Abstract
Nicotine dependence plays a critical role in addiction to tobacco products, and thus contributes to a variety of devastating tobacco-related diseases (SGR 2014). Annual costs associated with smoking in the US are estimated to be between $289 and $333 billion. Effective interventions for nicotine dependence, especially in smokers, are a critical barrier to the eradication of tobacco-related diseases. This overview highlights research presented at the Plenary Symposium of Behavior, Biology and Chemistry: Translational Research in Addiction Conference (BBC), hosted by the UT Health Science Center San Antonio, on March 9–10, 2013. The Plenary Symposium focused on tobacco addiction, and covered topics ranging from basic science to national policy. As in previous years, the meeting brought together globally-renowned scientists, graduate student recruits, and young scientists from underrepresented populations in Texas and other states with the goal of fostering interest in drug addiction research in young generations.
Keywords: Nicotine withdrawal, Nicotinic receptor antagonists, Nicotine dependence, Smoking cessation, Relapse, Nicotine analogs, Nicotine vaccine, Low nicotine content cigarettes, CHRNA5, Medial Habenula, Interpeduncular Nucleus, Tobacco product regulation
1. INTRODUCTION
Nicotine, the alkaloid primarily responsible for the addictive properties of tobacco products, acts at nicotinic acetylcholine receptors (nAChRs). Found throughout the nervous system, nAChRs comprise numerous combinations of α (α2-9) and β (β2-4) subunits in the form of homo- and heteromeric ion channels (Gotti et al., 2009). nAChRs can mediate either fast synaptic transmission - as they primarily do in the periphery - or modulate the function of other neurotransmitter systems, as is common in the central nervous system (Dani and Bertrand, 2007; De Biasi, 2002).
The majority of smokers desire to quit, but only a small fraction of attempts are ultimately successful (Benowitz, 2010). According to the Center for Disease Control (CDC), approximately 69% of smokers want to quit, and 52% of smokers attempted to quit in 2010 – but only 6.2% were successful (CDCP, 2011).
There are several issues to confront when considering smoking cessation. First, nicotine’s ability to interfere with the dopaminergic (DA) reward system is an important factor contributing to both the initiation and maintenance of nicotine use (Picciotto and Corrigall, 2002). Second, smoking is also motivated by the urge to alleviate affective, cognitive, and physical symptoms of withdrawal that emerge during periods of abstinence (De Biasi and Dani, 2011). Although driven by the pharmacological effects of nicotine, addiction to tobacco is influenced by non-pharmacological factors, including cue and context associations, and stress. Those non-pharmacological elements play a major role in cue reactivity, evoked craving, and relapse to smoking (Bedi et al., 2011; Ray et al., 2013; Wray et al., 2013). Therefore, to be successful, smoking cessation strategies must reduce both the motivation to smoke, the symptoms of withdrawal during quit attempts, and craving. Nicotine replacement therapy (NRT), bupropion, and varenicline are the most commonly applied pharmacological aids for smoking cessation. Although all three work better than placebo, long-term success rates remain low among smokers attempting to quit (Paolini and De Biasi 2011). The following summarizes progress that contributing authors’ labs are making toward understanding the molecular mechanisms of nicotine addiction, as well as the design of pharmacological and non-pharmacological strategies aimed at improving smoking cessation outcomes.
2. NICOTINIC RECEPTOR SUBUNITS AND THEIR INFLUENCE ON NICOTINE ADDICTION AND WITHDRAWAL
(Mariella De Biasi, Ian McLaughlin, Erika E. Perez)
2.1 Symptoms of nicotine withdrawal
Several pre-clinical behavioral tests are available to explore the circuit- and cell-based mechanisms underlying nicotine withdrawal symptoms. The unpleasant symptoms associated with nicotine withdrawal act as negative reinforcers that promote nicotine dependence (Koob and Volkow, 2010; Piper et al., 2011; Allen et al., 2008). These negative reinforcers include both affective (anxiety, depression, and irritability) and somatic (decreased heart rate, constipation, general restlessness) symptoms (Malin and Goyarzu, 2009; Salas et al., 2009). Mice chronically exposed to nicotine display withdrawal symptoms that develop spontaneously, peak 24hr following cessation of administration, and can last for several days. Withdrawal can also be precipitated by systemic injection of non-selective nAChR antagonists such as mecamylamine (Paolini and De Biasi, 2011). Affective signs of withdrawal can be examined in rodents using behavioral paradigms that test for anhedonia, conditioned place aversion, anxiety, and conditioned fear (De Biasi and Salas, 2008; Damaj et al., 2003; Epping-Jordan et al., 1998; Davis et al., 2005). Physical signs of withdrawal include chewing, teeth-chattering, shakes, tremors, writhing, palpebral ptosis, gasps, and yawns (De Biasi and Salas, 2008; Malin and Goyarzu, 2009).
2.2 A gene cluster on chromosome 15q25 influences nicotine addiction
Ample studies have demonstrated that genetic factors predispose individuals to younger smoking initiation, increased quantities of cigarettes smoked, nicotine dependence, and smoking persistence (Li et al., 2003; Rhee et al., 2003; Schnoll et al., 2007). A cluster of nicotinic receptor genes (CHRNA5/CHRNA3/CHRNB4) located on chromosome 15q25 has been repeatedly associated with nicotine dependence, smoking behaviors, and lung cancer (Amos et al., 2008; Berrettini et al., 2008; Furberg et al., 2010; Greenbaum and Lerer, 2009; Hung et al., 2008; Liu et al., 2010; Rose, 2007; Saccone et al., 2010, 2007; Thorgeirsson et al., 2008). SNPSs rs16969968, rs578776, and rs588765 represent three statistically distinct nicotine dependence loci associated with the CHRNA5/A3/B4 gene cluster (Saccone et al., 2010, 2009). The α5 risk variant, rs16969968 G/A, causes an Asp398Asn amino acid substitution, and the risk allele (Asn398) produces hypofunctional α5-containing nAChRs with reduced Ca2+ permeability and faster desensitization rates than non-risk alleles (Bierut et al., 2008; Kuryatow et al., 2011). Other polymorphisms are associated with different levels of α5 or α3 mRNA (Wang, 2009), and the functional significance of several other gene variants in the CHRNA5/A3/B4 gene cluster is currently being investigated (Flora et al., 2013).
2.3 nAChR mutant mice help reveal mechanisms of nicotine withdrawal symptoms
Pre-clinical rodent models can be used to elucidate the functions of genes and brain pathways involved in nicotine addiction. Our lab took advantage of genetically engineered mice carrying null mutations in nAChR subunit genes to examine the roles of various subunits in the mechanisms of withdrawal. We focused on physical symptoms of nicotine withdrawal - studied either 24 hr after nicotine deprivation, or upon systemic injection of mecamylamine in mice given free access to nicotine in drinking water. We found that mice lacking functional β4 nAChR subunits exhibited reduced somatic signs relative to wild-type mice undergoing nicotine withdrawal (Salas et al., 2004). Further experiments with additional mice lacking nAChR subunits revealed that physical symptoms of withdrawal also depend on α5, α2, and partially on α7 nAChR subunits (Salas et al., 2007, 2009). Interestingly, mice lacking the β2 nAChR subunit displayed symptoms of withdrawal resembling those of wild-type mice (Fig. 1). Mice carrying a null mutation for the α3 nAChR subunit were not studied due to perinatal mortality (Xu et al., 1999).
Fig. 1. Lack of β4, α5 or α2 nAChR subunits protects against increases in nicotine withdrawal-induced somatic signs.
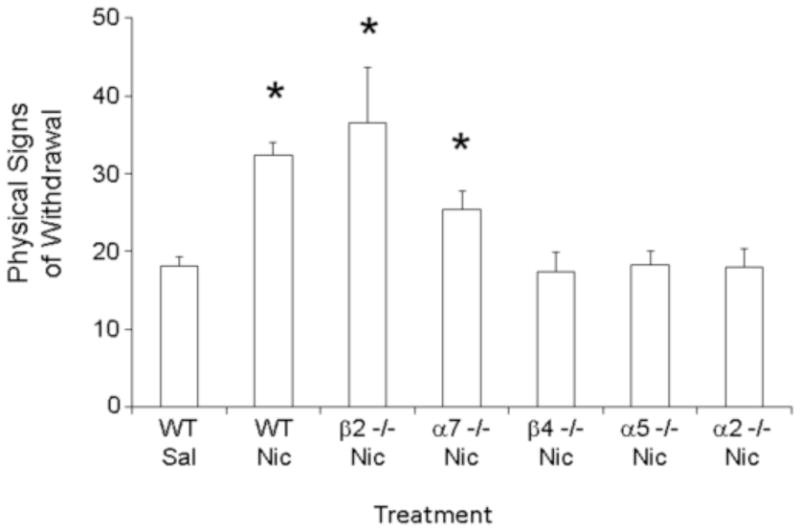
Various nAChR null mice and their wild type littermates were treated chronically with nicotine (24mg/kg/day free base) or saline, using a mini-osmotic pump, for two weeks. On day 14, each mouse received a 3 mg/kg injection of the nonselective nicotinic antagonist, mecamylamine. Somatic signs were measured for 20 min. Mecamylamine treatment precipitated increases in somatic signs in wild type, β2 null, and α7 null mice chronically treated with nicotine. Such increases were not observed in β4, α5, or α2 null mice -suggesting that nAChRs containing these subunits participate in the modulation of nicotine withdrawal symptoms. *p<0.05. The numbers within the bars indicate the number of animals tested for each strain. (Modified and adapted from Salas et al. 2004, 2007, 2009)
2.4 Medial Habenula (MHb) and Interpeduncular nucleus (IPN) are key brain areas for physical manifestations of nicotine withdrawal
We focused on the MHb-IPN pathway - which is among the brain areas with the highest co-expression of α5, α3, α2, and β4 – to pursue neuronal circuits associated with physical symptoms of withdrawal (De Biasi and Dani, 2011). The MHb, together with the lateral habenula (LHb), forms the habenular complex (Hb). The IPN is the main projection target of the MHb, while the LHb sends projections to the rostromedial tengmental nucleus (RMTg) in the midbrain. These brain areas play significant roles in aversion, negative reinforcement, negative prediction errors, and negative motivation (De Biasi and Dani, 2011; Fowler and Kenny, 2014). In mice chronically treated with nicotine, mecamylamine administration was sufficient to induce nicotine withdrawal behaviors only when microinjected into the MHb or the IPN, but not when microinjected into other brain areas, including the ventral tegmental area (VTA; Salas et al., 2009).
