Abstract
There is mounting evidence to suggest that protein glutathionylation is a key process contributing to the development of pathology. Glutathionylation occurs as a result of posttranslational modification of a protein and involves the addition of a glutathione moiety at cysteine residues. Such modification can occur on a number of proteins, and exerts a variety of functional consequences. The L-type Ca2+ channel has been identified as a glutathionylation target that participates in the development of cardiac pathology. Ca2+ influx via the L-type Ca2+ channel increases production of mitochondrial reactive oxygen species (ROS) in cardiomyocytes during periods of oxidative stress. This induces a persistent increase in channel open probability, and the resulting constitutive increase in Ca2+ influx amplifies the cross-talk between the mitochondria and the channel. Novel strategies utilising targeted peptide delivery to uncouple mitochondrial ROS and Ca2+ flux via the L-type Ca2+ channel following ischemia-reperfusion have delivered promising results, and have proven capable of restoring appropriate mitochondrial function in myocytes and in vivo.
Keywords: oxidative stress, L-type calcium channel, reactive oxygen species (ROS), glutathionylation, calcium, ROS-induced ROS release
1. Introduction
Oxidative stress is a key feature underlying many different forms of cardiac pathology and represents an imbalance between the production of reactive oxygen species (ROS) and the cell’s inherent antioxidant defense system [1]. Reactive oxygen species are chemically reactive molecules that are derived from the reduction of molecular oxygen [2]. Under normal conditions the cellular concentrations of ROS are maintained within a narrow range [3] and act as critical mediators of various processes, including cell proliferation, differentiation and apoptosis, gene expression and cell metabolism [4,5,6,7,8]. Increases in intracellular ROS during pathological states lead to alterations in the regulation of signaling pathways, coupled with redox-modification of critical cellular proteins [9]. Much attention has been paid to the L-type Ca2+ channel as a target, largely because it is the primary means of Ca2+ entry into cardiomyocytes. Calcium is vital for cardiac excitation-contraction coupling, but is also a key regulator of mitochondrial ROS production. The interplay between ROS and Ca2+ in cardiomyocytes is therefore a critical determinant of cardiac function [10,11]. In this review article, the cross-talk between ROS and Ca2+ in mediating cardiac pathology will be examined, and particular emphasis will be placed on ROS-dependent changes in L-type Ca2+ channel function including glutathionylation. Finally, the use of novel interventions to alleviate the effects of oxidative stress-induced changes in L-type calcium channel function will be discussed.
2. The Role of Calcium and Reactive Oxygen Species (ROS) in Oxidative Stress Responses
2.1. Calcium Homeostasis and Myocyte Contraction
Calcium is a critical regulator of cardiac excitation-contraction coupling. In ventricular myocytes, depolarisation during the rapid rising phase of the action potential induces inward flux of calcium via the L-type Ca2+ channel, and this leads to further downstream calcium release via ryanodine receptors on the sarcoplasmic reticulum [12]. This calcium-induced calcium release (CICR) results from a close association between the L-type Ca2+ channel and ryanodine receptors, and provides rapid amplification of the initial calcium trigger event, such that activation of a single channel may initiate release from an estimated 6–20 ryanodine receptors [12,13]. The rise in intracellular calcium activates contraction by the binding of calcium to troponin, a component of the thin filament complex. Muscle fibers are formed from overlapping strands of contractile proteins comprising a thick filament composed of myosin and a thin filament made of actin and troponin/tropomyosin. Calcium binding to troponin induces an allosteric modification of the troponin/tropomyosin complex. This unblocks the myosin binding sites on the actin filament, and myosin is then powered by hydrolysing adenosine triphosphate (ATP) to move along these binding sites, resulting in muscle contraction [13]. Contraction is closely followed by relaxation and removal of free cytosolic calcium. This is predominantly mediated by the sarcoplasmic reticulum Ca2+-ATPase, with additional extrusion via the Na+/Ca2+ exchanger, the sarcolemmal Ca2+-ATPase (and possibly the mitochondrial Ca2+ uniporter [14]) playing a comparatively minor role [12,15].
2.2. Ca2+ and ROS-Induced ROS-Release
Aside from its role in contraction, calcium is also critical for the production of ATP and ROS. Mitochondrial calcium entry via the mitochondrial Ca2+ uniporter (MCU) stimulates the tricarboxylic acid (TCA) cycle and oxidative phosphorylation [16,17,18,19], and is specifically required for activation of three key dehydrogenases of the TCA cycle, pyruvate dehydrogenase, isocitrate dehydrogenase and oxoglutarate dehydrogenase [19,20], as well as the ATP synthase (Complex V of the electron transport chain) [18] and the adenine nucleotide translocase [16]. Activation of the TCA cycle causes increased production of NADH, which triggers movement of electrons down Complexes I–IV of the electron transport chain. Electrons are also fed in to the electron transport chain via Complex II as a result of the conversion of succinate to fumarate within the TCA cycle. Complex IV is the terminal electron acceptor, which acts to convert oxygen to water. During this process Complexes I, III and IV pump protons into the intermembrane space from the mitochondrial matrix, resulting in an increased proton motive force that exists as an electrochemical potential, also known as mitochondrial membrane potential [21]. This potential difference allows for the production of ATP from ADP at Complex V [21,22,23]. During this process of electron transfer, electrons leak from the respiratory chain and react with molecular oxygen to form superoxide [9,11,24,25,26]. Superoxide is rapidly converted to more stable H2O2 by manganese superoxide dismutase, which is present at a high concentration within the mitochondrial matrix [9,24,27,28]. Hydrogen peroxide then readily diffuses into the cytoplasm, where it is either partially reduced to the hydroxyl radical or fully catabolised to water via activity of the enzyme catalase [2]. Reactive hydroxide and H2O2 are then free to modify the function of critical cellular proteins [29].
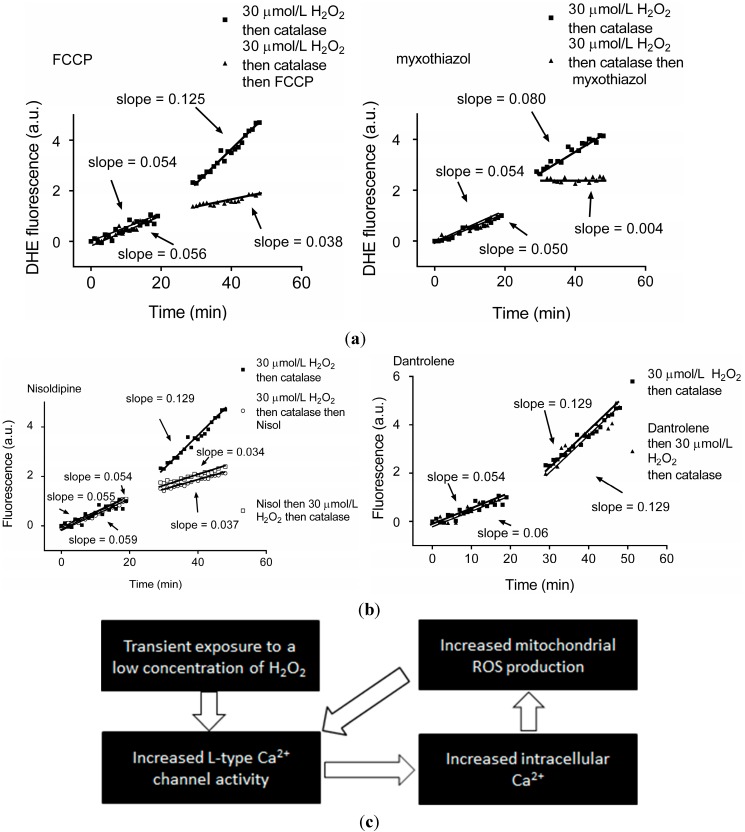
In pathological states, elevated levels of ROS can be amplified via a process known as ROS-induced ROS release (RIRR) [30,31,32]. The mechanisms underlying RIRR are commonly attributed to a positive feedback loop, in which the mitochondria are both the producer and the target of ROS [32,33,34]. However there is good evidence to suggest that an additional pathway of RIRR is via cross-talk between mitochondrial ROS and the L-type Ca2+ channel. The L-type Ca2+ channel contains a number of cysteines that are putative targets for modification under oxidising conditions, and acute exposure to H2O2 or thiol-oxidising compounds has been shown to increase L-type Ca2+ channel current [35,36,37,38]. Conversely, channel activity is decreased following exposure of the pore-forming α1C subunit of the human L-type Ca2+ channel expressed in HEK293 cells to a hypoxic insult [39]. Acute hypoxia decreases basal L-type Ca2+ channel current in isolated guinea pig ventricular myocytes without altering the current-voltage relationship [35]. Therefore there is good evidence that the channel is responsive to changes in the cell’s redox state. In order to further elucidate the interaction between the L-type Ca2+ channel and mitochondrial ROS production, we investigated the effect of a transient exposure of guinea pig cardiac myocytes to a concentration of H2O2 that was insufficient to induce apoptosis or necrosis, but mimicked an ischemia/reperfusion (I/R) injury, in which return of blood flow to the ischemic heart during reperfusion induces a burst of ROS [40]. Myocytes were exposed to 30 µM H2O2 for 5 min, followed by 10 U/mL catalase for 5 min to degrade the H2O2. Transient exposure of the myocytes to H2O2 significantly increased cellular superoxide levels, assessed by changes in dihydroethidium (DHE) fluorescence. The increase was attenuated when the myocytes were exposed to the electron transport chain uncoupler, carbonyl cyanide p-(trifluoromethoxy) phenyl-hydrazone (FCCP), or the mitochondrial inhibitor myxothiazol (Figure 1a) [40], identifying a mitochondrial origin for the increased superoxide consistent with the RIRR hypothesis. In addition, inhibition of mitochondrial calcium uptake using Ru360 (to block the MCU) resulted in an attenuation of the superoxide signal [40]. Consistent with this, acute treatment of myocytes with H2O2 induced an increase in free cytosolic calcium, assessed using the fluorescent free calcium indicator Fura-2 [40]. The calcium source required for the increase in cellular superoxide was subsequently identified as the L-type Ca2+ channel, as the increase in superoxide following transient exposure to H2O2 was abolished when the L-type Ca2+ channel was blocked with the channel antagonist nisoldipine, but not when calcium released from the sarcoplasmic reticulum via the ryanodine receptor was blocked with dantrolene (Figure 1b) [40]. A direct connection between the oxidative stress and alteration in L-type Ca2+ channel activity was subsequently established using whole-cell patch clamp recordings. Acute exposure of the myocytes to H2O2 increased basal activity of the L-type Ca2+ channel [29]. The increase in basal current density persisted for at least nine hours post-exposure due to the increase in mitochondrial superoxide, and was reversible.
