Summary
Agmatine is the decarboxylation product of arginine and a number of bacteria have devoted enzymatic pathways for its metabolism. Pseudomonas aeruginosa harbours the aguBA operon that metabolizes agmatine to putrescine, which can be subsequently converted into other polyamines or shunted into the TCA cycle for energy production. We discovered an alternate agmatine operon in the P. aeruginosa strain PA14 named agu2ABCA′ that contains two genes for agmatine deiminases (agu2A and agu2A′). This operon was found to be present in 25% of clinical P. aeruginosa isolates. Agu2A′ contains a twin-arginine translocation signal at its N-terminus and site-directed mutagenesis and cell fractionation experiments confirmed this protein is secreted to the periplasm. Analysis of the agu2ABCA′ promoter demonstrates that agmatine induces expression of the operon during the stationary phase of growth and during biofilm growth and agu2ABCA′ provides only weak complementation of aguBA, which is induced during log phase. Biofilm assays of mutants of all three agmatine deiminase genes in PA14 revealed that deletion of agu2ABCA′, specifically its secreted product Agu2A′, reduces biofilm production of PA14 following addition of exogenous agmatine. Together, these findings reveal a novel role for the agu2ABCA′ operon in the biofilm development of P. aeruginosa.
Introduction
Pseudomonas aeruginosa is a relatively infrequent cause of infection in healthy adults; however, it causes considerable morbidity in immunocompromised patients and is a leading cause of hospital-acquired infections (Richards et al., 1999). The majority of adult patients with cystic fibrosis (CF) have chronic lung infections dominated by P. aeruginosa which is widely believed to be the source of most of the mortality in this disease (Davies, 2002). P. aeruginosa is an environmental organism capable of infecting a wide range of life forms and has one of the most diversified metabolic arsenals of any described bacterium (Stover et al., 2000). Polyamines are a family of small cationic molecules which play important roles in cell cycle regulation in eukaryotes, and in prokaryotes they are implicated in oxidative stress responses, biofilm development and antibiotic resistance (Demady et al., 2001; Maeda et al., 2006; Patel et al., 2006; Battaglia et al., 2007; Kwon and Lu, 2007). One important precursor to polyamine formation, agmatine, is the product of the arginine decarboxylase pathway and is found throughout nature (Majumder et al., 1992; Raasch et al., 1995; Yanagisawa, 2001; Minic and Herve, 2003; Baumann et al., 2007). While agmatine can be a polyamine precursor in most life forms, diverse secondary roles have evolved. In humans, agmatine can function as a neurotransmitter with specificity to the α2-adrenoreceptor, and is also capable of nitric oxide regulation (Li et al., 1994; Regunathan and Piletz, 2003).
Pseudomonas aeruginosa contains a number of well-described enzymes in the polyamine synthesis pathway including arginine decarboxylase, agmatine deiminase (AgDI), carbamoylputrescine amidohydrolase and ornithine decarboxylase (Fig. 1) (Mercenier et al., 1980; Nakada et al., 2001; Lu et al., 2002). AgDI activity is pivotal in P. aeruginosa polyamine synthesis from arginine because it does not contain the speB product agmatinase that converts agmatine directly into putrescine as in other bacteria (Salas et al., 2002). AgDI is currently the only known enzyme that can metabolize agmatine in P. aeruginosa. The genetic organization of a P. aeruginosa AgDI (aguBA) operon has been described and contains a number of conserved features found in agmatine operons of other divergent bacteria (Nakada et al., 2001). Most operons with AgDI (encoded by aguA) appear to contain a corresponding N-carbamoylputrescine amidohydrolase (aguB) and some form of an agmatine inducible or derepressable promoter.
Fig. 1.
Polyamine synthesis in P. aeruginosa. All enzymes have been experimentally determined to function in P. aeruginosa except SpeE for which there is a putuative orf in the genome of PAO1. SpeE presence is also implied by the presence of spermidine in P. aeruginosa cells. dSAM, decarboxylated S-adenosylmethionine.