2.5 Future studies
One unanswered question is whether the MHb-IPN pathway and the nAChRs contained therein, are important for the affective manifestations of nicotine withdrawal. This is a key question, given the emerging role of the Hb in anxiety-related disorders, and the fact that both β4 and α5 null mice exhibit reduced anxiety-related behavior in the elevated plus maze (Mathuru and Jesuthasan, 2013; Paolini and De Biasi, 2011; Yamaguchi et al., 2013; Gill et al., 2013). In addition, given the role of the MHb-IPN pathway in nicotine aversion and withdrawal, it would be interesting to determine whether the same nAChR subtypes and the MHb-IPN pathway influence withdrawal from other drugs of abuse. Withdrawal from ethanol is a candidate, given similarities between certain manifestations of alcohol and nicotine withdrawal (Hughes et al., 1994). This hypothesis is corroborated by the observation that, although different from those influencing tobacco addiction, polymorphisms in the CHRNA5/A3/B4 gene region are independently associated with alcohol dependence (Wang et al., 2009; Sherva et al., 2010) -some of which are associated with altered levels of α5 mRNA (Wang et al., 2009). Furthermore, the rs16969968 α5 SNP has been linked to increased risk not only for nicotine, but also for alcohol dependence (Schlaepfer et al., 2008).
2.6 Conclusions
nAChRs, especially those in the CHRNA5/A3/B4 gene cluster, influence aversive manifestations of nicotine withdrawal - and thereby represent novel molecular targets for medications aimed at smoking cessation.
3. DISCOVERY OF NICOTINIC RECEPTOR ANTAGONISTS AS AGENTS FOR TREATING NICOTINE ADDICTION
(Peter A. Crooks, Linda P. Dwoskin, Michael T. Bardo)
3.1 Introduction
Activation of neuronal nicotinic acetylcholine receptors (nAChRs) evokes dopamine (DA) release within neuronal circuitry associated with reward (Picciotto and Corrigall, 2002). DA release is well known to underlie reinforcing properties of nicotine (Wise and Rompre, 1989). Therefore, tobacco smoking is reinforced and maintained, at least in part, by nicotine activation of nAChRs within DA reward circuitry. Results from a comprehensive molecular genetics study, in which an individual nAChR subunit gene (α4, α5, α6, α7, β2, β3, and β4) was deleted, suggest that nicotine-evoked DA release is mediated by 6 nAChR subtypes (Salminen et al., 2004; Gotti et al., 2005). These include α-conotoxin MII (α-CtxMII)-sensitive (α6β2β3*, α4α6β2β3*, α6β2*, and α4α6β2*) and α-CtxMII-insensitive (α4β2* and α4α5β2*) subtypes, while deletion of α7 and β4 subunits had no effect. Thus, multiple nAChR subtypes likely mediate nicotine-evoked DA release. Based on these data, we set out to develop smoking cessation therapies to identify subtype-selective nAChR antagonists that inhibit nAChRs mediating nicotine-evoked DA release (α6β2-containing nAChRs). We hypothesized that such subtype-selective nAChR antagonists would: i) be efficacious tobacco use cessation agents, ii) have limited side-effects due to their receptor selectivity, and iii) offer smokers who do not respond well to existing smoking cessation therapies alternative treatment options.
3.2 Target and Drug Discovery
Kulak and colleagues (1997) previously reported that the neuropeptide α-CtxMII potently blocks nicotine-stimulated DA release in rat striatal synaptosomes. While relevant to the development of smoking cessation therapies, neurotoxin peptides acting as subtype-selective nAChR antagonists are unlikely to be developed into treatments for tobacco addiction because they are high-molecular weight compounds, and are unlikely to cross the blood–brain barrier (BBB). Thus, our strategy was to develop small, drug-like molecules that are selective antagonists at α6- and β2-containing, α-CtxMII-sensitive nAChR subtypes (i.e., α6β2*, α6β2β3*, α6α4β2β3*, and α4α6β2*). Importantly, since these α-CtxMII-sensitive nAChRs are located on DA neurons that mediate nicotine-evoked DA release (Wickham et al., 2013; Gotti et al., 2010), they represent viable targets for the development of tobacco cessation agents.
We initially focused on a series of N-n-alkylnicotinium analogs and related compounds for their ability to inhibit nicotine-evoked DA release from rat striatal slices and to displace [3H]-nicotine binding to rat striatal membranes (α4β2* nAChRs) (Dwoskin et al., 1992; Crooks et al., 1995). The most potent compound that emerged was S-(−)-N-n-octylnicotinium iodide (NONI, Fig. 2). Though lacking selectivity, NONI exhibited good antagonist potency at α6β2* nAChRs (IC50 = 0.62 mM) (Crooks et al., 2004). NONI was also found to have good affinity for the BBB choline transporter (Crooks et al., 2004; Dwoskin et al., 2004; Lockman et al., 2008), suggesting good brain bioavailability due to its cationic quaternary ammonium structure.
Fig. 2. Chemical structure and pharmacological characteristics of potential smoking cessation agents.
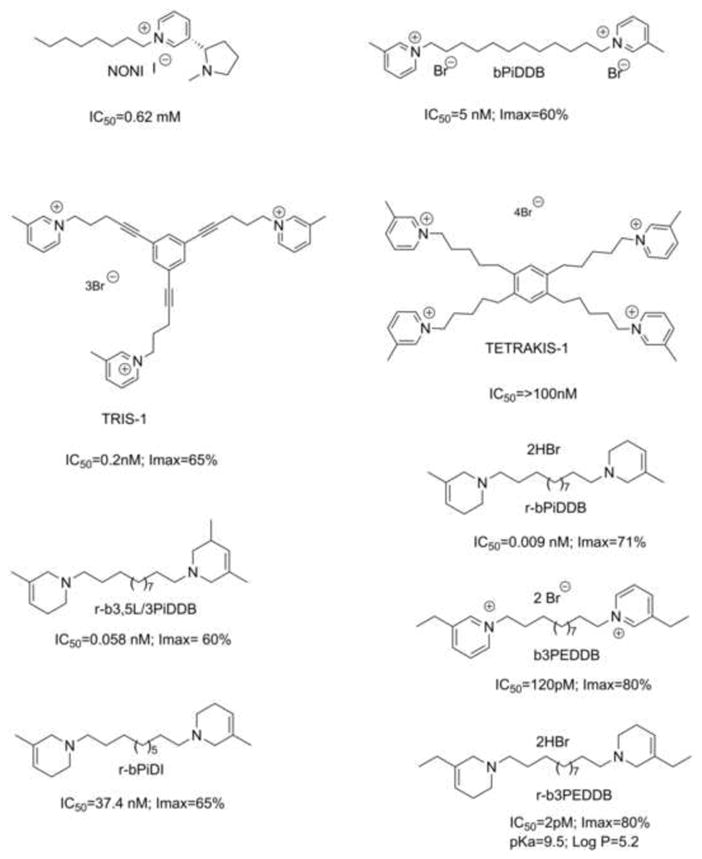
IC50 values of for the inhibition of NIC-evoked [3H]DA release from rat striatal slices for NONI, bis-, tris, and tetrakis quaternary ammonium compounds, and the bis-tertiary amino analogues (r-series compounds).
