embryonic day 14.5. Apoptosis was induced in MEFs by treating with etoposide, staurosporine or actinomycin D for 24 h. Cell death was assessed by flow cytometric analysis of propidium iodide (PI) uptake.
Retroviral infection
hBax, hBaxS184L or epitope (HA or FLAG)-tagged VDAC2 in the retroviral expression vector pMX-IRES-GFP (internal ribosome entry site-green fluorescent protein) or short hairpins targeting mBak or mBax in the MSCV LTRmir30 (LMP) vector were retrovirally transduced in MEFs using Phoenix ecotropic packaging cells.6 Infected cells were selected on the basis of GFP expression.
Subcellular fractionation and cytochrome c release
MEFs were treated as indicated, harvested and permeabilized in buffer (20 mM Hepes, pH 7.5, 100 mM KCl, 2.5 mM MgCl2, 100 mM sucrose) containing 0.025% digitonin and supplemented with Complete protease inhibitors without EDTA (Roche, Victoria, Australia). Permeabilization was confirmed by trypan blue uptake and cytosol and mitochondria-enriched heavy membrane fractions were separated by centrifugation at 13 000 g for 5 min prior to SDS-PAGE analysis. To assess cytochrome c release from isolated mitochondria, pelleted membrane fractions were resuspended in permeabilization buffer without digitonin and incubated for 30 min with or without caspase-8-cleaved human Bid (tBid), Bid BH3 peptide (SESQEDIIRNIARHLAQVGDSMDRSIPPGLVNGL) at 30 °C or without stimulus at 43 °C. Supernatant and membrane fractions were separated by centrifugation at 13 000 g for 5 min prior to SDS-PAGE.
Blue Native-PAGE
BN-PAGE was performed essentially as described.21 Membrane fractions were solubilized in 20 mM Bis-tris, pH 7.4, 50 mM NaCl, 10% glycerol with 1% w/v (or the indicated concentration) digitonin, 1% w/v CHAPS or 1% Triton X-100 for 20 min on ice before centrifugation at 13000 g to pellet detergent-insoluble debris. BN-PAGE loading dye (5% Coomassie Blue R-250 (Bio-Rad Laboratories, Gladesville, NSW, Australia) in 500 mM 6-aminohexanoic acid, 100 mM Bis-tris, pH 7.0) was then added to each sample. Gels were electrophoresed in anode buffer (50 mM Bis-tris, pH 7.0) and blue cathode buffer (50 mM Tricine, 15 mM Bis-tris unbuffered containing 0.02% Coomassie Blue G-250). Blue cathode buffer was replaced with clear buffer (without Coomassie Blue) when the dye front was 1/3 way through the resolving gel. Gels were transferred to PVDF in Tris-glycine transfer buffer containing 20% methanol and 0.037% SDS. Blots were destained in 50% methanol and 25% acetic acid and washed with TBS prior to immunoblotting.
Immunoprecipitation
Cells were solubilized on ice for 30 min in lysis buffer (20 mM Tris-HCl pH 7.4, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol) containing either 1% Triton X-100, 1% digitonin or 1% CHAPS. Immunoprecipitation was performed as described,6 with anti-HA (16B12, Covance, Princeton, NJ, USA). Immunoprecipitates and pre-IP cell lysates were run on SDS-PAGE under non-reducing or reducing conditions, and immunoblotted for HA (16B12, Covance,) or Bak (aa23-38, Cat. #B5897, Sigma-Aldrich, Castle Hill, NSW, Australia).
In vitro protein import into isolated mitochondria
Bak, Bcl-2 and Bcl-xL were amplified from pGEM4Z using M13 forward and reverse primers to generate RNA using the in vitro mMESSAGE mMachine SP6 kit (Ambion, Life Technologies, Mulgrave, VIC, Australia). RNA was isolated by LiCl precipitation according to the manufacturer's instructions and applied to in vitro translation reactions using rabbit reticulocyte lysate (Promega, Sydney, NSW, Australia) in the presence of [35S]methionine/cysteine protein labeling mix (PerkinElmer Life Sciences, Melbourne, VIC, Australia). Mitochondria from wild-type MEFs were isolated by differential centrifugation as described previously,44 and incubated with translation products in import buffer (20 mM HEPES-KOH, pH 7.4, 250 mM sucrose, 80 mM KOAc, 5 mM MgOAC, 10 mM sodium succinate, 5 mM methionine, 1 mM DTT, and 4 mM ATP) at 37 °C for the indicated times. Membranes were pelleted at 16 000 g for 5 min at 4 °C and subsequently solubilized in 1% digitonin for BN-PAGE or SDS-PAGE loading buffer. Where indicated, mitochondria were incubated in the presence of recombinant tBid (50 nM) in import buffer at 37 °C for 15 min.