Figure 1.
Transient exposure to H2O2 leads to L-type Ca2+ channel-dependent increase in mitochondrial superoxide, triggering ROS-induced-ROS release. (a) dihydroethidium (DHE) fluorescence recorded from individual guinea pig cardiomyocytes before and after exposure to 30 µM H2O2 for 5 min followed by 10 U/mL catalase for 5 min then 2 nM FCCP (an uncoupler of oxidative phosphorylation) as indicated (left panel) or 7 nM myxothiazol (to block electron transport at mitochondrial complex III) (right panel); (b) DHE fluorescence recorded from individual guinea pig cardiomyocytes before and after exposure to 30 µM H2O2 for 5 min followed by 10 U/mL catalase for 5 min and the L-type Ca2+ channel antagonist nisoldipine (nisol; 2 µM) as indicated (left panel) or ryanodine receptor antagonist dantrolene (20 µM; right panel); and (c) Schematic illustrating the persistent increase in intracellular ROS and intracellular Ca2+ following a transient H2O2 exposure or ROS-induced ROS-release. Reproduced with permission from [40].
These findings provide direct evidence that ROS generated by the mitochondria can act as potent and persistent regulators of L-type Ca2+ channel function (Figure 1c). It is worth noting that similar associations between ROS and voltage-gated calcium channels have been reported elsewhere. Neuronal P/Q-type Ca2+ channels expressed in Xenopus oocytes exhibit enhanced whole-cell currents following exposure to extracellular H2O2, as a result of an increase in channel open probability [41]. In other cell types there appears to be a reverse association between calcium and ROS under certain conditions. Exposure of L-type Ca2+ channels expressed in Chinese Hamster Ovary (CHO) cells to sulphydryl-oxidising agents inhibits channel activity as assessed using whole-cell and single channel recording techniques [42].
Cross-talk between calcium and ROS is therefore likely to be a mechanism that is highly conserved between systems, to allow for fine-tuning of cell redox state and calcium homeostasis.
2.3. Post-Ischemic Persistent Elevation of ROS and Ca2+ Is Pathological
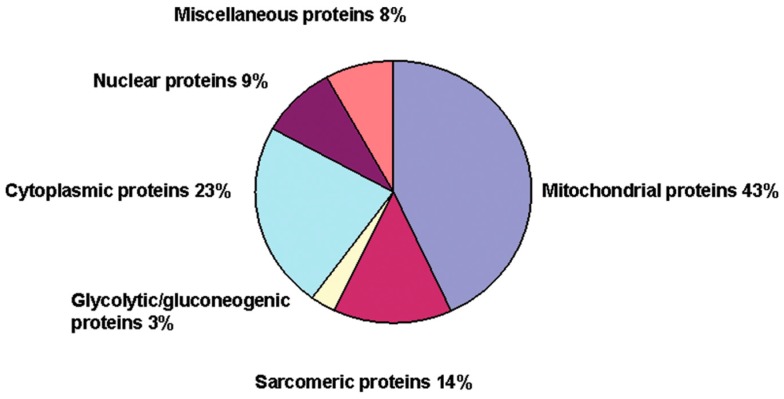
Cardiac hypertrophy is a common consequence of physiological increases in demand and/or pathological insults to the heart, and is characterised by an increase in the size of individual cardiac myocytes (without a change in myocyte number), enhanced protein synthesis and disorganisation of the sarcomere [43,44]. Many hypertrophic signaling pathways are triggered by an increase in intracellular calcium concentration and/or an elevation in ROS [45,46,47], and contribute to the development of ischemic heart disease and congestive heart failure [1,48,49]. Given the link between elevated calcium, ROS and cardiac dysfunction, we subsequently confirmed that the transient exposure to H2O2 and associated increase in superoxide [40] was sufficient to induce pathology at 48 h post-insult. Transient exposure of guinea pig myocytes to 30 µM H2O2 for 5 min resulted in a small but significant increase in the size of individual cardiomyocytes when imaged 48 h post-exposure [50]. Transient H2O2 exposure was also associated with an approximate two-fold increase in protein synthesis (assessed as incorporation of [3H]leucine). The increase was attenuated following L-type Ca2+ channel inhibition with nisoldipine but was unaffected by inhibition of the ryanodine receptor using dantrolene, indicating that the source of calcium was via the L-type Ca2+-channel and not intracellular Ca2+ stores [50]. Inhibition of downstream Ca2+-dependent signaling pathways using the Ca2+/calmodulin-dependent protein kinase II (CAMKII) inhibitor KN-62 also suppressed the enhancement of protein synthesis, consistent with previous reports identifying CAMKII signaling as a core mechanism underlying the development of cardiac hypertrophy and heart failure [51]. In order to identify and quantitate the expression levels of proteins 48 h after myocyte treatment with H2O2, a combined proteomics/isobaric tag for relative and absolute quantification (iTRAQ) based screening method was utilised. Using this approach, a total of 35 proteins were identified with altered expression, the majority of which were mitochondrial in origin (Figure 2) [50]. The proteins identified were predominantly involved in cellular metabolism, and included TCA cycle enzymes and oxidative phosphorylation proteins. Although there was no microscopic evidence of actin disorganisation at 48 h post-H2O2 exposure, iTRAQ analysis revealed alterations in the expression of sarcomeric proteins, including β-myosin heavy chain and troponin C, both of which are involved in sarcomeric disarray in advanced stages of cardiac hypertrophy [52]. The protein expression profile at 48 h after exposure to H2O2 offers a snapshot of the dynamic acute phase of induction of hypertrophy, and more pronounced increases in cell size and visible disorganisation of the sarcomere would likely become apparent at later time points. Therefore H2O2-induced persistent activation of the L-type Ca2+ channel is indeed sufficient to induce cardiac hypertrophy, and cross-talk between the L-type Ca2+ channel and mitochondrial ROS is critical in the progression of cardiac pathology.
Figure 2.
Transient exposure of myocytes to H2O2 alters mitochondrial protein synthesis. Exposure of guinea-pig myocytes to 30 µM H2O2 for 5 min followed by 10 U/mL catalase for 5 min is sufficient to induce alterations in cellular protein synthesis consistent with the development of myocyte hypertrophy. iTRAQ-facilitated proteomics analysis indicated that the majority of changes in protein synthesis are mitochondrial in origin. For further details see text. Reproduced with permission from [50].
2.4. Mitochondrial Complex III Is the Locus for Superoxide Generation
Under normal physiological conditions, leak of electrons from the electron transport chain occurs at mitochondrial Complexes I and III [2,53,54]. These electrons then react with molecular oxygen to form superoxide. To ascertain the site of increased superoxide production following transient H2O2 exposure, strategic pharmacological inhibition of the individual mitochondrial complexes was used in combination with fluorescent detection of changes in DHE signal to assess alterations in superoxide production. We had previously demonstrated that nicotinamide adenine dinucleotide phosphate NADPH-oxidase was not the source of superoxide [40]. Therefore we used diphenyleneiodonium chloride (DPI) to block electron flow prior to the superoxide generation site at Complex I, and this effectively attenuated the increase in DHE signal in response to transient H2O2 exposure [55]. Block of electron flow after the superoxide generation site of Complex I using rotenone also attenuated the H2O2-induced increase in superoxide signal [55]. Therefore the locus of increased superoxide was concluded to be either at or beyond Complex I. To examine whether Complex III was the site of enhanced superoxide production, myocytes were exposed to 7 nM myxothiazol and stigmatellin (a concentration that did not alter mitochondrial membrane potential), to block electron flow at the Q0 superoxide site of Complex III. Both compounds successfully attenuated the increase in superoxide [55]. Blocking of electron flow at the Qi superoxide generation site of Complex III (which is downstream from Q0) using antimycin A did not alter the increase in superoxide production, however, nor did application of sodium cyanide to block Complex IV [55]. This identified the Q0 superoxide generation site of Complex III as the locus of increased superoxide production, since blocking of electron flow beyond this site had no effect on the production of superoxide.