Second generation libraries incorporated a second quaternary ammonium moiety into the scaffold of the N-alkylnicotinium analogs, generating bis-quaternary ammonium analogs containing diverse headgroups separated by variable alkane, alkene, or alkyne linker units. The most potent among them was bPiDDB (N,N′-dodecane-1,12-diyl-bis-3-picolinium dibromide; Fig. 2) (IC50 = 5 nM; Imax = 60% at α6β2*-containing nAChRs) - which had little or no affinity for either α4β2* or α7* nAChRs (Crooks et al., 2004; Dwoskin et al., 2004), and was an excellent substrate for the BBB choline transporter (Lockman et al., 2008). Pharmacokinetic studies carried out in rats treated with 14C-bPiDDB confirmed its brain bioavailability (Albayati et al., 2008). bPiDDB dose-dependently decreased nicotine self-administration in Sprague-Dawley rats with no effect on sucrose-maintained responding (Neugebauer et al., 2006), and attenuated hyperactivity produced by acute and repeated nicotine dosing. Interestingly, in rats repeatedly administered nicotine, the in vitro inhibitory potency of bPiDDB at α6β2* nAChRs increases significantly compared to that exhibited in similar in vitro assays in naive rats (i.e., IC50 = 5 pM vs. 6 nM, respectively; Smith et al., 2010), demonstrating that repeated nicotine treatment may differentially regulate the stoichiometry and/or conformation of α6β2* nAChRs.
3.3 Exploring Analogs of bPiDDB
As part of an iterative SAR study, the effect of introducing an additional picolinium or other headgroups into the bPiDDB scaffold on inhibition of nicotine-evoked DA release was evaluated. Initially, three lead tris-quaternary ammonium molecules, TRIS-1, TRIS-2, and TRIS-3, emerged that were potent and selective inhibitors of α6β2* nAChRs (IC50 = 0.2–4 nM; e.g. TRIS-1, Fig. 2; Zheng et al., 2007). However, further development of these molecules was abandoned due to toxicity at higher doses in nicotine self-administration studies in rats, and poor affinity for the BBB choline transporter. The introduction of two additional picolinium headgroups into the bPiDDB scaffold resulted in tetrakis-analogs (e.g. TETRAKIS-1, Fig. 2) (Zhang et al., 2008), which were selective, lower potency antagonists at α6β2* nAChRs, but were not substrates for the BBB choline transporter, and were thus ineffective in decreasing nicotine self-administration in rats (unpublished results).
Replacement of the above quaternary ammonium head groups in the bis-, tris- and tetrakis-analogs with tertiary amine head groups is predicted to improve oral bioavailability and drug-likeness of such compounds. The resulting analogs should have good water-solubility, and cross the BBB independently of choline transporters due to passive diffusion through lipid membranes. Evaluation of a small library of these tertiary amine analogs as antagonists at α6β2* nAChRs identified several bis-analogs with IC50 values in the low nM or sub-nM range (Zhang et al., 2011).
Two of these bis molecules: r-b3,5L/3PiDDB and r-bPiDDB (Fig. 2) are chemically reduced analogs of bPiDDB, and both compounds potently and selectively inhibit nicotine-evoked [3H]-DA release (IC50 = 0.009–0.058 nM; Imax = 60–74%) at α-CtxMII-sensitive α6β2* nAChRs (Dwoskin et al., 2009; Smith et al., 2010). These analogs were more potent antagonists at α6β2* nAChRs compared to their corresponding quaternary ammonium counterparts (Zhang et al., 2011). Importantly, inhibition produced by a maximally effective concentration of r-b3,5L/3PiDDB and r-bPiDDB was not additive with a maximally effective concentration of α-CtxMII (Smith et al., 2010; Crooks et al., 2014), demonstrating interaction with the same nAChR subtypes with which α-CtxMII interacts. Also, r-b3,5L/3PiDDB and r-bPiDDB both decreased responding for i.v. nicotine in rats at doses that did not produce lethargy, weight loss, or other signs of toxicity, and that had no effect on food responding (Crooks et al., 2014).
r-bPiDI (Fig. 2), a structurally related analog of r-bPiDDB containing a C10 rather than a C12 n-alkane linker, exhibited decreased inhibitory potency (IC50 = 37.4 nM, Imax = 65%) compared to the above two C12 analogs, and did not inhibit [3H]nicotine or [3H]methyllycaconitine binding (Beckmann et al., 2013). Also, r-bPiDI inhibition of nicotine-evoked DA release was not different in the absence or presence of α-conotoxin MII, characterizing it as a potent and selective α6β2* nAChR antagonist. Acute systemic administration of r-bPiDI decreased nicotine self-administration with no effect on food-maintained behavior (Beckmann et al., 2013), indicating that r-bPiDI specifically decreases nicotine reinforcement. Thus, r-bPiDI, is another selective antagonist at α6β2* nAChRs.
The most recent drug-like antagonist identified in this series is r-b3EPDDB (Fig. 2) (pKa = 9.5; Log P = 5.2), a close structural analog of r-bPiDDB in which the two tetrahydro-3-picolino headgroups are chemically reduced to afford a tetrahydro-3-ethylpyridino headgroup. r-b3EPDDB exhibited a remarkable IC50 value of 2 pM in the nicotine-evoked [3H]-DA release assay (Imax = 80%) (Fig. 3). Data in Fig. 3 compare the IC50 and Imax values of r-b3EPDDB and b3EPDDB (the parent quaternary ammonium compound) in the nicotine-evoked [3H]-DA release assay, and demonstrate that chemical reduction of the bis-quaternary ammonium compound, b3EPDDB, affords an analog, r-b3EPDDB, that exhibits 60 times greater inhibitory potency at α6β2* nAChRs with no change in Imax. This highly potent inhibitor is being evaluated in nicotine self-administration studies in rats.
Fig. 3. b3EPDDB derivatives inhibit dopamine release in vitro.
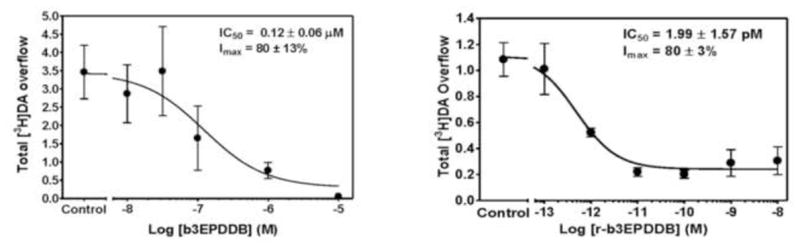
b3EPDDB (left) and r-b3EPDDB (right) both inhibit NIC-evoked [3H]DA release from rat striatum in vitro in a concentration-dependent manner. n = 5–8/analog. Data are mean ± SEM total [3H] overflow.
3.4 Summary
Striatal rat brain slices have been used to screen novel analogs that inhibit nicotine-evoked DA release in the search for potential smoking cessation agents. Several libraries of compounds were constructed and SAR generated. Evolution of the initial series of quaternary ammonium compounds into tertiary amino analogs provided lead candidates with IC50 values in the pM range. r-bPiDDB, r-bPiDB, r-b3,5L/3PiDDB and r-b3EPDDB have been identified as four promising new analogs with drug-like physicochemical properties. These molecules are potent antagonists at α6β2-containing nAChRs. r-bPiDDB, r-bPiDB and r-b3,5L/3PiDDB specifically decrease nicotine self-administration in a rat behavioral model with no effect on food-maintained responding, and are potential preclinical leads for development as smoking cessation agents.
The effects on food-maintained responding are not predictive to a potential lack of drug effect against weight loss after smoking cessation.
Our future aim is to enhance the drug-likeness of our lead compounds by improving water-solubility through introduction of hydrogen-bond acceptor moieties in the linker, and by decreasing conformational flexibility. We intend to identify orally bioavailable, drug-like preclinical leads for development as smoking cessation and/or relapse prevention pharmacotherapies. Current experiments are focused on determining if these analogues block cue-induced reinstatement of nicotine seeking.
4. VACCINES FOR THE TREATMENT OF TOBACCO ADDICTION
(Paul Pentel)
4.1 Introduction
Existing medications used for the treatment of tobacco addiction (nicotine replacement products, buproprion, varenicline) are valuable, but their efficacy is modest. The neuronal pathways they target - which include those mediating reward, cognition and affect - are diverse and essential to many normal functions. Interrupting their function in order to treat tobacco addiction can interfere with normal functions and cause side effects, which limit the usable dose of medication and its efficacy.
4.2 Nicotine vaccines
Vaccination against nicotine provides an alternative medication strategy, targeting the drug rather than the brain (Bevins et al., 2008; LeSage et al., 2006b; Raupach et al., 2012; Shen et al., 2012). The nicotine vaccines most thoroughly studied consist of nicotine conjugated (covalently attached) through a short linker to a foreign carrier protein. The nicotine binds and stimulates B lymphocytes, which will mature into antibody producing cells, and the carrier protein serves to activate T lymphocytes to provide signals required for B cell maturation (McHeyzer-Williams and McHeyzer-Williams, 2005). This construct is administered with an adjuvant, such as alum, which creates an immune-competent environment at the injection site (Awate et al., 2013).
Nicotine vaccines elicit production of nicotine-specific antibodies that can bind nicotine in serum or extracellular fluid, and reduce or slow its distribution to brain (Maurer et al., 2005; Pentel et al., 2006; Satoskar et al., 2003). The antibodies themselves are excluded from the brain because they are too large to cross the blood-brain barrier. In animals, vaccination blocks or attenuates a wide range of nicotine-related behaviors including the acquisition, maintenance, and reinstatement of nicotine self-administration at clinically relevant nicotine doses (LeSage et al., 2006a; Lindblom et al., 2002). Nicotine-specific antibodies bind nicotine and its minor but active metabolite nornicotine, but essentially nothing else, including acetylcholine (the natural ligand of nicotinic cholinergic receptors), other neurotransmitters, or medications with similar structures. This specificity, and the exclusion of antibodies from the brain, explains the absence of side effects (other than transient minor discomfort at the injection site) of this approach in both animals and humans.