Immunoblotting antibodies
SDS-PAGE gels were immunoblotted for Bak (aa23-38, Cat. #B5897, Sigma-Aldrich), Bax (N-20, Santa Cruz Biotechnology, Shanghai, China), cytochrome c (clone 7H8.2C12, BD Biosciences Pharmingen, CA, USA), Bcl-xL (#610212, BD Biosciences, North Ryde, NSW, Australia), Mcl-1 (#600-401-394, Rockland Immunochemicals, Gilbertsville, PA, USA), HSP70 (from W. Welch, UCSF), VDAC1 (Ab-2, Calbiochem, Billerica, MA, USA), FLAG (M2, Sigma-Aldrich), human VDAC2 (Boris Reljic) or haemagglutinin (HA, 16B12, Covance). Native-PAGE gels were immunoblotted for Bak (7D10, D. C. S. Huang, WEHI) or Bax (21C10, D. C. S. Huang, WEHI), VDAC1 (as above) or SDHA (complex II, Molecular Probes, Mulgrave, VIC, Australia, cat #A11142). Secondary antibodies were horseradish peroxidase-conjugated anti-rabbit IgG, anti-mouse IgG and anti-rat IgG (Southern Biotech, Birmingham, AL, USA).
TALEN gene targeting
VDAC2 was disrupted in wild-type and BAK−/− HCT116 colorectal cancer cells (a gift from Richard Youle, NIH) using TALEN (transcription activator-like effector nuclease) binding pairs (PMID 22484455) designed to target the common exon of the three isoforms of human VDAC2 as previously described.45 Clones were screened for VDAC2-deficiency using an antibody recognizing human VDAC2 (Boris Reljic).
Limited proteolysis
Membrane fractions were incubated with recombinant tBid in the absence of protease inhibitors prior to treatment with proteinase K (30 μg/ml) for 20 min on ice. Proteinase K was quenched with 100 mM PMSF and samples were boiled samples in SDS-PAGE sample buffer. Cleavage fragments were detected by immunoblotting with an internal antibody recognizing the Bak BH3 domain (4B5).6
Confocal analysis
MEFs were seeded overnight on cover slips and then transfected for 20 h with GFP-BaxS184L with Lipofectamine 2000 (Life Technologies) according to the manufacturer's instructions. Cells were incubated with 50 nM MitoTracker Red CMXRos (Molecular Probes) before being fixed in 4% (w/v) paraformaldehyde in PBS (pH 7.4) and incubated for 60 min at room temperature with primary antibody against the FLAG epitope (clone M2, Sigma-Aldrich) or TOM20 (Santa Cruz Biotechnology). Primary antibodies were labeled for 20 min at room temperature with Alexa Fluor 488-, Alexa Fluor 568-, or Alexa Fluor 647-conjugated anti-rabbit or anti-mouse (Molecular Probes). Confocal microscopy was performed with a Zeiss confocal microscope equipped with a ConfoCor 3 system containing an avalanche photodiode detector. GFP was detected using an argon laser, red fluorescence was detected using a DPSS laser, and deep red fluorescence was detected using a helium/neon laser. All images were processed using ImageJ (http://rsbweb.nih.gov/ij/index.html) and ZENlite 2011 (Blue edition, Zeiss, North Ryde, NSW, Australia).
Acknowledgments
We would like to thank Stephanie Fennell for technical assistance, Colin Hockings for the Bid and Bim BH3 peptides, Mark van Delft for the FLAG-VDAC2 construct, Gabriela Brumatti for fetal liver extraction, William Craigen for the Vdac2+/+(wild-type) and Vdac2−/− MEFs, Richard Youle for the wild-type, BAK−/− and BAX−/− HCT116 lines and Boris Reljic for the anti-human VDAC2 antibody. The work was supported by grants from the National Health and Medical Research Council of Australia (637335), and the Association for International Cancer Research (10–230), and operational infrastructure grants through the Australian Government IRISS and the Victorian State Government OIS.
