Abstract
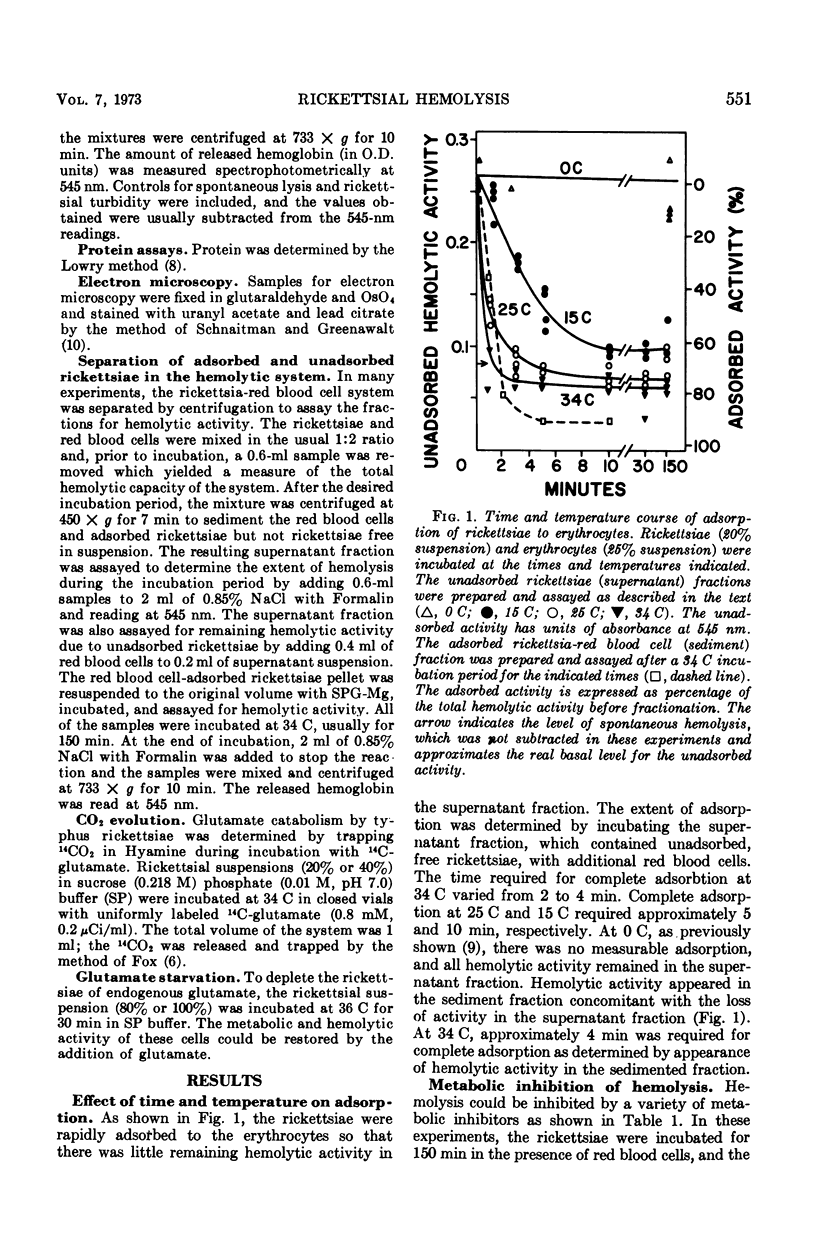
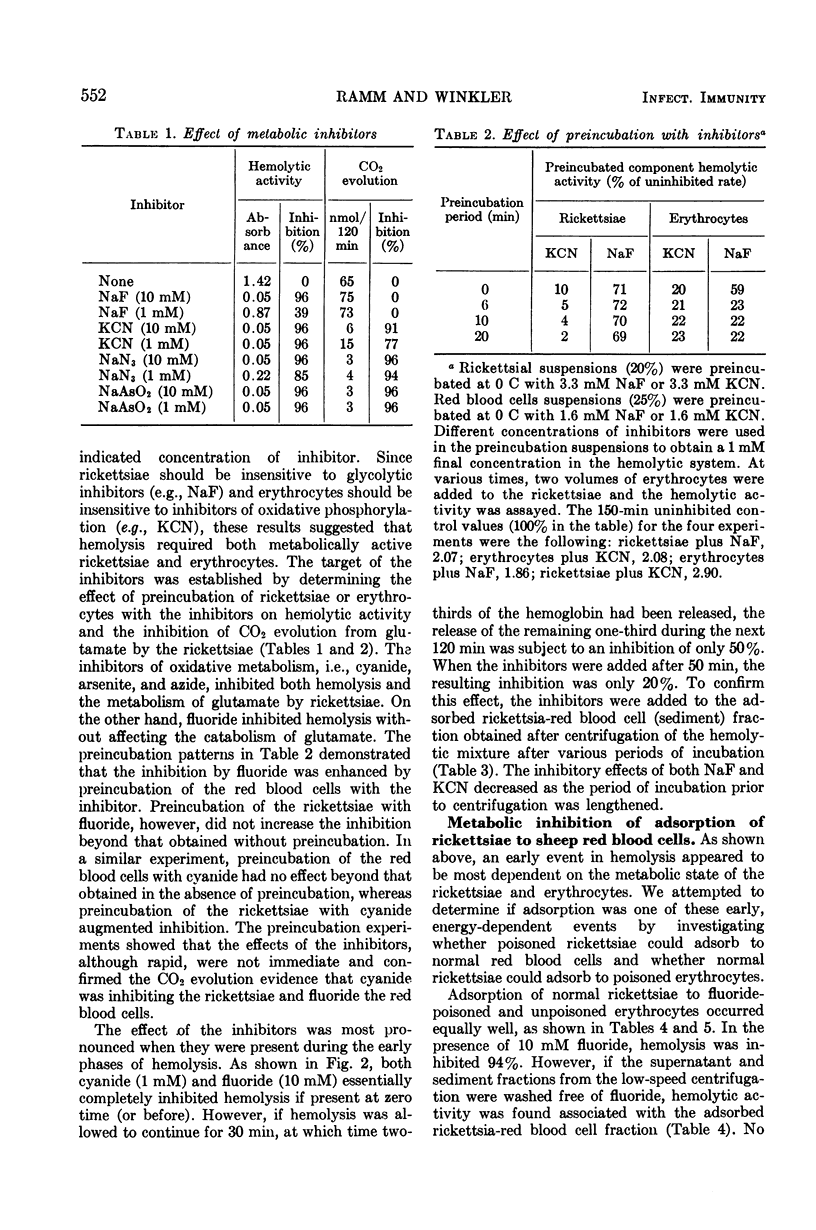
The hemolysis of sheep red blood cells by typhus rickettsiae is initiated by a temperature-sensitive adsorption process that is dependent on the energy-generating metabolism of the rickettsaie but not that of the erythrocyte. Adsorption is followed by events dependent on the metabolism of both the rickettsaie and red blood cells. At 34 C the adsorption step is complete after about 4 min, at lower temperatures the time required for adsorption is increased. At 0 C no adsorption occurred. The addition of cyanide inhibited the catabolism of glutamate by the rickettsiae and, consequently, both adsorption and the subsequent steps in hemolysis. The starvation of the rickettsiae for glutamate also prevented adsorption. Fluoride had no demonstrable effect on rickettsial metabolism nor the adsorption step but inhibited glycolysis in the erythrocytes and the hemolysis of the erythrocytes by rickettsiae.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of the toxicity and hemolytic activity of typhus rickettsiae by starvation. J Bacteriol. 1957 Nov;74(5):637–645. doi: 10.1128/jb.74.5.637-645.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of typhus Rickettsiae. I. Inactivation by freezing. J Gen Physiol. 