4.3 Vaccine efficacy
As the goal of vaccination is to bind as much drug as possible, efficacy is highly dependent upon the concentration (often estimated as titer) of antibody in serum. High antibody levels can be produced in rodents, but this often requires large vaccine doses, or routes of administration and adjuvants that are unacceptable for humans. Antibody levels achieved in clinical trials of nicotine vaccines have been considerably lower than in rodents (Keyler et al., 2008; Maurer et al., 2005). In addition, antibody levels in both animals and humans show high individual variability (Cornuz et al., 2008; Hatsukami et al., 2011). Reliably achieving high antibody concentrations has emerged as the principal challenge for translating vaccines into clinical use.
Clinical trials of nicotine vaccines have not shown the efficacy seen in animal studies. A phase II clinical trial of one nicotine vaccine (NicVax) showed a doubling of smoking cessation rates (Hatsukami et al., 2011), but follow-up Phase III trials of the same vaccine showed no treatment effect (Fahim et al., 2013). However, subgroup analysis of the phase II study showed efficacy in the 30% of subjects with the highest serum antibody levels, and a similar subgroup finding emerged from a phase II study of an unrelated nicotine vaccine (NicQb) (Cornuz et al., 2008). These analyses suggest that nicotine vaccines may be effective if sufficient antibody levels can be consistently achieved. Current efforts to improve vaccines involve modifications of conjugate vaccine design, development of nanoparticle vaccines, use of newer adjuvants, combining vaccines with each other or with other types of medications, and passive immunization through gene transfer.
4.4 Improving conjugate vaccines
Nicotine that is modified to allow placement of a linker is referred to as a hapten. Many nicotine haptens, with different linker positions, have proven useful, and none is clearly superior under all conditions (Isomura et al., 2001; Pravetoni et al., 2012). A conformationally constrained hapten that prevents rotation of the pyridine and pyrrolidine rings improved immunogenicity of one nicotine vaccine (Moreno et al., 2012). Fluorination, widely used in medicinal chemistry to improve ligand binding to receptors, also improved a model nicotine immunogen (Cai et al., 2013). Overall, however, modifications in hapten design have provided only modest benefit. Similarly, many linker lengths and structures have proven effective with particular haptens but no one structure emerges as consistently best.
A variety of carrier proteins have been used in nicotine vaccines, generally selected from proteins known to elicit strong immune responses on their own. Tetanus toxoid, recombinant diphtheria toxin (CRM197), and keyhole limpet hemocyanin are most commonly used, but others are also effective. Intact or disrupted viral capsid can serve the same purpose (Cornuz et al., 2008; De et al., 2013). The choice of carrier protein for a particular hapten remains highly empirical. T cell activation is provided by peptide sequences with the carrier proteins, and these peptides can serve in place of the full protein. To date, this approach has worked but has not been more effective than using the whole protein.
4.5 Nanoparticle vaccines
Nanoparticle vaccines are being developed based on liposomes, synthetic polymers, or novel materials such as DNA (Matyas et al., 2013; Peek et al., 2008). Several are effective in animals and have entered clinical trials, but results are not yet available (Kishimoto et al., 2012). Nanoparticles function as a scaffold to which essential vaccine components can be attached or incorporated with a high degree of control. Liposomes or polymer spheres can have their surfaces modified to allow attachment of nicotine or other vaccine components, enabling their display to immune cell surface receptors. Other components, such as T-cell help peptides, can be encapsulated for delivery to cytoplasmic receptors. Nanoparticles allow greater control of hapten, T cell help peptide, and adjuvant density and spacing than conjugate vaccines. Some, such as those based on DNA, can also achieve precise control of size and shape that may prove useful to optimize vaccine uptake by phagocytic cells or transport to regional lymph nodes (Liu et al., 2012).
4.6 Multivalent vaccines
It is well established that different vaccines can be combined and administered in the same injection with little or no loss of their individual activities, as is routinely done with common infectious disease vaccines such as MMR (measles, mumps, rubella). Some nicotine vaccines can be combined to address problems of low and variable antibody levels (Keyler et al., 2008). Nicotine haptens that have linkers at different positions can act as distinct immunogens, stimulating different populations of B cells, and eliciting antibodies that do not cross-react (Pravetoni et al., 2012). Each hapten elicits antibodies against nicotine, but these antibodies recognize different features of the nicotine molecule.
In rats, combining 3 nicotine immunogens based on haptens with linkers at the 1′ or 3′ position of the pyrrolidine ring, or 6 position of the pyridine ring, produced additive antibody titers and greater ability to prevent nicotine distribution to brain than a monovalent vaccine (de Villiers et al., 2013). In addition, some rats with poor responses to one immunogen had brisk responses to one of the others. This independence of response reduced the number of low- or non-responders that had ineffectual antibody titers. An appealing feature of this strategy is that it is quite general, and could be applied to other nicotine vaccines - even those based on very different designs such as nanoparticles, provided that the haptens used incorporate appropriate linker positions.
4.7 Passive immunization and gene transfer
Immunization can be accomplished actively by vaccination or passively through the transfer of pre-formed nicotine-specific monoclonal antibody. Passive immunization can achieve the same effects in animals as vaccination (Carrera et al., 2004; Keyler et al., 2005), but offers some advantages. The dose of monoclonal antibody, and resulting serum antibody levels, can be controlled to avoid the problem of non-responders, and the amount administered can be increased to provide a higher serum antibody concentration than that achievable with even the best vaccines. Passive immunization also has an immediate onset of effect, whereas vaccination takes weeks to months. The main limitation of passive immunization is the cost of monoclonal antibodies, and the doses needed for this application are high (Roiko et al., 2008). No clinical trials of passive immunization for nicotine have been reported, but if costs are reduced, it will merit further study.
An alternative means of delivering monoclonal antibody is administration of viral vectors containing genes for antibody expression. Vectors based on adeno-associated virus (AAV) have been used to produce very high levels of nicotine-specific antibody expression in mice, resulting in reduced nicotine distribution to brain and attenuated physiologic and behavioral effects (Hicks et al., 2012). These vectors have shown no important toxicity in clinical trials for the transfer of other types of genes (Mingozzi and High, 2011). If safety is confirmed with more extensive use, and high levels of expression can be achieved in humans, gene therapy could prove an effective means of providing therapeutic antibodies for tobacco or other addictions.
4.8 Combining immunotherapy with medications
There are compelling reasons to consider combining vaccines or passive immunization with medications that act via different mechanisms to assist with treating tobacco dependence. Even if very high nicotine-specific antibody levels can be reliably achieved through one or more of the strategies described above, immunotherapy is likely to have limits on both its magnitude and spectrum of therapeutic effects. Reasons include: 1) Immunotherapies reduce, but do not completely prevent, nicotine distribution to brain, and even low brain concentrations of nicotine can produce substantial nicotinic receptor occupancy (Brody et al., 2009); 2) Immunotherapies can act only when nicotine is present for antibodies to bind. Effects that may occur when nicotine is no longer present, such as withdrawal and craving, cannot be directly blocked using vaccines; 3) Minor alkaloids and other components of tobacco may contribute to tobacco addiction, and are not bound or blocked by nicotine-specific antibodies (Hoffman and Evans, 2013).
As proof-of principle, a combination of the nicotinic receptor antagonist mecamylamine and passive immunization with a nicotine-specific monoclonal antibody (Nic311) was studied and these were found to have strong synergistic effects in rats (LeSage et al., 2012). Mecamylamine can block nicotine actions, but use in humans for this purpose is not feasible because it has side effects due to blocking the actions of endogenous acetylcholine. However combining a low dose of mecamylamine with a low dose of antibody produced complete blockade of nicotine discrimination even though the individual therapies at these doses were essentially without any effect (Figure 4). These preliminary data support further study of immunotherapy/medication combinations to enhance treatment efficacy.
Fig. 4. Synergistic effect of mecamylamine and the nicotine-specific monoclonal antibody Nic311 for blocking the subjective effects of nicotine.