Glossary
- BH
Bcl-2 homology
- BN-PAGE
blue native-polyacrylamide gel electrophoresis
- C-terminus
carboxy terminus
- DTT
dithiothreitol
- GFP
green fluorescent protein
- HA
haemagglutinin
- hBax
human Bax
- IRES
internal ribosome entry site
- mBax
mouse Bax
- MEFs
mouse embryonic fibroblasts
- MOM
mitochondrial outer membrane
- N-terminus
amino terminus
- PI
propidium iodide
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- sh
short hairpin
- TALEN
transcription activator-like effector nuclease
- tBid
truncated Bid
- VDAC
voltage-dependent anion channel
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by C Borner
Supplementary Material
References
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813:521–531. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, et al. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Ma S, Frederick P, Hockings C, Tan I, Kratina T, et al. Bax dimerizes via a symmetric BH3:groove interface during apoptosis. Cell Death Differ. 2012;19:661–670. doi: 10.1038/cdd.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, et al. To trigger apoptosis Bak exposes its BH3 domain and homo-dimerizes via BH3:grooove interactions. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Moldoveanu T, Liu Q, Tocilj A, Watson M, Shore G, Gehring K. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell. 2006;24:677–688. doi: 10.1016/j.molcel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y-T, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, et al. Bcl-x(L) Retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg B, Wang P, Keeble JA, Rodriguez-Enriquez R, Walker S, Owens TW, et al. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol Cell. 2013;49:959–971. doi: 10.1016/j.molcel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Roy SS, Ehrlich AM, Craigen WJ, Hajnoczky G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 2009;10:1341–1347. doi: 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. VDAC regulation by the Bcl-2 family of proteins. Cell Death Differ. 2000;7:1174–1181. doi: 10.1038/sj.cdd.4400780. [DOI] [PubMed] [Google Scholar]
- Huckabee DB, Jekabsons MB. Identification of Bax-voltage-dependent anion channel 1 complexes in digitonin-solubilized cerebellar granule neurons. J Neurochem. 2011;119:1137–1150. doi: 10.1111/j.1471-4159.2011.07499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M, Stojanovski D, Frazier AE, Kotevski A, Dewson G, Craigen WJ, et al. Inhibition of Bak activation by VDAC2 is dependent on the Bak transmembrane anchor. J Biol Chem. 2010;285:36876–36883. doi: 10.1074/jbc.M110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Hockings C, Anwari K, Kratina T, Fennell S, Lazarou M, et al. Assembly of the Bak apoptotic pore: A critical role for the Bak alpha6 helix in the multimerization of homodimers during apoptosis. J Biol Chem. 2013;288:26027–26038. doi: 10.1074/jbc.M113.490094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crichton PG, Harding M, Ruprecht JJ, Lee Y, Kunji ER. Lipid, detergent, and Coomassie Blue G-250 affect the migration of small membrane proteins in blue native gels: mitochondrial carriers migrate as monomers not dimers. J Biol Chem. 2013;288:22163–22173. doi: 10.1074/jbc.M113.484329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S, Raulf N, Bregenhorn S, Biniossek ML, Maurer U, Czabotar P, et al. Cytosolic Bax: does it require binding proteins to keep its pro-apoptotic activity in check. J Biol Chem. 2012;287:9112–9127. doi: 10.1074/jbc.M111.248906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn AJ, Upton JP, Gilmore AP. Analysis of endogenous Bax complexes during apoptosis using blue native PAGE: implications for Bax activation and oligomerization. Biochem J. 2008;412:347–357. doi: 10.1042/BJ20071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KJ, Singh P, Lee K, Foss K, Lee S, Park M, et al. Conformational changes in BAK, a pore-forming proapoptotic Bcl-2 family member, upon membrane insertion and direct evidence for the existence of BH3-BH3 contact interface in BAK homo-oligomers. J Biol Chem. 2010;285:28924–28937. doi: 10.1074/jbc.M110.135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Classen M, Padmavathi PV, Ishikawa T, Zeth K, Steinhoff HJ, et al. Molecular details of Bax activation, oligomerization, and membrane insertion. J Biol Chem. 2010;285:6636–6647. doi: 10.1074/jbc.M109.081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Fletcher JI, Meusburger S, Hawkins CJ, Riglar DT, Lee EF, Fairlie WD, et al. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci USA. 2008;105:18081–18087. doi: 10.1073/pnas.0808691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K, Rudel T, Kozjak-Pavlovic V. TOM-independent complex formation of Bax and Bak in mammalian mitochondria during TNFalpha-induced apoptosis. Cell Death Differ. 2009;16:697–707. doi: 10.1038/cdd.2008.194. [DOI] [PubMed] [Google Scholar]
- Llambi F, Moldoveanu T, Tait SWG, Bouchier-Hayes L, Temirov J, McCormick LL, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setoguchi K, Otera H, Mihara K. Cytosolic factor- and TOM-independent import of C-tail-anchored mitochondrial outer membrane proteins. EMBO J. 2006;25:5635–5647. doi: 10.1038/sj.emboj.7601438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbel N, Ben-Hail D, Shoshan-Barmatz V. Mediation of the antiapoptotic activity of Bcl-xL protein upon interaction with VDAC1 protein. J Biol Chem. 2012;287:23152–23161. doi: 10.1074/jbc.M112.345918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Hu X, Eno CO, Zhao G, Li C, White C. An interaction between Bcl-xL and the voltage-dependent anion channel (VDAC) promotes mitochondrial Ca2+ uptake. J Biol Chem. 2013;288:19870–19881. doi: 10.1074/jbc.M112.448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todt F, Cakir Z, Reichenbach F, Youle RJ, Edlich F. The C-terminal helix of Bcl-x(L) mediates Bax retrotranslocation from the mitochondria. Cell Death Differ. 2013;20:333–342. doi: 10.1038/cdd.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn AJ, Upton JP, Bates N, Gilmore AP. Bax targeting to mitochondria occurs via both tail anchor-dependent and -independent mechanisms. Cell Death Differ. 2008;15:1243–1254. doi: 10.1038/cdd.2008.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellot G, Cartron PF, Er E, Oliver L, Juin P, Armstrong LC, et al. TOM22, a core component of the mitochondria outer membrane protein translocation pore, is a mitochondrial receptor for the proapoptotic protein Bax. Cell Death Differ. 2007;14:785–794. doi: 10.1038/sj.cdd.4402055. [DOI] [PubMed] [Google Scholar]
- Yamagata H, Shimizu S, Nishida Y, Watanabe Y, Craigen WJ, Tsujimoto Y. Requirement of voltage-dependent anion channel 2 for pro-apoptotic activity of Bax. Oncogene. 2009;28:3563–3572. doi: 10.1038/onc.2009.213. [DOI] [PubMed] [Google Scholar]
- Willis SN, Chen L, DEWSON G, Wei A, Naik E, Fletcher JI, et al. Pro-apoptotic Bak is sequestered by Mc1-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Kim H, Tu HC, Westergard TD, Fisher JK, Rubens JA, et al. The VDAC2-BAK rheostat controls thymocyte survival. Sci Signal. 2009;2:ra48. doi: 10.1126/scisignal.2000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Lindsten T, Zong W-X, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Deshmukh M, Johnson EM., Jr Inhibition of apoptotic signaling cascades causes loss of trophic factor dependence during neuronal maturation. J Cell Biol. 2000;149:1011–1018. doi: 10.1083/jcb.149.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M, McKenzie M, Ohtake A, Thorburn DR, Ryan MT. Analysis of the assembly profiles for mitochondrial- and nuclear-DNA-encoded subunits into complex I. Mol Cell Biol. 2007;27:4228–4237. doi: 10.1128/MCB.00074-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud DA, Formosa LE, Wijeyeratne XW, Nguyen TN, Ryan MT. Gene knockout using transcription activator-like effector nucleases (TALENs) reveals that human NDUFA9 protein is essential for stabilizing the junction between membrane and matrix arms of complex I. J Biol Chem. 2013;288:1685–1690. doi: 10.1074/jbc.C112.436766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.