In order to provide unequivocal confirmation of the Q0 site of Complex III as the predominant source of superoxide, simultaneous whole-cell patch clamp and fluorescence imaging experiments were performed in which the Q0 site was pharmacologically isolated. To achieve this, cardiac myocytes were perfused with the Complex II substrate succinate intracellularly via the patch pipette, while the extracellular medium was supplemented with the Complex I inhibitor rotenone and the Complex III Qi site inhibitor antimycin A. This approach allowed for block of electron flow from Complex I and from Complex III Qi site onwards, leaving the Q0 site of complex III intact. Under these conditions, transient exposure to H2O2 resulted in a similar increase in superoxide signal as had previously been recorded from cells that were not patch-clamped. The increase in superoxide was blocked when the myocytes were exposed to extracellular myxothiazol (to block electron flow at Q0) prior to H2O2 insult.
In order to develop appropriate therapies to effectively limit the development of ROS-induced cardiac hypertrophy, it is necessary to first understand the progression of events involved during the acute phase of oxidative stress. Identification of the Q0 site of Complex III as the locus for increased superoxide production is an important step towards the development of therapeutic strategies for ROS-induced hypertrophy, which have otherwise proven elusive. To that end, there is limited evidence to suggest that myxothiazol is capable of decreasing mitochondrial ROS production during an ischemic insult in an experimental setting [56]. However targeting the electron transport chain may yet prove to be a viable strategy.
3. Glutathionylation of the L-type Ca2+ Channel during Oxidative Stress
3.1. Overview of Protein Glutathionylation
Hydrogen peroxide is responsible for mediating many cellular redox reactions, and acts as an efficient second messenger that provides rapid communication within and between cells [57]. One of the most common outcomes of a rise in cellular oxidant levels is the modification of redox-sensitive proteins via a process known as S-glutathionylation [58]. Glutathionylation occurs as a posttranslational modification of proteins at the cysteine residues by adding a glutathione (GSH, γ-glutamylcysteinylglycine) moiety [58,59,60]. Glutathione is a ubiquitous tripeptide that acts as an inherent antioxidant, and works in conjunction with oxidised glutathione (glutathione disulphide, GSSG) as an intracellular redox buffer [58,61]. The ratio of GSH to GSSG therefore contributes to the redox potential of the cell, and during periods of oxidative stress, ROS oxidises GSH to GSSG (causing a decrease in the GSH/GSSG ratio) via Equation (1) [62].
| (1) |
A critical role of the GSH-GSSG redox couple is to facilitate reversible S-glutathionylation of proteins (Pr) [60]. One means by which glutathionylated proteins (PrSSG) are formed is via direct reaction of a cysteine residue on a protein (PrSH) with GSSG via a thiol-disulfide exchange reaction. (Equation (2)) [61,63,64]. Alternatively, proteins can be glutathionylated via interaction between oxidised protein thiols and GSH [61].
| (2) |
During periods of oxidative stress, ROS oxidises GSH to GSSG (resulting in a decrease in the ratio of GSH to GSSG), and leading to increased glutathionylation of proteins [62]. It is important to note however that the kinetics of protein glutathionylation are influenced by other factors beyond the redox state of the cell, including the pKa of the sulfhydryl (–SH) group of protein cysteine residues and the steric location of cysteine residues within the protein [61]. These factors in conjunction with the cell redox potential dictate the probability of protein glutathionylation. To date, a number of proteins have been identified that undergo S-glutathionylation, often in response to oxidative stress. S-Glutathionylation of proteins can induce a range of consequences [59,60,65], including a downregulation [66,67] or upregulation [68] of enzymatic activity, altered DNA binding by transcription factors [69,70] and increased [71] or decreased [72] protein stability. Whilst there are many cellular modifications that occur as a result of oxidative stress, available evidence suggests that glutathionylation may predominate as a critical signaling mechanism in cardiac disease [73].
3.2. Glutathionylation of the L-type Ca2+ Channel
Given the strong evidence that protein glutathionylation is a key process underlying oxidative stress-induced disease, we hypothesised that the H2O2-induced persistent increase in L-type Ca2+ channel activity [40] was due to glutathionylation of the channel. To assess this, we first examined whether the purified channel protein was a candidate for glutathionylation in vitro. The channel was isolated from guinea pig heart homogenates and exposed to 1 mM GSH and 30 µM H2O2. Immunoblot analysis revealed that the channel was significantly glutathionylated following this exposure [74].
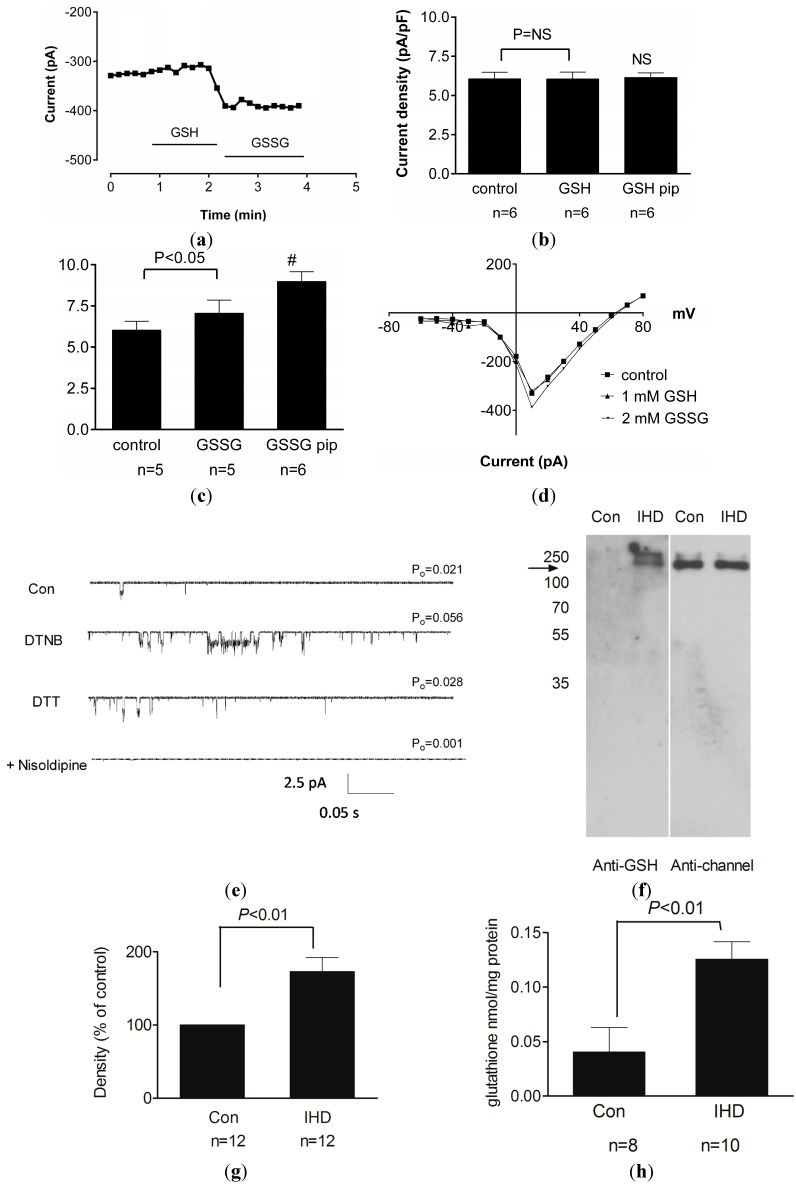
We next examined the effects of GSH and GSSG on the function of the native channel using the patch clamp technique in vitro. Exposure of ventricular myocytes to 1 mM GSH in the extracellular solution resulted in a small decrease in basal channel activity, with no change in activity when the GSH was applied intracellularly via the patch pipette (Figure 3a,b) [74]. External application of GSSG produced a significant increase in the peak inward current when the cell voltage was stepped to +10 mV, with no change in the current-voltage relationship of the channel (Figure 3a,c,d) [74]. This effect was amplified when GSSG was applied intracellularly via the patch pipette, suggesting that the cytoplasmic portion of the L-type Ca2+ channel is more sensitive to redox modification (Figure 3c). The increase in current observed with GSSG application is consistent with prior reports by us, and others, that H2O2 exposure induces a persistent enhancement of channel function [38,40,75,76]. We subsequently confirmed that GSSG application was sufficient to increase intracellular calcium, assessed as changes in Fura-2 fluorescence, consistent with a ROS-induced potentiation of L-type Ca2+ channel activity. This increase in intracellular calcium was attenuated with glutaredoxin, an agent that induces reduction of GSSG to GSH [77]. In contrast GSH application had no effect on intracellular calcium [74]. Therefore glutathionylation of the L-type Ca2+ channel alters macroscopic channel function and intracellular calcium during oxidative stress.
Figure 3.