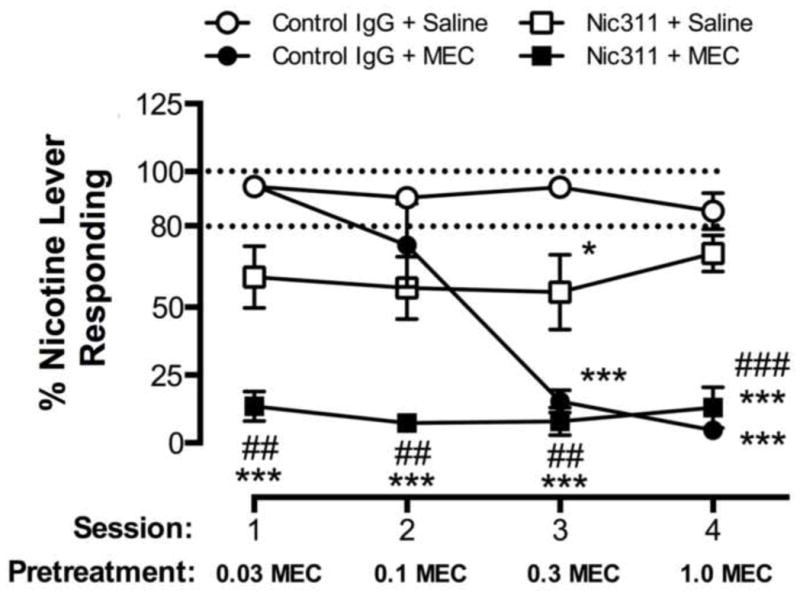
Rats were trained in a two-lever nicotine discrimination assay. Data are the mean ± SE % responding on the nicotine lever during consecutive daily sessions with the 0.4 mg/kg s.c. nicotine training dose. Nic311 or control antibody was administered i.v. one day prior to session 1, and mecamylamine or saline was administered s.c. 15 minutes prior to each session. Dashed lines indicate criterion levels of performance for discrimination of the 0.4 mg/kg nicotine training dose. Significantly different from Control antibody+Saline, *p<0.05, ***p<0.001. Significantly different from Nic311+Saline, ##p<0.01, ###p<0.001. Reproduced from (LeSage et al., 2012).
4.9 Summary
Nicotine vaccines appear quite effective in animals, but have been disappointing in clinical trials for smoking cessation. Many options are under development for improving vaccine efficacy or providing antibody through alternative strategies. These advances are likely to provide the higher serum nicotine-specific antibody levels needed to test the therapeutic potential of immunotherapy.
5. REDUCING LEVELS OF NICOTINE IN CIGARETTES
(Dorothy Hatsukami)
5.1 Introduction
Rather than targeting the smoker, another area of tobacco addiction intervention is altering the tobacco product itself. Reducing the addictiveness or appeal of cigarettes as a national regulatory measure can potentially lead to significant public health benefits by reducing the prevalence of smoking. Prior reports have stated that prevention of tobacco use, cessation of its use, and reduction of tobacco-caused mortality are greatly impeded by the addictive nature of cigarettes (WHO Study Group on Tobacco Product Regulation, 2012; USDHHS, 1988). As a result, Benowitz and Henningfield (1994) proposed a gradual reduction of nicotine levels in all marketed cigarettes over 10–15 years, with the goal of preventing nicotine addiction in youth. This proposal can also facilitate abstinence among those who are already addicted to cigarettes. For many years, this concept was not fully embraced by the public health community because no governmental agency had regulatory authority to require the reduction of nicotine levels in tobacco products. However, since 2009, when the Family Smoking Prevention and Tobacco Control Act (FSPTCA) was enacted, the U.S. Food and Drug Administration now has jurisdiction over some tobacco products, and the law allows a reduction in levels of nicotine in these products to the point that they are rendered non-addictive, but not to zero.
5.2 Reducing nicotine content
Research in animals (e.g., Donny et al., 2012) and humans (e.g., Hatsukami et al., 2010a) have been conducted that supports the viability of reducing nicotine to non-addictive levels in cigarettes. Most animal studies have shown an inverted U-shaped dose-response curve for nicotine self-administration and that generally doses less than 10 μg/kg are not self-administered more than saline, although in some studies the dose needed to be lower than 3.75 μg/kg (Donny et al., 2012; Smith et al., 2013; Grebenstein et al., 2014). In humans, four clinical trials examined the effects of reducing nicotine in cigarettes either gradually (Benowitz et al., 2007, 2012) or immediately to very low levels (Hatsukami et al., 2010b, 2012). In general, these studies have shown that cigarette consumption and carbon monoxide (CO) exposure were significantly reduced with very low nicotine content (VLNC) cigarettes (< 0.1 mg nicotine yield), with some compensatory smoking occurring with cigarettes containing > 0.2 mg nicotine yield. There was no evidence of increased exposure to toxicants, nor evidence of adverse effects on cardiovascular biomarkers, even at higher doses of reduced nicotine content (RNC) cigarettes (Benowitz, 2007, 2012). Decreased exposure was seen at the lowest nicotine content cigarettes (< 0.05 mg; Hatsukami et al., 2010, 2013). It is important to note that these cigarettes are unlike the “light” and “ultralight” cigarettes, marketed as generating reduced tar and nicotine yield, but resulted in levels of nicotine and toxicant exposure that were similar to “regular” cigarettes (NCI monograph 13). The “light” and “ultralight” cigarettes had the same amount of nicotine content as regular cigarettes, but were reduced in machine-determined nicotine yields, primarily through filter ventilation holes. The RNC cigarettes have reduced nicotine in the tobacco itself, making compensation at very low doses difficult.
These clinical trials also demonstrated no substantial withdrawal symptoms with the switch to RNC cigarettes, and minimal withdrawal when smokers quit using VLNC cigarettes (Benotwitz 2012; Hatsukami et al., 2010b, 2013). Similarly, smokers reported reduced dependence scores with VLNC cigarettes. Although the primary outcome of these studies was not cessation, in the Hatsukami et al. studies, in which participants were motivated to quit smoking, the 7-day point prevalence biochemically verified (cotinine and CO) quit rates with the VLNC was 36% at 6 weeks post-treatment in one study (Hatsukami et al., 2010b), and 24% at 6 weeks post-product assignment in another study (Hatsukami et al., 2013). For the Benowtiz studies, in which smokers were not motivated to quit, rates ranged from 4% at the end of taper, when nicotine content was changed on a monthly basis (Benowtiz et al., 2012), to 25% when nicotine content was reduced on a weekly basis in a separate study (Benowitz et al., 2007).
5.3 Gradual reduction vs. immediate reduction
To date, minimal studies have examined the best approach to reducing levels of nicotine in cigarettes - that is, gradual reduction over time, or an immediate reduction to non-addictive levels on a specified date. In one study, Smith et al., (2013) showed that whether nicotine doses were reduced immediately or gradually in rats, reduction to 3.75 μg/kg led to significant changes in nicotine self-administration with no significant compensation (increased infusions in response to lowered nicotine dose). Reduction in nicotine doses above 3.75 μg/kg led to limited and temporary compensation. Although, no human clinical trials have been conducted to directly compare these two approaches. Nevertheless, similar to the findings in the animal study, in the Benowitz et al. studies (2007, 2012), with gradual reduction, modest increases in numbers of RNC cigarettes smoked and CO exposure were observed, with no significant change in exposure to toxicants in the initial phases of reduction. No substantial decrease would be seen until nicotine content is reduced significantly. However, no major withdrawal or discomfort may be experienced during the reduction period. On the other hand, with the abrupt reduction to VLNC cigarettes, a more immediate effect is observed in the reduction in numbers of VLNC cigarettes smoked, and consequently more immediate reduction in exposure to toxicants (Hatsukami et al., 2010b, 2013). This approach has the advantage of benefiting public health sooner. Furthermore, the number of individuals likely to engage in significant compensatory smoking is likely to be limited because the dose of nicotine is so low, whereas with higher RNC cigarettes, greater variability in compensatory smoking is likely to be experienced. However, an immediate reduction in nicotine to very low levels may generate greater discomfort in smokers, particularly in heavily dependent smokers.
5.4 Moderating responses to VLNC cigarettes
Several factors may moderate or mitigate discomfort experienced when switching to a VLNC cigarette, including use of medicinal nicotine products, other medications to reduce withdrawal, or alternative less harmful non-combusted tobacco products (e.g., electronic cigarettes). Few studies have examined this area. One study compared the effects of VLNC (0.05–0.9 mg nicotine yield) cigarettes alone, nicotine patch (NP) alone, and a combination of NP and VLNC cigarettes over the course of 6 weeks (Hatsukami et al., 2013). The combination approach led to fewer VLNC cigarettes smoked and lower CO exposure, than VLNC cigarettes alone, and fewer usual brand cigarettes smoked than the other two conditions. In addition, significantly less severe withdrawal was experienced when smokers were switched from usual brand cigarettes to combination compared to NP condition, and near significant differences when compared to VLNC cigarettes alone. Another study also observed that the combination approach leads to lower consumption of usual brand cigarettes and total number of cigarettes smoked than VLNC alone (Rose et al., 2006). In another short-term 10-day study (Donny and Jones, 2009), smokers were assigned to one of four conditions: 1) placebo patch (PP) plus 0.6 mg nicotine yield cigarette; 2) 7 mg NP plus 0.05 mg nicotine yield (VLNC) cigarette; 3) 21 mg NP plus VLNC cigarette. This study also found that subjects assigned to NP (21 mg and 7 mg) plus VLNC cigarettes, compared to PP plus VLNC cigarettes, showed greater reductions in the numbers of VLNC cigarettes smoked, in total volume of inhaled cigarette smoke, and fewer withdrawal symptoms. These studies show beneficial effects when combining VLNC with medicinal nicotine, demonstrating less discomfort and need to smoke either usual or experimental cigarettes.