Yersinia pestis has been shown to use polyamines to augment its biofilm formation and the enzymatic pathways for polyamine metabolism are similar to those of P. aeruginosa (Patel et al., 2006). Both species contain the speA and speC genes for arginine and ornithine decarboxylase, respectively, and neither contains an agmatinase gene, suggesting they rely solely on AgDI for agmatine metabolism. While speA/speC double mutants of Y. pestis show a marked reduction in biofilm development, they appear to have near normal growth in a defined, polyamine-free media. P. aeruginosa speA/speC double mutants do not grow unless supplemented with putrescine (Nakada and Itoh, 2003). The role of polyamines in the development of the P. aeruginosa biofilm is unknown.
Agmatine deiminase belongs to a superfamily of enzymes called the guanidino-group modifying enzymes (GME) which include a wide range of enzymes spanning prokaryotes to higher eukaryotes, including plants and mammals (Shirai et al., 2006). These enzymes all catalyse the modification of a guanidino group and appear to have strikingly similar functional elements despite very divergent amino acid sequences. Crystallography studies of these enzymes suggest a highly conserved C-terminal cysteine active site is likely critical to their activity (Lu et al., 2006). The genome of P. aeruginosa strain PAO1 contains three previously identified examples of the GME superfamily – AgDI (PA0292), arginine deiminase (ADI, PA5171) and dimethylarginase (PA1195) (Tricot et al., 1990). This work describes another group of GME genes labelled ‘porphyromonas peptidyl-arginine deiminase’ (PPAD) found in the genome sequence of the pathogenic P. aeruginosa strain PA14 but not PAO1 (Shirai et al., 2001). We demonstrate that these genes are part of a putative alternative AgDI operon, henceforth named agu2ABCA′, and do not have PADI enzyme activity as their GenBank naming suggests. This work confirms the speculation of a prior comparative analysis that suggests most of the PPAD-designated genes of bacteria are likely AgDI (Shirai et al., 2006). Furthermore we demonstrate that one of the AgDI enzymes is secreted at least to the periplasm, which has not been demonstrated in any previously described bacterial enzyme in the GME superfamily. Promoter expression analyses demonstrate that agu2ABCA′ is induced by agmatine, but preferentially in the stationary phase and during biofilm growth. We speculated this may have evolved to provide polyamines for biofilm development. Mutant analysis of PA14 revealed a dynamic role for agmatine metabolism in the development of the PA14 biofilm. The inclusion of agu2ABCA′ into the Pseudomonas arsenal of arginine and polyamine metabolism and its function in biofilm development highlight the importance of these enzymatic pathways to the survival of P. aeruginosa in the diverse niches it occupies.
Results
Identification of the agu2ABCA′ operon
The sequence of the putative agu2ABCA′ operon can be found on GenBank and the PA14 genome project at http://ausubellab.mgh.harvard.edu/cgi-bin/pa14/home.cgi (Liberati et al., 2006). The operon spans the PA14 chromosome from base pair 4306779-4311980 as annotated in GenBank. The proposed open reading frames are shown in Fig. 2A. The designation of the first and last ORF in the forward transcript is based on the PFAM motif of ‘putative peptidyl arginine deiminase’ and corresponds to gene designations PA14 48490 (henceforth named agu2A) and PA14 48450 (agu2A′). Agu2A′ contains a genetic sequence to encode for a twin-arginine translocation (tat) signal sequence at its 5′ end as predicted by the online program TatP 1.0 located at http://www.cbs.dtu.dk/services/TatP/ (Bendtsen et al., 2005). The ORFs between agu2A and agu2A′ code for a putative N-carbamoylputrescine amidohydrolase (PA14 48470/ agu2B) and a probable polyamine binding/transport protein (PA14 48450/agu2C). There is a ‘lysR’ transcriptional regulator (PA14 48500/agu2R) coded on the opposite reading strand of these four genes. Two potential promoters with their transcription start sites (TSS) are identified in the intergenic region between agu2R and agu2A (Fig. 2B) by the online neural network promoter prediction available at http://www.fruitfly.org/seq_tools/promoter.html (Reese, 2001). The transcription start site for the mRNA containing agu2A and beyond was experimentally determined and found to agree with the predicted TSS. The DNA flanking this operon is nearly identical to a long stretch of PAO1 genome and the entire operon appears inserted into a probable NADPH dehydrogenase gene designated PAO11225. The PAO1 genome base pairs show homology to position 1327795 before the operon and position 1327861 after the operon which is a loss of 66 base pairs in the acquisition of this operon into a conserved ‘backbone’. The agu2ABCA′ operon is not prevalent in many other sequenced organisms and its best match is found in a sequence from Pseudomonas entomophila strain L48 showing 78% nucleotide identity over the entire operon.