Oxidative stress causes persistent enhancement of the L-type Ca2+ channel via ROS-mediated glutathionylation. (a) Time course of changes in membrane current recorded from guinea pig cardiac myocytes during extracellular exposure to 1 mM GSH followed by 2 mM GSSG as indicated; (b) Mean (±SEM) of L-type Ca2+ channel current density under control conditions (no drugs) and after exposure to GSH applied either extracellularly or in the patch pipette (GSH pip). NS, not significant; (c) Mean (±SEM) of L-type Ca2+ channel current density under control conditions (no drugs) and after exposure to GSSG applied either extracellularly or in the patch pipette (GSSG pip). #, p < 0.05 vs. control; (d) Current-voltage relationship for representative myocytes during voltage steps from −60 to +80 mV and exposure to GSH or GSSG as indicated; (e) Representative single channel currents recorded at −100 mV in the absence and presence of 200 µM DTNB followed by 1 mM DTT and then 2 µM nisoldipine. The open probability (P0) for each treatment is indicated; (f) Immunoblot demonstrating glutathionylation of protein after probing with anti-GSH antibody (left) and anti-channel antibody (right) in immunoprecipitated Cav1.2 channel protein samples from control nonischemic human heart (Con) and ischemic human heart (IHD); (g) Densitometry analysis of immunoblots for glutathionylated protein band normalised to the channel protein in the same lane for Con samples and IHD samples (mean ± SEM); and (h) Concentration of protein-glutathione mixed-disulfides in channel protein from Con and IHD heart samples (mean ± SEM). Reproduced with permission from [74].
3.3. Functional and Clinical Consequences of L-type Ca2+ Channel Glutathionylation
To verify that the effects of GSSG and H2O2 were due to a direct effect on the channel protein, we reconstituted the purified long N-terminal (NT) isoform of the human Cav1.2 (α1C subunit) into proteoliposomes [74]. This strategy allowed for functional analysis of channel currents to be performed in the absence of other cellular proteins, such as the auxiliary subunits that can modulate biophysical channel properties and are reported to be vulnerable to glutathionylation [78]. Exposure of the channel to either GSH, nisoldipine, or the thiol reducing agent dithiothreitol (DTT) significantly decreased channel open probability, whilst GSSG, H2O2 and the thiol oxidising agent 5,5'-dithio-bis(2-nitrobenzoic acid) (DTNB) induced an increase in channel open probability (Figure 3e). These changes occurred in the absence of any change in the current-voltage sensitivity of the channel.
We subsequently confirmed that an experimental ischemia reperfusion (I/R) protocol was sufficient to induce glutathionylation of the L-type Ca2+ channel [74]. In these experiments, guinea pig hearts were retrogradely perfused via the aorta on a Langendorff apparatus and exposed to 30 min of no-flow ischemia followed by 30 min of reperfusion. Elevated levels of creatine kinase (CK) and lactate dehydrogenase (LDH) were measured in the coronary effluent, indicative of myocardial damage [79]. Further analysis revealed an increase in the level of glutathionylation of the L-type Ca2+ channel following I/R injury, and a glutathione recycling assay was used to confirm a significant increase in the level of protein-glutathione mixed disulfides [74]. The modifications were also confirmed to occur in human hearts following oxidative stress. A significant increase in the level of glutathionylation of the L-type Ca2+ channel was found in ventricular samples from ischemic human hearts when compared to non-ischemic controls (Figure 3f–h) [74]. This finding provides confirmation that the persistent redox-dependent alterations in channel function we described in the laboratory setting are clinically relevant.
A number of proteins have been demonstrated to undergo reversible glutathionylation. These proteins often tend to cluster into several categories, and are frequently involved in energy metabolism, cell signaling, cytoskeletal function, protein folding and editing, redox balance and ion channel modification [61,80]. Thus far we have limited knowledge of how structure and function of these proteins are altered by glutathionylation. In the case of ion channels, there is good evidence to suggest that glutathionylation can inhibit ion flux via an alteration in channel conductance. For example, the vascular ATP-sensitive potassium channel (KATP) is glutathionylated at Cys176, which is located in the critical region of the inner helix, close to both the activation and hinge gate residues [81]. In silico modelling of the channel has demonstrated that binding of the GSH moiety to Cys176 prevents the channel from entering its open state and retains it in a closed conformation. Other channels exhibit enhanced activity after glutathionylation, and structural changes that affect channel kinetics are likely to underlie the enhancement. For example, the neuronal Kv4 voltage-gated potassium channel contains an auxiliary subunit (dipeptidyl peptidase-like protein 6; DPP6a) which is glutathionylated at Cys13, resulting in a slowing of inactivation kinetics and increase in peak current amplitude [78]. These effects can be fully reversed using the thiol reducing agent DTT [78].
The α1C subunit of the L-type Ca2+ channel contains 48 cysteines, approximately 38 of which are located at sites that could be accessible for glutathionylation. It is likely that glutathionylation at one or more of these sites therefore induces a structural change that results in an increase in the open probability of the channel, without altering the voltage sensitivity. This would allow for a greater amount of calcium influx into the cell, without altering single channel conductance. The L-type Ca2+ channel therefore represents a viable clinical target following ischemia reperfusion injury, and tailoring therapies to reduce or inhibit redox-dependent modifications of the channel may prevent the activation of signaling pathways and development of hypertrophy.
3.4. S-Nitrosylation of the L-type Ca2+ Channel as an Alternate Means of Channel Modification during Oxidative Stress
Nitric oxide (NO) signaling is important for a diverse range of physiological processes [82]. Signaling is mediated by a range of molecules, including S-nitrosothiols which are derivatives of the free radical NO [83]. S-Nitrosothiols serve as donors of the nitrosonium ion (NO+), and can facilitate S-nitrosylation of proteins via the addition of a nitroso group to a sulfur atom of an amino acid. This process is reversible and results in post-translational modification of the target protein. There is some evidence to suggest that S-nitrosylation of the L-type Ca2+ channel can occur following changes in oxygen tension, and that this leads to an inhibition of channel current [84,85]. Contrary to these reports, we find no evidence for a role for NO in the regulation of basal L-type Ca2+ currents or the inhibition of current induced by hypoxia [35]. In addition we report no effect of NO inhibitors on the L-type Ca2+ current in the presence of acute exposure to H2O2 [40]. It is possible that variation in the extent of oxidative stress and in the experimental preparation used may account for these differences.
4. Novel Interventions to Alleviate Ischemic Injury
4.1. Overview of Ischemia-Reperfusion Injury
Ischemic heart disease is characterised by reduced coronary blood flow and is a leading cause of mortality and morbidity worldwide [43,86]. Ischemic injury is associated with atherosclerosis, but is also an unfortunate consequence of some clinical procedures (e.g., angioplasty, cardiopulmonary bypass). In such circumstances timely reperfusion of the heart is essential to reduce further cardiac damage, however reflow into the ischemic heart paradoxically leads to additional myocardial injury [87,88]. Cardiac tissue has been characterised histologically following reperfusion injury and is typified by unique features, including contraction bands in the contractile proteins, calcific granules within mitochondria and the disruption of the sarcoplasmic and mitochondrial membranes [89,90,91,92,93]. Functional changes to cardiac tissue following reperfusion include depressed contractility, reduced coronary flow and altered vascular reactivity [94,95,96]. A number of mechanisms have been proposed as contributors to I/R injury, including activation of inflammatory cascades [88], calcium overload [97], the no reflow phenomenon [98,99] and endothelial dysfunction [100,101], but there is good evidence to suggest that the increased production of ROS is a primary factor [102]. A critical study by Bolli and colleagues [103] provided unequivocal confirmation that large quantities of ROS are produced in the first few minutes following reperfusion, and that this is an important determinant of reperfusion injury. Increased ROS production following reperfusion alters the redox state of the cell, and induces a number of cellular effects including an upregulation of protein glutathionylation and impaired calcium homeostasis.
4.2. Reduced Ischemia-Reperfusion Injury Using Targeted Peptide Delivery
Our experimental work has identified that persistent activation of the L-type Ca2+ channel via redox-dependent glutathionylation is critical in triggering downstream cascades that lead to myocyte dysfunction [74]. Features of this dysfunction include impaired calcium homeostasis and mitochondrial function, and increased protein synthesis and cell size, consistent with the development of myocyte hypertrophy [40,50,74,104,105]. We have demonstrated that some of these features can be attenuated using targeted peptide delivery to prevent increased activation of the L-type Ca2+ channel during periods of oxidative stress.
The cardiac L-type Ca2+ channel is a heterotetramer comprised of the α1C, α2δ and β2 subunits. The α1C subunit contains the pore-forming and voltage-sensing regions responsible for ion conductance, and contains the binding sites for channel modulating drugs and toxins [15,106,107]. The α2δ subunit regulates channel turnover at the membrane and is required for appropriate channel trafficking [108]. The β2 subunit binds to the α1 subunit at the alpha-interacting domain (AID) and assists with the trafficking and insertion of the α1C subunit into the membrane [109]. The β2 subunit also regulates channel inactivation, and couples the channel to cytoskeletal proteins [110]. There is good evidence to suggest that the β2 subunit of the channel interacts with F-actin via a 700 kDa stabilizing protein known as AHNAK [110,111,112]. Additionally, channel-activated increases in mitochondrial membrane potential are abolished when cells are exposed to latrunculin A, which disrupts actin assembly [104]. These findings have led to the proposal that a conformational change during activation of the L-type Ca2+ channel results in movement of the β2 subunit, which is transmitted to the mitochondria via the actin cytoskeleton. In this manner, activation of the channel can lead to actin-mediated increases in mitochondrial membrane potential.