Relatively few studies have examined the effects of a combination approach relative to VLNC cigarettes to facilitate cessation. In the study conducted by Hatsukami (2013), an exploratory analysis of the data showed no differences in cessation rates between the combination vs. VLNC or NP alone. A large clinical trial had been conducted, in smokers who called a telephone-based cessation support system. Abstinence rates were compared in smokers who were randomly assigned to usual care involving both medicinal nicotine products and behavioral treatment plus VLNC (0.05 mg nicotine yield) cigarettes versus usual care alone. The usual care plus VLNC cigarettes had higher 7-day point prevalence abstinence rate at the 6-month follow-up compared to usual care (33% vs. 28%, RR=1.18, 95% CI 1.01, 1.39), and higher continuous abstinence rates (23% vs. 15%, RR=1.50, 95% CI 1.20, 1.87).
5.5 Reducing nicotine in vulnerable populations
One concern is the effect of reducing nicotine content cigarettes in vulnerable populations, such as smokers who have serious mental illnesses. While no clinical trials have been conducted, a laboratory study showed no adverse effects of these cigarettes in smokers with schizophrenia (Tidey et al., 2013). In this study, smokers with schizophrenia and a control group of smokers without psychiatric diagnoses underwent 5 laboratory sessions, during which products were used under controlled conditions for 5 hours: 1) usual brand cigarettes; 2) VLNC cigarettes with 42 mg NP; 3) VLNC cigarettes with PP; 4) no cigarettes with 42 mg NP; and 5) no cigarettes with PP. After the session of product use, participants were allowed to smoke cigarettes ad libitum during a 90-minute period. The results showed that smoking VLNC cigarettes reduced usual-brand smoking and withdrawal symptoms, did not worsen psychiatric or performance measures, and were reported to be acceptable among smokers with schizophrenia. The addition of an active patch had no effect in this population, which may be a function of the short duration of this study or the fact for smokers with schizophrenia, the act of smoking has greater valence.
5.6 Remaining questions and future considerations
Although studies thus far support a reduction of nicotine content in cigarettes, many issues remain. These include:
Determining the dose of nicotine that will facilitate cessation in smokers and/or minimize the development of addiction among those who experiment with cigarettes.
Directly comparing a gradual reduction in nicotine content of cigarettes with an immediate reduction to a non-addictive level of nicotine content.
Determining the effects of RNC cigarettes in vulnerable populations of smokers (e.g., those with co-morbid disorders) in clinical trials.
Examining other factors that might moderate responses to RNC cigarettes and determining the variability in response.
Identifying and examining ways to mitigate negative consequences resulting from reducing nicotine levels in cigarettes.
Some of these gaps are already being addressed by NIH grants oriented towards developing tobacco regulatory science. It is important to recognize that reducing level of nicotine in cigarettes alone may not be sufficient. Levels of nicotine in all combustible tobacco products will be required to dramatically reduce death and disease associated with smoking. Should a policy to reduce nicotine to non-addictive levels in all combustible tobacco products marketed in the U.S. be enacted, it would be critical to establish a surveillance system able to track its impact.
5.7 Summary
The future for tobacco control is bright. We not only have effective existing methods of reducing the prevalence of smoking (e.g., comprehensive smoking bans, increased taxes on cigarettes, anti-smoking media campaigns), we can now regulate the contents of cigarettes under the FSPTCA and Article 9 of the World Health Organization Framework Convention on Tobacco Control. Regulation of nicotine content can reduce or eliminate the use of the most deadly tobacco products sold to consumers (i.e., combusted tobacco products), which would lead to a substantial reduction in tobacco-caused death and disease.
6. OVERALL CONCLUSION
The neurophysiological etiology of tobacco addiction is complex, involving both genetic predispositions and environmental influence. Therefore, a broad based set of interventions ranging from specific neurobiological targets for medications to policies must be considered. The wide range of signaling pathways and circuits that undergo neuroplastic adaptation during prolonged use render quitting very difficult. Effective treatments will need to confront the spectrum of systems involved in dependence, withdrawal, and relapse. Accordingly, studies of approaches to treating different components of addiction will be critical to revealing comprehensive therapies. Through work with genetically modified mice, we have learned that particular nAChR subunits appear to mediate specific symptoms of nicotine withdrawal, representing potentially druggable targets for more effective pharmacological intervention. The development of highly specific drugs, capable of attenuating or abolishing downstream dopaminergic signaling in response to nicotine intake, may present the ability to prevent the appeal of cigarettes by blocking their rewarding effects. Innovative approaches to preventing the effects of nicotine by taking advantage of immune system-mediated elimination of the drug may represent another method by which tobacco might be rendered ineffective – and, therefore, unappealing to individuals attempting to quit. As research continues to find new ways to effectively treat smokers, the prospect of reducing nicotine levels in tobacco products through federal regulation may reduce consumption of combusted tobacco products – and, ultimately, reduce the prevalence of tobacco-associated disease.
As this research begins to yield a wider variety of options for smokers attempting to quit, clinicians will be capable of providing treatments with fewer side-effects that are better tailored to the unique characteristics of patients. Additionally, the ability to combine therapies will assist in the treatment of physical, emotional, and cognitive withdrawal symptoms -as well as cravings. Such targeted therapies, or combination of targeted therapies, represent opportunities to more effectively ameliorate the burden of tobacco-associated disease on public health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albayati ZAF, Dwoskin LP, Crooks PA. Pharmacokinetics of the novel nicotinic receptor antagonist N, N-dodecane-1,12-diyl-bis-3-picolinium dibromide in the rat. Drug Metab Dispos. 2008;18:3870–3873. doi: 10.1124/dmd.108.020354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Bade T, Hatsukami D, Center B. Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine Tob Res. 2008;10:35–45. doi: 10.1080/14622200701705076. [DOI] [PubMed] [Google Scholar]
- Amos CI, Wu X, Broderick P, Gorlov IP, Gu J, Eisen T, Dong Q, Zhang Q, Gu X, Vijayakrishnan J, Sullivan K, Matakidou A, Wang Y, Mills G, Doheny K, Tsai YY, Chen WV, Shete S, Spitz MR, Houlston RS. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40:616–622. doi: 10.1038/ng.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awate S, Babiuk LA, Mutwiri G. Mechanisms of action of adjuvants. Front Immunol. 2013;4:114. doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann JS, Meyer AC, Pivavarchyk M, Horton DB, Zheng G, Smith AM, McIntosh M, Crooks PA, Bardo MT, Dwoskin LP. r-bPiDI acts selectively at α6β2* nicotinic receptors to decrease nicotine-evoked dopamine release and nicotine reinforcement. Neuropharmacology. 2013 doi: 10.1007/s11064-015-1680-4. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N Engl J Med. 1994;331:123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev. 2007;16:8479–8485. doi: 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. N Engl J of Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Dains KM, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P. Smoking behavior and exposure to tobacco toxicants during 6 months of smoking progressively reduced nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev. 2012;21:761–769. doi: 10.1158/1055-9965.EPI-11-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry. 2008;13:368–373. doi: 10.1038/sj.mp.4002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Wilkinson JL, Sanderson SD. Vaccines to combat smoking. Expert Opin Biol Ther. 2008;8:379–383. doi: 10.1517/14712598.8.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, Horton WJ, Breslau N, Budde J, Cloninger CR, Dick DM, Foroud T, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Kuperman S, Madden PA, Mayo K, Nurnberger J, Jr, Pomerleau O, Porjesz B, Reyes O, Schuckit M, Swan G, Tischfield JA, Edenberg HJ, Rice JP, Goate AM. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165:1163–1171. doi: 10.1176/appi.ajp.2008.07111711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, London ED, Olmstead RE, Rose JE, Mukhin AG. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol. 2009;12:305–316. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Wedenoja J, Largeau MR, Korhonen T, Pitkaniemi J, Keskitalo-Vuokko K, Happola A, Heikkila KH, Heikkila K, Ripatti S, Sarin AP, Salminen O, Paunio T, Pergadia ML, Cai X, Tsuchikama K, Janda KD. Modulating cocaine vaccine potency through hapten fluorination. J Am Chem Soc. 2013;135:2971–2974. doi: 10.1021/ja400356g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Hoffman TZ, Isomura S, Wirsching P, Koob GF, Janda KD. Investigations using immunization to attenuate the psychoactive effects of nicotine. Bioorg Med Chem. 2004;12:563–570. doi: 10.1016/j.bmc.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Quitting Smoking Among Adults—United States, 2001–2010. MMWR. 2011;60:1513–1519. [PubMed] [Google Scholar]