Fig. 2. The agu2ABCA′ and aguBA operons of PA14 and select mutants.
A. The agu2ABCA′ operon contains two agmatine deiminase genes agu2A and agu2A′. The black box in gene agu2A′ designates a tat secretion peptide. The promoter region for this operon is denoted with arrows between agu2R and agu2A and is shown in B. The aguBA operon has been described (Nakada et al., 2001) and is shown for comparison. Open reading frames shown in approximate scale to relative base pair length. The agu2ABCA′::Tet construct (not shown) is the same as the agu2ABCA′::Gm except a TetR cassette replaces the GmR cassette.
B. The intergenic region between agu2R and agu2A contains two putative promoters designated by thin arrows. The transcription starts sites (TSS) are designated as +1 and the TSS for agu2A has been experimentally verified.
The prevalence of the agu2ABCA′ operon in P. aeruginosa was determined in a panel of 64 clinical isolates, 16 of which were from the airways of patients with CF. We performed colony PCR screening of all of these strains as well as the laboratory strain PA103 for aguA, agu2A and agu2A′. While every P. aeruginosa tested was positive for aguA, 25% were positive for both agu2A and agu2A′ as was PA103 suggesting the entire operon is conserved in these strains. While the clinical details of all 64 isolates are not known, the isolates harbouring agu2ABCA′ operon represent a wide range of infections including those of the urine, sputum, tracheostomoy site and a skin ulcer. Only one of the 16 CF isolates was positive for the agu2ABCA′ operon, and this strain was mucoidy.
Enzymatic properties of Agu2A and Agu2A′
While the GenBank nomenclature suggested the purpose of this operon may involve PPAD activity, its genetic arrangement suggested it is likely involved in agmatine metabolism. To investigate substrate specificity, both agu2A and agu2A′ were cloned into expression vectors bestowing a C-terminal 6-His tag for identification and purification. The purification of Agu2A, Agu2A′ and an active site mutant of Agu2A′ is shown in Fig. 3A and the resulting enzymatic activities in Table 1. Agu2A with the C-terminal 6-His tag is predicted to be 40.1 kDa while Agu2A′ with the C-terminal 6-His tag is predicted to be 43.4 kDa with its tat signal peptide intact, or 39.9 kDa when cleaved. Given that the purified protein bands of Agu2A and Agu2A′ are very similar, it is likely that most of Agu2A′ is present in its cleaved form. Both of these purified enzymes were tested against the appropriate substrates for arginine deiminase activity (ADI) (data not shown), peptidylarginine deiminase activity (PADI) (data not shown) and AgDI activity. Only agmatine served as a substrate in this assay for guanidino conversion to carbamido.
Fig. 3. Agu2A and Agu2A′ are agmatine deiminase enzymes with a conserved C-terminal cysteine active site.

A. Coomassie stained gel with crude cell lysates (1–5) or nickel column purified fractions (6–8) of arabinose induced TOP10 cultures harbouring plasmids expressing the following proteins: 1. Agu2A, 2. Agu2A′, 3. Agu2A′:C364G, 4. Agu2A′ with no arabinose induction, 5. TOP10 cloning strain, 6. Agu2A eluate, 7. Agu2A′ eluate, 8. Agu2A′ C364G eluate. See Table 1 for corresponding enzymatic activities.
B. Comparison of the C-terminal amino acid sequences of members of the guanidino modifying enzymes found in P. aeruginosa. Conserved amino acids are highlighted and have been described (Shirai et al., 2006).
Table 1.