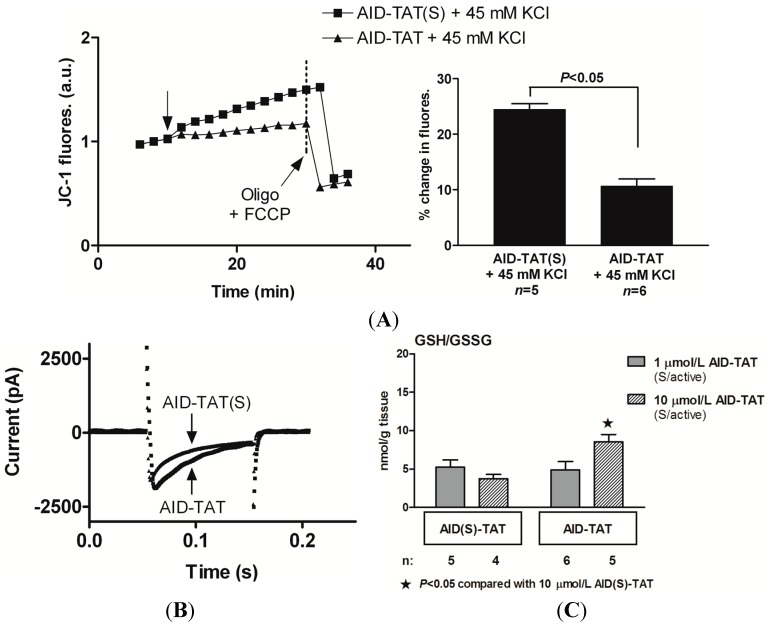
To assess whether specific pharmacological interventions might be equally effective at disrupting communication between the channel and mitochondria, we applied a peptide derived against the AID of the channel using a transactivator of transcription (TAT) sequence to facilitate membrane transfer (AID–TAT peptide) [104]. The peptide immobilises the β2 subunit [113] and induces uncoupling from the actin cytoskeleton, allowing for normal calcium conductance to be maintained while altering mitochondrial and metabolic responses [104]. Application of the AID–TAT peptide to guinea pig ventricular myocytes effectively attenuates the increase in metabolic activity after activation of the channel. This includes the increase in oxidation of flavoprotein (flavin adenine dinucleotide) that is measured as autofluorescence and mitochondrial membrane potential upon activation of the channel [104] (Figure 4a). Using the whole-cell patch clamp technique we confirmed that exposure to AID–TAT modified the biophysical properties of the channel, such that it exhibited a delayed rate of inactivation of current as would be expected from binding of the peptide to the AID region of the α1C subunit [104] (Figure 4b).
Figure 4.
Targeted peptide delivery disrupts cross-talk between the L-type Ca2+ channel and the mitochondria and reduces ischemia-reperfusion injury. (A) Exposure to AID–TAT attenuates the increase in mitochondrial membrane potential (Ψm) associated with activation of the channel. Ψm assessed as changes in JC-1 fluorescence recorded from two representative guinea pig myocytes before and after exposure to either 1 µM scrambled AID–TAT (AID–TAT(S)) or 1 µM AID–TAT followed by 45 mM KCl to activate the L-type Ca2+ channel. Arrow indicates where KCl was added. Mean ± SEM of changes in JC-1 fluorescence for myocytes exposed to AID–TAT(S) and AID–TAT shown on the right; (B) L-type Ca2+ channel current traces activated by voltage step to +10 mV from a holding potential of −30 mV from representative myocytes that were pre-incubated with either AID–TAT(S) or AID–TAT; and (C) GSH/GSSG ratio of guinea pig hearts treated with 1 or 10 µM scrambled AID–TAT (AID(S)–TAT) or active AID–TAT within 5 min after commencement of reperfusion following 30 min of no-flow ischemia. Panels A and B reproduced with permission from [104] and panel C reproduced with permission from [114].
We additionally reported the therapeutic benefits of this construct, and demonstrated a dose-dependent alleviation of oxidative stress following coronary injection of the AID–TAT peptide in guinea pig hearts. Exposure to 10 µM of active peptide following I/R injury resulted in an increase in GSH to GSSG ratio [114] (Figure 4c), whereas a lower concentration of the peptide (1 µM) was insufficient to alter the GSH to GSSG ratio but still effective in reducing infarct size and CK and LDH levels. Application of the AID–TAT peptide was also found to improve contractility following oxidative stress. In untreated hearts, I/R injury caused a reduced left ventricular developed pressure which failed to improve by 30 min post-ischemia. Application of 10 µM AID–TAT peptide significantly improved developed pressure in reperfused hearts at 20–30 min post-reperfusion [114].
4.3. Targeted Peptide Delivery Decreases Infarct Size and Restores Contractility in Vivo
We have demonstrated that the L-type Ca2+ channel regulates mitochondrial function via a calcium-dependent mechanism. Calcium influx via the channel increases NADH production, superoxide production and metabolic activity [104]. L-type Ca2+ channel activation also induces an increase in mitochondrial membrane potential and metabolic activity, and this is mediated via the actin cytoskeleton. [104,115]. Given that increased production of ROS is thought to be a primary contributor to ischemia-reperfusion injury [49,102], we predicted that attenuation of ischemia-reperfusion injury using the AID–TAT peptide would result from an effect on mitochondrial function.
We examined the effect of application of AID–TAT peptide after ischemia-reperfusion in rats [114]. In these experiments the AID–TAT peptide was injected into the left ventricle after reperfusion of ischemic hearts. Following a recovery period of up to 12 weeks, infarct size, heart weight and left ventricular function were assessed. Both the low and high doses of the AID–TAT peptide were sufficient to reduce infarct size, and this was evident as early as 6 weeks post-infarction. The high dose of AID–TAT was also sufficient to prevent development of hypertrophy associated with ischemia-reperfusion injury, as well as restore left ventricular function.
These in vivo findings in combination with our ex vivo and in vitro data suggest that 10 µM AID–TAT is an effective therapeutic dose to reduce I/R injury. We propose that the effects are mediated by disruption of the functional communication that exists between the L-type Ca2+ channel and the mitochondria via cytoskeletal proteins, preventing increases in mitochondrial function that are induced by increased activation of the channel. This ultimately translates into reduced injury pathology when assessed in the short-term (reduced CK and LDH, increased GSH:GSSG) and in the long-term improved contractility [114]. Importantly, a 10 µM dose would still allow for calcium influx to be maintained under these conditions, enabling appropriate excitation-contraction to be sustained.
Prior clinical use of calcium modulating drugs have largely been spurred on by early animal studies demonstrating that blockade of the L-type Ca2+ channel using antagonists such as nisoldipine [116,117,118] and diltiazem [119,120] or by blocking with MgSO4 [121,122] limits myocardial damage following ischemia and reperfusion. Despite these positive preclinical findings, subsequent clinical trials using these blockers have produced mostly disappointing results [123,124,125]. This may be in part due to the substantial negative inotropic effects of these agents, a serious drawback in scenarios in which contractility may already be impaired following an ischemic insult. Therapeutic delivery of agents that allow for appropriate excitation-contraction to be maintained following reperfusion (such as the AID–TAT peptide) may overcome this shortcoming. Indeed, mibefradil (Ro 40-5967) is one such agent that does not evoke the substantial negative inotropic effects of other classical L-type Ca2+ channel blockers and was found to exhibit positive outcomes in experimental animals [126,127] as well as in a series of phase I, II and III clinical trials [128]. The agent was subsequently removed from the market as a result of adverse drug interactions, but provided convincing evidence that restoration of contractility post-ischemia is critical to reduce morbidity and mortality. Use of L-type Ca2+ channel modifying peptides, such as AID–TAT, therefore warrants further investigation to assess their efficacy in limiting myocardial damage following ischemia-reperfusion.
5. Concluding Remarks
Calcium influx via the L-type calcium channel is the predominant means of calcium entry into cardiomyocytes and provides the trigger for appropriate excitation contraction in the heart. Our data has verified that there is cross-talk between calcium flux via the L-type calcium channel and ROS produced by the mitochondria, such that increased intracellular calcium induces increased ROS production. In parallel, increased mitochondrial ROS leads to glutathionylation of the L-type calcium channel, resulting in a persistent increase in channel open probability. This constitutive channel activity leads to further increases in mitochondrial function, and thereby amplifies the cross-talk between ROS and the L-type calcium channel. We have good evidence to suggest that ROS-induced effects on channel function are likely to contribute to the development of pathology in vivo, and indeed preliminary experiments indicate that use of targeted peptide therapies that disrupt cross-talk between the channel and the mitochondria are capable of limiting reperfusion injury and restoring normal mitochondrial function.
Acknowledgments
The work presented in this article was supported by the National Health and Medical Research Council of Australia (NHMRC) and the Australian Research Council (ARC). Livia Hool is an ARC Future Fellow and an Honorary NHMRC Senior Research Fellow.
