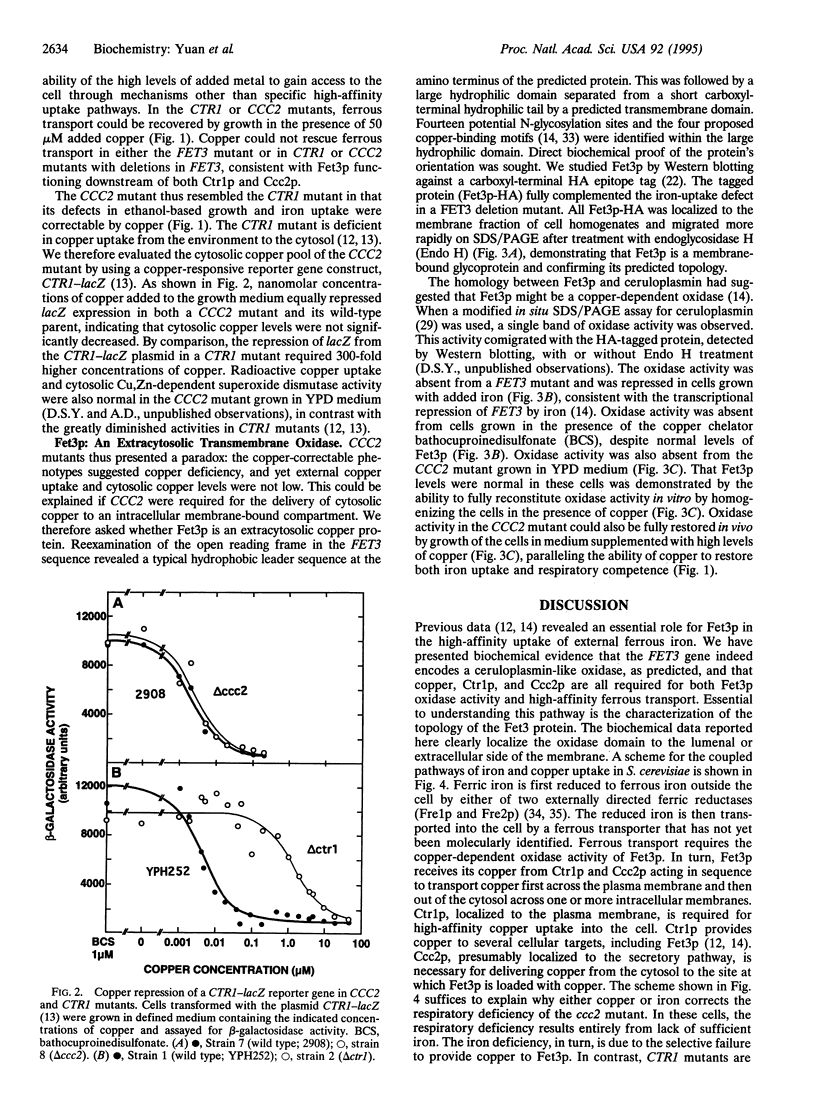
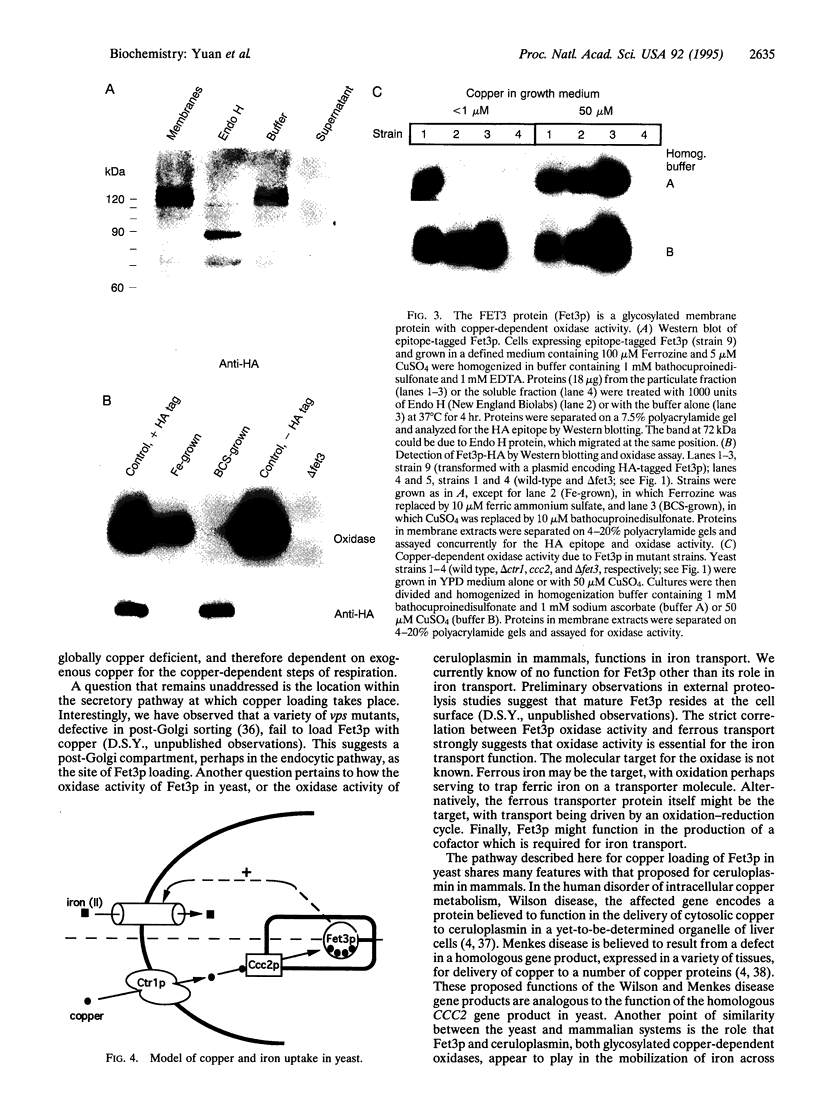
Abstract
The CCC2 gene of the yeast Saccharomyces cerevisiae is homologous to the human genes defective in Wilson disease and Menkes disease. A biochemical hallmark of these diseases is a deficiency of copper in ceruloplasmin and other copper proteins found in extracytosolic compartments. Here we demonstrate that disruption of the yeast CCC2 gene results in defects in respiration and iron uptake. These defects could be reversed by supplementing cells with copper, suggesting that CCC2 mutant cells were copper deficient. However, cytosolic copper levels and copper uptake were normal. Instead, CCC2 mutant cells lacked a copper-dependent oxidase activity associated with the extracytosolic domain of the FET3-encoded protein, a ceruloplasmin homologue previously shown to be necessary for high-affinity iron uptake in yeast. Copper restored oxidase activity both in vitro and in vivo, paralleling the ability of copper to restore respiration and iron uptake. These results suggest that the CCC2-encoded protein is required for the export of copper from the cytosol into an extracytosolic compartment, supporting the proposal that intracellular copper transport is impaired in Wilson disease and Menkes disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold F. H., Haymore B. L. Engineered metal-binding proteins: purification to protein folding. Science. 1991 Jun 28;252(5014):1796–1797. doi: 10.1126/science.1648261. [DOI] [PubMed] [Google Scholar]
- Askwith C., Eide D., Van Ho A., Bernard P. S., Li L., Davis-Kaplan S., Sipe D. M., Kaplan J. The FET3 gene of S. cerevisiae encodes a multicopper oxidase required for ferrous iron uptake. Cell. 1994 Jan 28;76(2):403–410. doi: 10.1016/0092-8674(94)90346-8. [DOI] [PubMed] [Google Scholar]
- Baudin A., Ozier-Kalogeropoulos O., Denouel A., Lacroute F., Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993 Jul 11;21(14):3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler T., Gable K., Zhao C., Dunn T. A novel protein, CSG2p, is required for Ca2+ regulation in Saccharomyces cerevisiae. J Biol Chem. 1994 Mar 11;269(10):7279–7284. [PubMed] [Google Scholar]
- Bull P. C., Cox D. W. Wilson disease and Menkes disease: new handles on heavy-metal transport. Trends Genet. 1994 Jul;10(7):246–252. doi: 10.1016/0168-9525(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Bull P. C., Thomas G. R., Rommens J. M., Forbes J. R., Cox D. W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993 Dec;5(4):327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- Chelly J., Monaco A. P. Cloning the Wilson disease gene. Nat Genet. 1993 Dec;5(4):317–318. doi: 10.1038/ng1293-317. [DOI] [PubMed] [Google Scholar]