- Cornuz J, Zwahlen S, Jungi WF, Osterwalder J, Klingler K, van Melle G, Bangala Y, Guessous I, Müller P, Willers J, Maurer P, Bachmann MF, Cerny T. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS One. 2008;3:e2547. doi: 10.1371/journal.pone.0002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks PA, Ravard A, Teng LH, Dwoskin LP. Inhibition of nicotine-evoked [3H]dopamine release by pyridine N-substituted nicotine analogues: a new class of nicotinic antagonist. Drug Dev Res. 1995;36:71–82. [Google Scholar]
- Crooks PA, Ayers JT, Rui X, Sumithran SP, Grinevich VP, Wilkins LW, Deaciuc AG, Allen DD, Dwoshkin LP. Development of subtype-selective nicotinic receptor ligands as receptor antagonists. Bioorg Med Chem Lett. 2004;14:1869–1874. doi: 10.1016/j.bmcl.2003.10.074. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Bardo MT, Dwoskin LP. Nicotinic receptor antagonists as treatments for nicotine abuse. In: Dwoskin LP, editor. Emerging Targets and Therapeutics in the Treatment of Psychostimulant Abuse. Elsevier; New York: 2014. [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Ann Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M. Nicotinic mechanisms in the autonomic control of organ systems. J Neurobiol. 2002;53:568–579. doi: 10.1002/neu.10145. [DOI] [PubMed] [Google Scholar]
- De Biasi M, Salas R. Influence of neuronal nicotinic receptors over nicotine addiction and withdrawal. Exp Biol Med (Maywood) 2008;233:917–929. doi: 10.3181/0712-MR-355. [DOI] [PubMed] [Google Scholar]
- De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Ann Rev Neurosci. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De BP, Pagovich OE, Hicks MJ, Rosenberg JB, Moreno AY, Janda KD, Koob GF, Worgall, Kaminsky SM, Sondhi D, Crystal RG. Disrupted adenovirus-based vaccines against small addictive molecules circumvent anti-adenovirus immunity. Hum Gene Ther. 2013;24:58–66. doi: 10.1089/hum.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers S, Cornish KE, Troska AJ, Praveton M, Pentel PR. Increased efficacy of a trivalent nicotine vaccine compared to a dose-matched monovalent vaccine when formulated with alum. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.10.051. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug Alcohol Depend. 2009;104:23–33. doi: 10.1016/j.drugalcdep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Taylor TG, LeSage MG, Levin M, Buffalari DM, Joel D, Sved AF. Impact of tobacco regulation on animal research: new perspectives and opportunities. Nicotine Tob Res. 2012;14:1319–1338. doi: 10.1093/ntr/nts162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Leibee LL, Jewell AL, Fang Z-X, Crooks PA. Inhibition of [3H]-dopamine uptake into rat striatal slices by quaternary N-methylated nicotine metabolites. Life Sci. 1992;50:PL233–PL237. doi: 10.1016/0024-3205(92)90533-u. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Smith AM, Wooters TE, Zhang Z, Crooks PA, Bardo MT. Nicotinic receptor-based therapeutics and candidates for smoking cessation. Biochem Pharmacol. 2009;78:732–743. doi: 10.1016/j.bcp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Sumithran SP, Zhu J, Deaciuc AG, Ayers JT, Crooks PA. Subtype-selective nicotinic receptor antagonists: potential as tobacco use cessation agents. Bioorg Med Chem Lett. 2004;14:1863–1867. doi: 10.1016/j.bmcl.2003.10.073. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fahim RE, Kessler PD, Kalnik MW. Therapeutic vaccines against tobacco addiction. Expert Rev Vaccines. 2013;12:333–342. doi: 10.1586/erv.13.13. [DOI] [PubMed] [Google Scholar]
- Flora AV, Zambrano CA, Gallego X, Miyamoto JH, Johnson KA, Cowan KA, Stitzel JA, Ehringer MA. Functional characterization of SNPs in CHRNA3/B4 intergenic region associated with drug behaviors. Brain Res. 2013;1529:1–15. doi: 10.1016/j.brainres.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Nicotine aversion: neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology. 2014;76:533–544. doi: 10.1016/j.neuropharm.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furberg H, Kim Y, Dackor J, Boerwinkle E, Franceshini N, Ardissino D, Bernardinelli L, Mannucci PM, Mauri F, Merlini PA, et al. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42:441–447. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MJ, Ghee SM, Harper SM, See RE. Inactivation of the lateral habenula reduces anxiogenic behavior and cocaine seeking under conditions of heightened stress. Pharmacol Biochem Behav. 2013;111:24–29. doi: 10.1016/j.pbb.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti F, McIntosh JM, Collins AC, Marks MJ, Whiteaker P. Expression of nigrostriatal α6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by β3 subunit gene deletion. Mol Pharmacol. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;14:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharm Bio Behav. 2014;114–115:70–81. doi: 10.1016/j.pbb.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum L, Lerer B. Differential contribution of genetic variation in multiple brain nicotinic cholinergic receptors to nicotine dependence: recent progress and emerging open questions. Mol Psychiatry. 2009;14:912–945. doi: 10.1038/mp.2009.59. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Perkins KA, Lesage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger CL, Zeller M. Nicotine reduction revisited: science and future directions. Tob Control. 2010a;19:1–10. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Allen SS, Shields PG, Murphy SE, Stepanov I, Hecht SS. Reduced nicotine content cigarettes: effects on toxicant exposure, dependence and cessation. Addiction. 2010b;105:343–355. doi: 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, Kessler PD, Niknian M, Kalnik MW, Rennard SI. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther. 2011;89:392–399. doi: 10.1038/clpt.2010.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Hertsgaard LA, Vogel RI, Jensen JA, Murphy SE, Hecht SS, Carmella SG, al’Absi M, Joseph AM, Allen SS. Reduced nicotine content cigarettes and nicotine patch. Cancer Epidemiol Biomarkers Prev. 2013;22:1015–1024. doi: 10.1158/1055-9965.EPI-12-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks MJ, Rosenberg JB, De BP, Pagovich OE, Young CN, Qiu JP, Kaminsky SM, Hackett NR, Worgall S, Janda KD, Davisson RL, Crystal RG. AAV-directed persistent expression of a gene encoding anti-nicotine antibody for smoking cessation. Sci Transl Med. 2012;4:140ra187. doi: 10.1126/scitranslmed.3003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob Res. 2013;15:622–632. doi: 10.1093/ntr/nts192. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–1470. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Hung RJ, McKay JD, Gaborieau V, Boffetta P, Hashibe M, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, Lissowska J, Rudnai P, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452:633–637. doi: 10.1038/nature06885. [DOI] [PubMed] [Google Scholar]
- Isomura S, Wirsching P, Janda KD. An immunotherapeutic program for the treatment of nicotine addiction: hapten design and synthesis. J Org Chem. 2001;66:4115–4121. doi: 10.1021/jo001442w. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Benlhabib E, LeSage MG, St Peter JV, Stewart S, Fuller S, Le CT, Pentel PR. Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: dose- and affinity-response relationships. Drug Metab Dispos. 2005;33:1056–1061. doi: 10.1124/dmd.105.004234. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Earley CA, Murtaugh MP, Pentel PR. Enhanced immunogenicity of a bivalent nicotine vaccine. Int Immunopharmacol. 2008;8:1589–1594. doi: 10.1016/j.intimp.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto TK. Rational Design of a Fully Synthetic Nanoparticle-based Vaccine. 2nd International Conference on Vaccines and Vaccinations; Aug 20–27; Chicago. 2012. [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. α-Conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. J Neurosci. 1997;17:5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J. Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol Pharmacol. 2011;79:119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Hieda Y, Collins G, Burroughs D, Le C, Pentel PR. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology. 2006a;184:409–416. doi: 10.1007/s00213-005-0027-2. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Pentel PR. Current status of immunologic approaches to treating tobacco dependence: vaccines and nicotine-specific antibodies. AAPS J. 2006b;8:E65–75. doi: 10.1208/aapsj080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Pravetoni M, Pentel PR. Enhanced attenuation of nicotine discrimination in rats by combining nicotine-specific antibodies with a nicotinic receptor antagonist. Pharmacol Biochem Behav. 2012;102:157–162. doi: 10.1016/j.pbb.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Lindblom N, de Villiers SH, Kalayanov G, Gordon S, Johansson AM, Svensson TH. Active immunization against nicotine prevents reinstatement of nicotine-seeking behavior in rats. Respiration. 2002;69:254–260. doi: 10.1159/000063629. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, Berrettini W, Knouff CW, Yuan X, Waeber G, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet. 2010;42:436–440. doi: 10.1038/ng.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Xu Y, Yu T, Clifford C, Liu Y, Yan H, Chang Y. A DNA nanostructure platform for directed assembly of synthetic vaccines. Nano Lett. 2012;12:4254–4259. doi: 10.1021/nl301877k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman PR, Geldenhuys WJ, Manda V, Thomas F, Crooks PA, Dwoskin LP, Allen DD. Carrier mediated transport at the blood–brain barrier for the quaternary ammonium nicotinic receptor antagonist, N, N-dodecyl-bis-picolinium bromide (bPiDDB) J Pharmacol Exp Ther. 2008;324:244–250. doi: 10.1124/jpet.107.130906. [DOI] [PubMed] [Google Scholar]
- Malin DH, Goyarzu P. Rodent models of nicotine withdrawal syndrome. Handb Exp Pharmacol. 2009;192:401–434. doi: 10.1007/978-3-540-69248-5_14. [DOI] [PubMed] [Google Scholar]