Author Contributions
Livia C. Hool analysed data and interpreted results of experiments. Victoria P. A. Johnstone and Livia C. Hool prepared figures, drafted, edited and revised manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dhalla N.S., Temsah R.F., Netticadan T. Role of oxidative stress in cardiovascular diseases. J. Hyperten. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schafer F.Q., Buettner G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/S0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 4.Sauer H., Wartenberg M., Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 5.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 6.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 8.Turpaev K.T. Reactive oxygen species and regulation of gene expression. Biochemistry. 2002;67:281–292. doi: 10.1023/a:1014819832003. [DOI] [PubMed] [Google Scholar]
- 9.Finkel T. Signal transduction by reactive oxygen species. J. Cell. Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Y., Wei C.L., Zhang W.R., Cheng H.P., Liu J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol. Sin. 2006;27:821–826. doi: 10.1111/j.1745-7254.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 11.Feissner R.F., Skalska J., Gaum W.E., Sheu S.S. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front. Biosci. 2009;14:1197–1218. doi: 10.2741/3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bers D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 13.Winslow R.L., Greenstein J.L. Cardiac myocytes and local signaling in nano-domains. Prog. Biophys. Mol. Biol. 2011;107:48–59. doi: 10.1016/j.pbiomolbio.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirichok Y., Krapivinsky G., Clapham D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 15.Bodi I., Mikala G., Koch S.E., Akhter S.A., Schwartz A. The L-type calcium channel in the heart: The beat goes on. J. Clin. Investig. 2005;115:3306–3317. doi: 10.1172/JCI27167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mildaziene V., Baniene R., Nauciene Z., Bakker B.M., Brown G.C., Westerhoff H.V., Kholodenko B.N. Calcium indirectly increases the control exerted by the adenine nucleotide translocator over 2-oxoglutarate oxidation in rat heart mitochondria. Arch. Biochem. Biophys. 1995;324:130–134. doi: 10.1006/abbi.1995.9918. [DOI] [PubMed] [Google Scholar]
- 17.Hansford R.G., Zorov D. Role of mitochondrial calcium transport in the control of substrate oxidation. Mol. Cell. Biochem. 1998;184:359–369. doi: 10.1023/A:1006893903113. [DOI] [PubMed] [Google Scholar]
- 18.Das A.M., Harris D.A. Control of mitochondrial ATP synthase in heart cells: Inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc. Res. 1990;24:411–417. doi: 10.1093/cvr/24.5.411. [DOI] [PubMed] [Google Scholar]
- 19.Traaseth N., Elfering S., Solien J., Haynes V., Giulivi C. Role of calcium signaling in the activation of mitochondrial nitric oxide synthase and citric acid cycle. Biochim. Biophys. Acta. 2004;1658:64–71. doi: 10.1016/j.bbabio.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 20.McCormack J.G., Denton R.M. Mitochondrial Ca2+ transport and the role of intramitochondrial Ca2+ in the regulation of energy metabolism. Dev. Neurosci. 1993;15:165–173. doi: 10.1159/000111332. [DOI] [PubMed] [Google Scholar]
- 21.Chen L.B. Mitochondrial membrane potential in living cells. Annu. Rev. Cell. Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- 22.Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- 23.Chaban Y., Boekema E.J., Dudkina N.V. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim. Biophys. Acta. 2014;1837:418–426. doi: 10.1016/j.bbabio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheu S.S., Nauduri D., Anders M.W. Targeting antioxidants to mitochondria: A new therapeutic direction. Biochim. Biophys. Acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Gunter T.E., Sheu S.S. Characteristics and possible functions of mitochondrial Ca2+ transport mechanisms. Biochim. Biophys. Acta. 2009;1787:1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Chance B., Sies H., Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 29.Rasmussen H.H., Hamilton E.J., Liu C.C., Figtree G.A. Reversible oxidative modification: Implications for cardiovascular physiology and pathophysiology. Trends Cardiovasc. Med. 2010;20:85–90. doi: 10.1016/j.tcm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ROS)-induced ros release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aon M.A., Cortassa S., Marban E., O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 32.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Zinkevich N.S., Gutterman D.D. ROS-induced ROS release in vascular biology: Redox-redox signaling. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H647–H653. doi: 10.1152/ajpheart.01271.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brady N.R., Hamacher-Brady A., Westerhoff H.V., Gottlieb R.A. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid. Redox Signal. 2006;8:1651–1665. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]
- 35.Hool L.C. Hypoxia increases the sensitivity of the L-type Ca2+ current to β-adrenergic receptor stimulation via a C2 region-containing protein kinase C isoform. Circ. Res. 2000;87:1164–1171. doi: 10.1161/01.RES.87.12.1164. [DOI] [PubMed] [Google Scholar]
- 36.Hool L.C. Hypoxia alters the sensitivity of the L-type Ca2+ channel to α-adrenergic receptor stimulation in the presence of β-adrenergic receptor stimulation. Circ. Res. 2001;88:1036–1043. doi: 10.1161/hh1001.090841. [DOI] [PubMed] [Google Scholar]
- 37.Sims C., Harvey R.D. Redox modulation of basal and β-adrenergically stimulated cardiac L-type Ca2+ channel activity by phenylarsine oxide. Br. J. Pharmacol. 2004;142:797–807. doi: 10.1038/sj.bjp.0705845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akaishi T., Nakazawa K., Sato K., Saito H., Ohno Y., Ito Y. Hydrogen peroxide modulates whole cell Ca2+ currents through L-type channels in cultured rat dentate granule cells. Neurosci. Lett. 2004;356:25–28. doi: 10.1016/j.neulet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Fearon I.M., Palmer A.C., Balmforth A.J., Ball S.G., Varadi G., Peers C. Hypoxic and redox inhibition of the human cardiac L-type Ca2+ channel. Adv. Exp. Med. Biol. 2000;475:209–218. doi: 10.1007/0-306-46825-5_20. [DOI] [PubMed] [Google Scholar]
- 40.Viola H.M., Arthur P.G., Hool L.C. Transient exposure to hydrogen peroxide causes an increase in mitochondria-derived superoxide as a result of sustained alteration in L-type Ca2+ channel function in the absence of apoptosis in ventricular myocytes. Circ. Res. 2007;100:1036–1044. doi: 10.1161/01.RES.0000263010.19273.48. [DOI] [PubMed] [Google Scholar]
- 41.Li A., Segui J., Heinemann S.H., Hoshi T. Oxidation regulates cloned neuronal voltage-dependent Ca2+ channels expressed in xenopus oocytes. J. Neurosci. 1998;18:6740–6747. doi: 10.1523/JNEUROSCI.18-17-06740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiamvimonvat N., O’Rourke B., Kamp T.J., Kallen R.G., Hofmann F., Flockerzi V., Marban E. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ. Res. 1995;76:325–334. doi: 10.1161/01.RES.76.3.325. [DOI] [PubMed] [Google Scholar]
- 43.Frey N., Olson E.N. Cardiac hypertrophy: The good, the bad, and the ugly. Annu. Rev. Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 44.Heineke J., Molkentin J.D. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat. Rev. Mol. Cell. Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 45.Wilkins B.J., Molkentin J.D. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem. Biophys. Res. Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 46.Takimoto E., Kass D.A. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 47.Sag C.M., Santos C.X., Shah A.M. Redox regulation of cardiac hypertrophy. J. Mol. Cell. Cardiol. 2014;73:103–111. doi: 10.1016/j.yjmcc.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Hafstad A.D., Nabeebaccus A.A., Shah A.M. Novel aspects of ROS signalling in heart failure. Basic Res. Cardiol. 2013;108:359. doi: 10.1007/s00395-013-0359-8. [DOI] [PubMed] [Google Scholar]
- 49.Becker L.B. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc. Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 50.Seenarain V., Viola H.M., Ravenscroft G., Casey T.M., Lipscombe R.J., Ingley E., Laing N.G., Bringans S.D., Hool L.C. Evidence of altered guinea pig ventricular cardiomyocyte protein expression and growth in response to a 5 min in vitro exposure to H2O2. J. Proteome Res. 2010;9:1985–1994. doi: 10.1021/pr9011393. [DOI] [PubMed] [Google Scholar]
- 51.Anderson M.E., Brown J.H., Bers D.M. Camkii in myocardial hypertrophy and heart failure. J. Mol. Cell. Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pokharel S., Sharma U.C., Pinto Y.M. Left ventricular hypertrophy: Virtuous intentions, malign consequences. Int. J. Biochem. Cell. Biol. 2003;35:802–806. doi: 10.1016/S1357-2725(02)00344-8. [DOI] [PubMed] [Google Scholar]