1954 Nov 20;38(2):169–179. doi: 10.1085/jgp.38.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BOZEMAN F. M., CAMPBELL J. M., HUMPHRIES J. W., SAWYER T. K. Study on growth of Rickettsia. V. Penetration of Rickettsia tsutsugamushi into mammalian cells in vitro. J Exp Med. 1959 Mar 1;109(3):271–292. doi: 10.1084/jem.109.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R. M. A simple incubation flask for 14CO2 collection. Anal Biochem. 1971 Jun;41(2):578–580. doi: 10.1016/0003-2697(71)90180-1. [DOI] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Rickettsial hemolysis: adsorption of rickettsiae to erythrocytes. Infect Immun. 1973 Jan;7(1):93–99. doi: 10.1128/iai.7.1.93-99.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER J. C., BOVARNICK M. R., MILLER J. C., CHANG R. S. M. Observations on the hemolytic properties of typhus rickettsiae. J Bacteriol. 1954 Jun;67(6):724–730. doi: 10.1128/jb.67.6.724-730.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, JACKSON E. B., HAHN F. E., LEY A. C., SMADEL J. E. Metabolic studies of rickettsiae. I. The effects of antimicrobial substances and enzyme inhibitors on the oxidation of glutamate by purified rickettsiae. J Immunol. 1951 Aug;67(2):123–136. [PubMed] [Google Scholar]