- Matyas GR, Mayorov AV, Rice KC, Jacobson AE, Cheng K, Iyer MR, Li F, Beck Z, Janda KD, Alving CA. Liposomes containing monophosphoryl lipid a: a potent adjuvant system for inducing antibodies to heroin hapten analogs. Vaccine. 2013;31:2804–2810. doi: 10.1016/j.vaccine.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathuru AS, Jesuthasan S. The medial habenula as a regulator of anxiety in adult zebrafish. Front Neural Circuits. 2013;7:99. doi: 10.3389/fncir.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, Renner WA, Muller P, Bachmann MF. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and phase i safety and immunogenicity. Eur J Immunol. 2005;35:2031–2040. doi: 10.1002/eji.200526285. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory b cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using aav: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- Moreno AY, Azar MR, Koob GF, Janda KD. Probing the protective effects of a conformationally constrained nicotine vaccine. Vaccine. 2012;30:6665–6670. doi: 10.1016/j.vaccine.2012.08.064. [DOI] [PubMed] [Google Scholar]
- Neugenbauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT. Effect of a novel nicotinic receptor antagonist, N, N-dodecane-1,12-diyl-bis-3-picolinium dibromide (bPiDDB), on nicotine self-administration and hyperactivity in rats. Psychopharmacology. 2006;184:426–434. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- Paolini M, De Biasi M. Mechanistic insights into nicotine withdrawal. Biochem Pharmacol. 2011;82:996–1007. doi: 10.1016/j.bcp.2011.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60:915–928. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentel PR, Dufek MB, Roiko SA, Lesage MG, Keyler DE. Differential effects of passive immunization with nicotine-specific antibodies on the acute and chronic distribution of nicotine to brain in rats. J Pharmacol Exp Ther. 2006;317:660–666. doi: 10.1124/jpet.105.097873. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Baker TB. Anxiety diagnoses in smokers seeking cessation treatment: relations with tobacco dependence, withdrawal, outcome and response to treatment. Addiction. 2011;106:418–427. doi: 10.1111/j.1360-0443.2010.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Keyler DE, Pidaparthi RR, Carroll FI, Runyon SP, Murtaugh MP, Earley CA, Pentel PR. Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochem Pharmacol. 2012;83:543–550. doi: 10.1016/j.bcp.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raupach T, Hoogsteder PH, Onno van Schayck CP. Nicotine vaccines to assist with smoking cessation: current status of research. Drugs. 2012;72:e1–16. doi: 10.2165/11599900-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Lunny K, Bujarski S, Moallem N, Krull JL, Miotto K. The effects of varenicline on stress-induced and cue-induced craving for cigarettes. Drug Alcohol Depend. 2013;131:136–142. doi: 10.1016/j.drugalcdep.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hewitt JK, Young SE, Corley RP, Crowley TJ, Stallings MC. Genetic and environmental influences on substance initiation, use, and problem use in adolescents. Arch Gen Psychiatry. 2003;60:1256–1264. doi: 10.1001/archpsyc.60.12.1256. [DOI] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, Keyler DE, Lesage MG, Zhang Y, Pentel PR. Combined active and passive immunization enhances the efficacy of immunotherapy against nicotine in rats. J Pharmacol Exp Ther. 2008;325:985–993. doi: 10.1124/jpet.107.135111. [DOI] [PubMed] [Google Scholar]
- Rose JE. Multiple brain pathways and receptors underlying tobacco addiction. Biochem Pharmacol. 2007;74:1263–1270. doi: 10.1016/j.bcp.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Kukovich P. Pre-cessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob Res. 2006;8:89–101. doi: 10.1080/14622200500431866. [DOI] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16:36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Saccone SF, Hinrichs AL, Stitzel JA, Duan W, Pergadia ML, Agrawal A, Breslau N, Grucza RA, Hatsukami D, Johnson EO, Madden PA, Swan GE, Wang JC, Goate AM, Rice JP, Bierut LJ. Multiple distinct risk loci for nicotine dependence identified by dense coverage of the complete family of nicotinic receptor subunit (CHRN) genes. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:453–466. doi: 10.1002/ajmg.b.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone NL, Culverhouse RC, Schwantes-An TH, Cannon DS, Chen X, Cichon S, Giegling I, Han S, Han Y, Keskitalo-Vuokko K, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6:e1001053. doi: 10.1371/journal.pgen.1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, De Biasi M. Decreased signs of nicotine withdrawal in mice null for the β4 nicotinic acetylcholine receptor subunit. J Neurosci. 2004;24:10035–10039. doi: 10.1523/JNEUROSCI.1939-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Main A, Gangitano D, De Biasi M. Decreased withdrawal symptoms but normal tolerance to nicotine in mice null for the alpha7 nicotinic acetylcholine receptor subunit. Neuropharmacol. 2007;53:863–869. doi: 10.1016/j.neuropharm.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci. 2009;29:3014–3018. doi: 10.1523/JNEUROSCI.4934-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Satoskar SD, Keyler DE, LeSage MG, Raphael DE, Ross CA, Pentel PR. Tissue-dependent effects of immunization with a nicotine conjugate vaccine on the distribution of nicotine in rats. Int Immunopharmacol. 2003;3:957–970. doi: 10.1016/S1567-5769(03)00094-8. [DOI] [PubMed] [Google Scholar]
- Schlaepfer IR, Hoft NR, Collins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA. The chrna5/a3/b4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry. 2008;63:1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Johnson TA, Lerman C. Genetics and smoking behavior. Curr Psych Rep. 2007;9:349–357. doi: 10.1007/s11920-007-0045-3. [DOI] [PubMed] [Google Scholar]
- Sherva R, Kranzler HR, Yu Y, Logue MW, Poling J, Arias AJ, Anton RF, Oslin D, Farrer LA, Gelernter J. Variation in nicotinic acetylcholine receptor genes is associated with multiple substance dependence phenotypes. Neuropsychopharmacology. 2010;35:1921–1931. doi: 10.1038/npp.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clin Pharmacol Ther. 2012;91:60–70. doi: 10.1038/clpt.2011.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Levin ME, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Gradual and immediate nicotine reduction result in similar low-dose nicotine self-administration. Nicotine Tob Res. 2013;15:1918–1925. doi: 10.1093/ntr/ntt082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Pivavarchyk M, Wooters TE, Zhang Z, McIntosh JM, Crooks PA, Bardo MT, Dwoskin LP. Repeated nicotine increases bPiDDB potency to inhibit nicotine-evoked DA release from rat striatum. Biochem Pharmacol. 2010;80:402–409. doi: 10.1016/j.bcp.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Ahnallen CG. Separate and combined effects of very low nicotine cigarettes and nicotine replacement in smokers with schizophrenia and controls. Nicotine Tob Res. 2013;15:121–29. doi: 10.1093/ntr/nts098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452:638–642. doi: 10.1038/nature06846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: Nicotine and Addiction. A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Center for Health Promotion and Education, Office on Smoking and Health, U.S. Government Printing Office; Rockville, MD: 1988. [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress. A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, U.S. Government Printing Office; Washington, DC: 2014. [Google Scholar]
- Wang JC, Grucza R, Cruchaga C, Hinrichs AL, Bertelsen S, Budde JP, Fox L, Goldstein E, Reyes O, Saccone N, Saccone S, Xuei X, Bucholz K, Kuperman S, Nurnberger J, Jr, Rice JP, Schuckit M, Tischfield J, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate AM. Genetic variation in the chrna5 gene affects mrna levels and is associated with risk for alcohol dependence. Mol Psychiatry. 2009;14:501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Study Group on Tobacco Product Regulation. Report on the Scientific Basis of Tobacco Product Regulation: Fourth Report of a WHO Study Group. WHO; Geneva: 2012. [Google Scholar]
- Wickham R, Solecki W, Rathbun L, McIntosh JM, Addy NA. Ventral tegmental area α6β2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core. Psychopharmacology (Berl) 2013;229:73–82. doi: 10.1007/s00213-013-3082-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wray JM, Gass JC, Tiffany ST. A systematic review of the relationships between craving and smoking cessation. Nicotine Tob Res. 2013;15:1167–1182. doi: 10.1093/ntr/nts268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Gelber S, Orr-Urtreger A, Armstrong D, Lewis RA, Ou CN, Patrick J, Role L, De Biasi M, Beaudet AL. Megacystis, mydriasis, and ion channel defect in mice lacking the alpha3 neuronal nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1999;96:5746–5751. doi: 10.1073/pnas.96.10.5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Danjo T, Pastan I, Hikida T, Nakanishi S. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. Neuron. 2013;78:537–544. doi: 10.1016/j.neuron.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng G, Sumithran SP, Deaciuc AG, Dwoskin LP, Crooks PA. tris-Azaaromatic quaternary ammonium salts: novel templates as antagonists at nicotinic receptors mediating nicotine-evoked dopamine release. Bioorg Med Chem Lett. 2007;17:6701–6706. doi: 10.1016/j.bmcl.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zheng G, Pivavarchyk M, Deaciuc AG, Dwoskin LP, Crooks PA. tetrakis-Azaaromatic quaternary ammonium salts: novel subtype-selective antagonists at neuronal nicotinic receptors that mediate nicotine-evoked dopamine release. Bioorg Med Chem Lett. 2008;18:5753–5757. doi: 10.1016/j.bmcl.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zheng G, Pivavarchyk M, Deaciuc AG, Dwoskin LP, Crooks PA. Novel bis-, tris-, and tetrakis-tertiary amino analogs as antagonists at neuronal nicotinic receptors that mediate nicotine-evoked dopamine release. Bioorg Med Chem Lett. 2011;21:88–91. doi: 10.1016/j.bmcl.2010.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]