- 53.St-Pierre J., Buckingham J.A., Roebuck S.J., Brand M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 54.Jezek P., Hlavata L. Mitochondria in homeostasis of reactive oxygen species in cell, tissues, and organism. Int. J. Biochem. Cell. Biol. 2005;37:2478–2503. doi: 10.1016/j.biocel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 55.Viola H.M., Hool L.C. Qo site of mitochondrial Complex III is the source of increased superoxide after transient exposure to hydrogen peroxide. J. Mol. Cell. Cardiol. 2010;49:875–885. doi: 10.1016/j.yjmcc.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Vanden Hoek T.L., Becker L.B., Shao Z., Li C., Schumacker P.T. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J. Biol. Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 57.Collins Y., Chouchani E.T., James A.M., Menger K.E., Cocheme H.M., Murphy M.P. Mitochondrial redox signalling at a glance. J. Cell. Sci. 2012;125:801–806. doi: 10.1242/jcs.098475. [DOI] [PubMed] [Google Scholar]
- 58.Groitl B., Jakob U. Thiol-based redox switches. Biochim. Biophys. Acta. 2014;1844:1335–1343. doi: 10.1016/j.bbapap.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghezzi P. Oxidoreduction of protein thiols in redox regulation. Biochem. Soc. Trans. 2005;33:1378–1381. doi: 10.1042/BST20051378. [DOI] [PubMed] [Google Scholar]
- 60.Ghezzi P. Protein glutathionylation in health and disease. Biochim. Biophys. Acta. 2013;1830:3165–3172. doi: 10.1016/j.bbagen.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Cooper A.J., Pinto J.T., Callery P.S. Reversible and irreversible protein glutathionylation: Biological and clinical aspects. Expert Opin. Drug Metab. Toxicol. 2011;7:891–910. doi: 10.1517/17425255.2011.577738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meister A., Anderson M.E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 63.Hurd T.R., Costa N.J., Dahm C.C., Beer S.M., Brown S.E., Filipovska A., Murphy M.P. Glutathionylation of mitochondrial proteins. Antioxid. Redox Signal. 2005;7:999–1010. doi: 10.1089/ars.2005.7.999. [DOI] [PubMed] [Google Scholar]
- 64.Hurd T.R., Filipovska A., Costa N.J., Dahm C.C., Murphy M.P. Disulphide formation on mitochondrial protein thiols. Biochem. Soc. Trans. 2005;33:1390–1393. doi: 10.1042/BST20051390. [DOI] [PubMed] [Google Scholar]
- 65.Ghezzi P. Regulation of protein function by glutathionylation. Free Radic. Res. 2005;39:573–580. doi: 10.1080/10715760500072172. [DOI] [PubMed] [Google Scholar]
- 66.Petrushanko I.Y., Yakushev S., Mitkevich V.A., Kamanina Y.V., Ziganshin R.H., Meng X., Anashkina A.A., Makhro A., Lopina O.D., Gassmann M., et al. S-Glutathionylation of the Na, k-atpase catalytic α subunit is a determinant of the enzyme redox sensitivity. J. Biol. Chem. 2012;287:32195–32205. doi: 10.1074/jbc.M112.391094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fratelli M., Demol H., Puype M., Casagrande S., Eberini I., Salmona M., Bonetto V., Mengozzi M., Duffieux F., Miclet E., et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human t lymphocytes. Proc. Natl. Acad. Sci. USA. 2002;99:3505–3510. doi: 10.1073/pnas.052592699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cabiscol E., Levine R.L. The phosphatase activity of carbonic anhydrase III is reversibly regulated by glutathiolation. Proc. Natl. Acad. Sci. USA. 1996;93:4170–4174. doi: 10.1073/pnas.93.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Klatt P., Molina E.P., Lamas S. Nitric oxide inhibits c-jun DNA binding by specifically targeted S-glutathionylation. J. Biol. Chem. 1999;274:15857–15864. doi: 10.1074/jbc.274.22.15857. [DOI] [PubMed] [Google Scholar]
- 70.Pineda-Molina E., Klatt P., Vazquez J., Marina A., Garcia de Lacoba M., Perez-Sala D., Lamas S. Glutathionylation of the p50 subunit of NF-κB: A mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 71.Davis D.A., Newcomb F.M., Starke D.W., Ott D.E., Mieyal J.J., Yarchoan R. Thioltransferase (glutaredoxin) is detected within HIV-1 and can regulate the activity of glutathionylated HIV-1 protease in vitro. J. Biol. Chem. 1997;272:25935–25940. doi: 10.1074/jbc.272.41.25935. [DOI] [PubMed] [Google Scholar]
- 72.Liang J.N., Pelletier M.R. Destabilization of lens protein conformation by glutathione mixed disulfide. Exp. Eye Res. 1988;47:17–25. doi: 10.1016/0014-4835(88)90020-6. [DOI] [PubMed] [Google Scholar]
- 73.Pastore A., Piemonte F. Protein glutathionylation in cardiovascular diseases. Int. J. Mol. Sci. 2013;14:20845–20876. doi: 10.3390/ijms141020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang H., Viola H.M., Filipovska A., Hool L.C. Cav1.2 calcium channel is glutathionylated during oxidative stress in guinea pig and ischemic human heart. Free Radic. Biol. Med. 2011;51:1501–1511. doi: 10.1016/j.freeradbiomed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Hudasek K., Brown S.T., Fearon I.M. H2O2 regulates recombinant Ca2+ channel α1c subunits but does not mediate their sensitivity to acute hypoxia. Biochem. Biophys. Res. Commun. 2004;318:135–141. doi: 10.1016/j.bbrc.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 76.Yang L., Xu J., Minobe E., Yu L., Feng R., Kameyama A., Yazawa K., Kameyama M. Mechanisms underlying the modulation of L-type Ca2+ channel by hydrogen peroxide in guinea pig ventricular myocytes. J. Physiol. Sci. 2013;63:419–426. doi: 10.1007/s12576-013-0279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fernandes A.P., Holmgren A. Glutaredoxins: Glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid. Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 78.Jerng H.H., Pfaffinger P.J. S-Glutathionylation of an auxiliary subunit confers redox sensitivity to Kv4 channel inactivation. PLoS One. 2014;9:e93315. doi: 10.1371/journal.pone.0093315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kemp M., Donovan J., Higham H., Hooper J. Biochemical markers of myocardial injury. Br. J. Anaesth. 2004;93:63–73. doi: 10.1093/bja/aeh148. [DOI] [PubMed] [Google Scholar]
- 80.Dalle-Donne I., Rossi R., Colombo G., Giustarini D., Milzani A. Protein S-glutathionylation: A regulatory device from bacteria to humans. Trends Biochem. Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 81.Yang Y., Shi W., Chen X., Cui N., Konduru A.S., Shi Y., Trower T.C., Zhang S., Jiang C. Molecular basis and structural insight of vascular KATP channel gating by S-glutathionylation. J. Biol. Chem. 2011;286:9298–9307. doi: 10.1074/jbc.M110.195123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bredt D.S., Snyder S.H. Nitric oxide: A physiologic messenger molecule. Annu. Rev. Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- 83.Broniowska K.A., Hogg N. The chemical biology of S-nitrosothiols. Antioxid. Redox Signal. 2012;17:969–980. doi: 10.1089/ars.2012.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell D.L., Stamler J.S., Strauss H.C. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J. Gen. Physiol. 1996;108:277–293. doi: 10.1085/jgp.108.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hu H., Chiamvimonvat N., Yamagishi T., Marban E. Direct inhibition of expressed cardiac L-type Ca2+ channels by S-nitrosothiol nitric oxide donors. Circ. Res. 1997;81:742–752. doi: 10.1161/01.RES.81.5.742. [DOI] [PubMed] [Google Scholar]
- 86.Grech E.D. Pathophysiology and investigation of coronary artery disease. BMJ. 2003;326:1027–1030. doi: 10.1136/bmj.326.7397.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carden D.L., Granger D.N. Pathophysiology of ischaemia-reperfusion injury. J. Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 88.Eltzschig H.K., Collard C.D. Vascular ischaemia and reperfusion injury. Br. Med. Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 89.Solares J., Garcia-Dorado D., Oliveras J., Gonzalez M.A., Ruiz-Meana M., Barrabes J.A., Gonzalez-Bravo C., Soler-Soler J. Contraction band necrosis at the lateral borders of the area at risk in reperfused infarcts. Observations in a pig model of in situ coronary occlusion. Virchows Arch. 1995;426:393–399. doi: 10.1007/BF00191349. [DOI] [PubMed] [Google Scholar]
- 90.Whalen D.A., Hamilton D.G., Ganote C.E., Jennings R.B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am. J. Pathol. 1974;74:381–398. [PMC free article] [PubMed] [Google Scholar]
- 91.Kloner R.A., Ganote C.E., Whalen D.A., Jr., Jennings R.B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am. J. Pathol. 1974;74:399–422. [PMC free article] [PubMed] [Google Scholar]
- 92.Gross G.J., Kersten J.R., Warltier D.C. Mechanisms of postischemic contractile dysfunction. Ann. Thorac. Surg. 1999;68:1898–1904. doi: 10.1016/S0003-4975(99)01035-8. [DOI] [PubMed] [Google Scholar]
- 93.Piper H.M., Garcia-Dorado D. Prime causes of rapid cardiomyocyte death during reperfusion. Ann. Thorac. Surg. 1999;68:1913–1919. doi: 10.1016/S0003-4975(99)01025-5. [DOI] [PubMed] [Google Scholar]