- Chelly J., Tümer Z., Tønnesen T., Petterson A., Ishikawa-Brush Y., Tommerup N., Horn N., Monaco A. P. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet. 1993 Jan;3(1):14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- Dancis A., Haile D., Yuan D. S., Klausner R. D. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem. 1994 Oct 14;269(41):25660–25667. [PubMed] [Google Scholar]
- Dancis A., Klausner R. D., Hinnebusch A. G., Barriocanal J. G. Genetic evidence that ferric reductase is required for iron uptake in Saccharomyces cerevisiae. Mol Cell Biol. 1990 May;10(5):2294–2301. doi: 10.1128/mcb.10.5.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A., Roman D. G., Anderson G. J., Hinnebusch A. G., Klausner R. D. Ferric reductase of Saccharomyces cerevisiae: molecular characterization, role in iron uptake, and transcriptional control by iron. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3869–3873. doi: 10.1073/pnas.89.9.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis A., Yuan D. S., Haile D., Askwith C., Eide D., Moehle C., Kaplan J., Klausner R. D. Molecular characterization of a copper transport protein in S. cerevisiae: an unexpected role for copper in iron transport. Cell. 1994 Jan 28;76(2):393–402. doi: 10.1016/0092-8674(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox T. D., Folley L. S., Mulero J. J., McMullin T. W., Thorsness P. E., Hedin L. O., Costanzo M. C. Analysis and manipulation of yeast mitochondrial genes. Methods Enzymol. 1991;194:149–165. doi: 10.1016/0076-6879(91)94013-3. [DOI] [PubMed] [Google Scholar]
- GITLIN D., JANEWAY C. A. Turnover of the copper and protein moieties of ceruloplasmin. Nature. 1960 Mar 5;185:693–693. doi: 10.1038/185693a0. [DOI] [PubMed] [Google Scholar]
- Georgatsou E., Alexandraki D. Two distinctly regulated genes are required for ferric reduction, the first step of iron uptake in Saccharomyces cerevisiae. Mol Cell Biol. 1994 May;14(5):3065–3073. doi: 10.1128/mcb.14.5.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L., Ptashne M. Fusion of Escherichia coli lacZ to the cytochrome c gene of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2199–2203. doi: 10.1073/pnas.78.4.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H. 'Kinky hair' disease sheds light on copper metabolism. Nat Genet. 1993 Jan;3(1):3–4. doi: 10.1038/ng0193-3. [DOI] [PubMed] [Google Scholar]