- 94.Ambrosio G., Weisfeldt M.L., Jacobus W.E., Flaherty J.T. Evidence for a reversible oxygen radical-mediated component of reperfusion injury: Reduction by recombinant human superoxide dismutase administered at the time of reflow. Circulation. 1987;75:282–291. doi: 10.1161/01.CIR.75.1.282. [DOI] [PubMed] [Google Scholar]
- 95.VanBenthuysen K.M., McMurtry I.F., Horwitz L.D. Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J. Clin. Investig. 1987;79:265–274. doi: 10.1172/JCI112793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pearson P.J., Schaff H.V., Vanhoutte P.M. Long-term impairment of endothelium-dependent relaxations to aggregating platelets after reperfusion injury in canine coronary arteries. Circulation. 1990;81:1921–1927. doi: 10.1161/01.CIR.81.6.1921. [DOI] [PubMed] [Google Scholar]
- 97.Bolli R., Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol. Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 98.Wong D.T., Puri R., Richardson J.D., Worthley M.I., Worthley S.G. Myocardial “no-reflow”—Diagnosis, pathophysiology and treatment. Int. J. Cardiol. 2013;167:1798–1806. doi: 10.1016/j.ijcard.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 99.Galasso G., Schiekofer S., D’Anna C., Gioia G.D., Piccolo R., Niglio T., Rosa R.D., Strisciuglio T., Cirillo P., Piscione F., et al. No-reflow phenomenon: Pathophysiology, diagnosis, prevention, and treatment. A review of the current literature and future perspectives. Angiology. 2014;65:180–189. doi: 10.1177/0003319712474336. [DOI] [PubMed] [Google Scholar]
- 100.Lefer A.M., Tsao P.S., Lefer D.J., Ma X.L. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991;5:2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- 101.Szocs K. Endothelial dysfunction and reactive oxygen species production in ischemia/reperfusion and nitrate tolerance. Gen. Physiol. Biophys. 2004;23:265–295. [PubMed] [Google Scholar]
- 102.Zweier J.L., Talukder M.A. The role of oxidants and free radicals in reperfusion injury. Cardiovasc. Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 103.Bolli R., Jeroudi M.O., Patel B.S., DuBose C.M., Lai E.K., Roberts R., McCay P.B. Direct evidence that oxygen-derived free radicals contribute to postischemic myocardial dysfunction in the intact dog. Proc. Natl. Acad. Sci. USA. 1989;86:4695–4699. doi: 10.1073/pnas.86.12.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Viola H.M., Arthur P.G., Hool L.C. Evidence for regulation of mitochondrial function by the L-type Ca2+ channel in ventricular myocytes. J. Mol. Cell. Cardiol. 2009;46:1016–1026. doi: 10.1016/j.yjmcc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 105.Hool L.C. Evidence for the regulation of L-type Ca2+ channels in the heart by reactive oxygen species: Mechanism for mediating pathology. Clin. Exp. Pharmacol. Physiol. 2008;35:229–234. doi: 10.1111/j.1440-1681.2007.04727.x. [DOI] [PubMed] [Google Scholar]
- 106.Catterall W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell. Dev. Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 107.Takahashi M., Catterall W.A. Dihydropyridine-sensitive calcium channels in cardiac and skeletal muscle membranes: Studies with antibodies against the α subunits. Biochemistry. 1987;26:5518–5526. doi: 10.1021/bi00391a046. [DOI] [PubMed] [Google Scholar]
- 108.Davies A., Hendrich J., van Minh A.T., Wratten J., Douglas L., Dolphin A.C. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 109.Dolphin A.C. β Subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 2003;35:599–620. doi: 10.1023/B:JOBB.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 110.Hohaus A., Person V., Behlke J., Schaper J., Morano I., Haase H. The carboxyl-terminal region of ahnak provides a link between cardiac L-type Ca2+ channels and the actin-based cytoskeleton. FASEB J. 2002;16:1205–1216. doi: 10.1096/fj.01-0855com. [DOI] [PubMed] [Google Scholar]
- 111.Haase H., Alvarez J., Petzhold D., Doller A., Behlke J., Erdmann J., Hetzer R., Regitz-Zagrosek V., Vassort G., Morano I. Ahnak is critical for cardiac Cav1.2 calcium channel function and its β-adrenergic regulation. FASEB J. 2005;19:1969–1977. doi: 10.1096/fj.05-3997com. [DOI] [PubMed] [Google Scholar]
- 112.Alvarez J., Hamplova J., Hohaus A., Morano I., Haase H., Vassort G. Calcium current in rat cardiomyocytes is modulated by the carboxyl-terminal ahnak domain. J. Biol. Chem. 2004;279:12456–12461. doi: 10.1074/jbc.M312177200. [DOI] [PubMed] [Google Scholar]
- 113.Hohaus A., Poteser M., Romanin C., Klugbauer N., Hofmann F., Morano I., Haase H., Groschner K. Modulation of the smooth-muscle L-type Ca2+ channel α1 subunit (α1c-b) by the β2a subunit: A peptide which inhibits binding of β to the I–II linker of α1 induces functional uncoupling. Biochem. J. 2000;348:657–665. doi: 10.1042/0264-6021:3480657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Viola H.M., Jordan M.C., Roos K.R., Hool L.C. Decreased myocardial injury and improved contractility after administration of a peptide derived against the α-interacting domain of the L-type calcium channel. J. Am. Heart Assoc. 2014;3:e000961. doi: 10.1161/JAHA.114.000961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Viola H.M., Adams A.M., Davies S.M., Fletcher S., Filipovska A., Hool L.C. Impaired functional communication between the L-type calcium channel and mitochondria contributes to metabolic inhibition in the mdx heart. Proc. Natl. Acad. Sci. USA. 2014;111:E2905–E2914. doi: 10.1073/pnas.1402544111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hammerman H., Moscovitz M., Hir J. Beneficial effect of nisoldipine in repeated coronary reperfusion. Coron. Artery Dis. 1997;8:97–100. doi: 10.1097/00019501-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 117.Sheiban I., Tonni S., Chizzoni A., Marini A., Trevi G. Recovery of left ventricular function following early reperfusion in acute myocardial infarction: A potential role for the calcium antagonist nisoldipine. Cardiovasc. Drugs Ther. 1997;11:5–16. doi: 10.1023/A:1007713118941. [DOI] [PubMed] [Google Scholar]
- 118.Liu X., Engelman R.M., Wei Z., Bagchi D., Rousou J.A., Nath D., Das D.K. Attenuation of myocardial reperfusion injury by reducing intracellular calcium overloading with dihydropyridines. Biochem. Pharmacol. 1993;45:1333–1341. doi: 10.1016/0006-2952(93)90287-7. [DOI] [PubMed] [Google Scholar]
- 119.Akita T., Abe T., Kato S., Kodama I., Toyama J. Protective effects of diltiazem and ryanodine against ischemia-reperfusion injury in neonatal rabbit hearts. J. Thorac. Cardiovasc. Surg. 1993;106:55–66. [PubMed] [Google Scholar]
- 120.Herzog W.R., Vogel R.A., Schlossberg M.L., Edenbaum L.R., Scott H.J., Serebruany V.L. Short-term low dose intracoronary diltiazem administered at the onset of reperfusion reduces myocardial infarct size. Int. J. Cardiol. 1997;59:21–27. doi: 10.1016/S0167-5273(96)02883-5. [DOI] [PubMed] [Google Scholar]
- 121.Borchgrevink P.C., Bergan A.S., Bakoy O.E., Jynge P. Magnesium and reperfusion of ischemic rat heart as assessed by 31p-NMR. Am. J. Physiol. 1989;256:H195–H204. doi: 10.1152/ajpheart.1989.256.1.H195. [DOI] [PubMed] [Google Scholar]
- 122.Hara A., Matsumura H., Abiko Y. Beneficial effect of magnesium on the isolated perfused rat heart during reperfusion after ischaemia: Comparison between pre-ischaemic and post-ischaemic administration of magnesium. N-S Arch. Pharmacol. 1990;342:100–106. doi: 10.1007/BF00178980. [DOI] [PubMed] [Google Scholar]
- 123.Bolli R., Becker L., Gross G., Mentzer R., Jr., Balshaw D., Lathrop D.A. Myocardial protection at a crossroads: The need for translation into clinical therapy. Circ. Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 124.Cannon R.O., 3rd. Mechanisms, management and future directions for reperfusion injury after acute myocardial infarction. Nat. Clin. Pract. Cardiovasc. Med. 2005;2:88–94. doi: 10.1038/ncpcardio0096. [DOI] [PubMed] [Google Scholar]
- 125.Dirksen M.T., Laarman G.J., Simoons M.L., Duncker D.J. Reperfusion injury in humans: A review of clinical trials on reperfusion injury inhibitory strategies. Cardiovasc. Res. 2007;74:343–355. doi: 10.1016/j.cardiores.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 126.Clozel J.P., Veniant M., Osterrieder W. The structurally novel Ca2+ channel blocker Ro 40–5967, which binds to the [3h] desmethoxyverapamil receptor, is devoid of the negative inotropic effects of verapamil in normal and failing rat hearts. Cardiovasc. Drugs Ther. 1990;4:731–736. doi: 10.1007/BF01856562. [DOI] [PubMed] [Google Scholar]
- 127.Billman G.E. Ro 40–5967, a novel calcium channel antagonist, protects against ventricular fibrillation. Eur. J. Pharmacol. 1992;229:179–187. doi: 10.1016/0014-2999(92)90553-G. [DOI] [PubMed] [Google Scholar]
- 128.Reimer K.A., Califf R.M. Good news for experimental concept but bad news for clinically effective therapy. Circulation. 1999;99:198–200. doi: 10.1161/01.CIR.99.2.198. [DOI] [PubMed] [Google Scholar]