- Harris Z. L., Takahashi Y., Miyajima H., Serizawa M., MacGillivray R. T., Gitlin J. D. Aceruloplasminemia: molecular characterization of this disorder of iron metabolism. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2539–2543. doi: 10.1073/pnas.92.7.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler S. G., Gallo L. K., Proud V. K., Percy A. K., Mark Y., Segal N. A., Goldstein D. S., Holmes C. S., Gahl W. A. Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nat Genet. 1994 Oct;8(2):195–202. doi: 10.1038/ng1094-195. [DOI] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990 Sep;54(3):266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Lee G. R., Nacht S., Lukens J. N., Cartwright G. E. Iron metabolism in copper-deficient swine. J Clin Invest. 1968 Sep;47(9):2058–2069. doi: 10.1172/JCI105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. F., Livingston J., Hall B., Paynter J. A., Begy C., Chandrasekharappa S., Lockhart P., Grimes A., Bhave M., Siemieniak D. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet. 1993 Jan;3(1):20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeser H. P., Lee G. R., Nacht S., Cartwright G. E. The role of ceruloplasmin in iron metabolism. J Clin Invest. 1970 Dec;49(12):2408–2417. doi: 10.1172/JCI106460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Gitlin J. D. Mechanisms of copper incorporation during the biosynthesis of human ceruloplasmin. J Biol Chem. 1991 Mar 15;266(8):5128–5134. [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R. E., Petrukhin K., Chernov I., Pellequer J. L., Wasco W., Ross B., Romano D. M., Parano E., Pavone L., Brzustowicz L. M. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993 Dec;5(4):344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- Vulpe C., Levinson B., Whitney S., Packman S., Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993 Jan;3(1):7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- Wach A., Schlesser A., Goffeau A. An alignment of 17 deduced protein sequences from plant, fungi, and ciliate H(+)-ATPase genes. J Bioenerg Biomembr. 1992 Jun;24(3):309–317. doi: 10.1007/BF00768851. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Heiny M. E., Gitlin J. D. Isolation and characterization of a human liver cDNA as a candidate gene for Wilson disease. Biochem Biophys Res Commun. 1993 Nov 30;197(1):271–277. doi: 10.1006/bbrc.1993.2471. [DOI] [PubMed] [Google Scholar]
- Zubenko G. S., Mitchell A. P., Jones E. W. Mapping of the proteinase b structural gene PRB1, in Saccharomyces cerevisiae and identification of nonsense alleles within the locus. Genetics. 1980 Sep;96(1):137–146. doi: 10.1093/genetics/96.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]