Specific activities and enzymatic rates of AgDI enzymes.
| Induced protein/sample | Specific activitya | Km/Kcatb |
|---|---|---|
| Agu2A/crude cell lysate | 0.0298 ± 0.0003 | ND |
| Agu2A′/crude cell lysate | 0.135 ± 0.001 | ND |
| Agu2A′ C364G/crude cell lysate | < 0.0001 | ND |
| Agu2A/column eluate | 0.901 ± 0.061 | 0.136 ± 0.040 / 14.12 ± 0.49 |
| Agu2A′/column eluate | 0.633 ± 0.030 | 2.624 ± 1.125 / 33.27 ± 4.22 |
| Agu2A′ C364G/column eluate | < 0.0001 | ND |
Specific activity is in nmol citrulline equivalents h−1 μg−1 protein ± SEM.
Km is expressed in mM ± SEM and Kcat is s−1± SEM.
ND – not determined.
The Km and Kcat values of Agu2A and Agu2A′ are shown in Table 1. The Km and Kcat of AguA has been previously shown to be 0.6 ± 0.05 mM and 4.2 ± 0.2 s−1 respectively (Nakada and Itoh, 2003). These calculations were performed at 37°C for Agu2A and 25°C for Agu2A′ to overcome enzymatic instability during initial velocity determinations. Agu2A appears closely related to AguA in affinity for agmatine and given its proximity to agu2B and lack of secretory signal it would appear these cytosolic enzymes are more closely related than either is to the unique Agu2A′. A protein BLAST search at NCBI reveals the protein sequences of Agu2A and Agu2A′ only share 41% and 33% identity, respectively, to AguA and only 36% identity between each other. Despite the lack of amino acid homology the functional relationship between AguA and Agu2A is also supported by the conserved poly-glutamine region just after the cysteine active site (Fig. 3B) which is absent in Agu2A′.
The predicted protein sequences of Agu2A and Agu2A′ both share C-terminal residues important for enzymatic activity in other GME superfamily members (Fig. 3B). To define the active site in Agu2A′, and to serve as a purification control, we mutated the plasmid pBW421 harbouring agu2A′ to express glycine instead of cysteine at residue 364, the putative active site of this enzyme. The protein from this mutant was expressed and purified in an identical fashion to its non-mutated parent (Fig. 3A), but has no AgDI activity in either the crude cell lysate or in the purified mutant protein (Table 1).
The comparative, enzymatic and mutational analyses all supported the hypothesis that the AguA orthologues of the agu2ABCA′ operon both functioned as AgDI enzymes, not PADI enzymes, and Agu2A′ absolutely requires the C-terminal cysteine at position 364 for its activity.
Secretion of Agu2A′
The proposed protein sequence of Agu2A′ contains a twin-arginine translocation (tat) sequence at its N-terminus shown in Fig. 4A. The twin-arginine motif is so named as the arginines appear to be required for appropriate secretion by the tat system (Lee et al., 2006). To determine if this signal sequence directs secretion of Agu2A′, the twin arginine motif was mutated to glycine-serine in plasmid pBW42101 harbouring an inducible, 6-His-tagged Agu2A′ in an E. coli/P. aeruginosa shuttle vector. The resultant plasmid, pBW42151, and its parent plasmid were transformed into PAO1 and induced with 0.2% arabinose in Luria–Bertani (LB). The cells were fractionated into spheroplasts, which contain the inner membrane and cytosol, periplasm/outer membrane and culture supernatant. Protein precipitates of these fractions were analysed by Western blot using the anti-C-terminal 6-His antibody as demonstrated in Fig. 4B. NADH oxidase was measured as a cytoplasmic control enzyme and β-lactamase as a periplasmic protein as previously described (Snyder et al., 2006). The samples used in Fig. 4B show > 90% of the total NADH oxidase activity was retained in the spheroplast fraction and > 99% of the β-lactamase activity was present in the periplasmic and spent media fractions of both strains (data not shown). The wild type Agu2A′ is much more abundant in the peri-plasmic fraction than its twin-arginine mutant demonstrating that Agu2A′ is secreted through the inner membrane by the twin-arginine translocase system. A faint band is also seen in the supernatant fraction suggesting it may also traverse the outer membrane in limited quantities. While the predicted secretion product of Agu2A′ is 39.9 kDa compared with 43.4 kDa for the unsecreted form, a distinct size difference was not seen between the cytoplasmic and periplasmic fractions. While this may simply have been an inability to resolve a 3.5 kDa difference in our electrophoresis system, it also suggests cleavage occurs in the inner membrane in both wild-type and mutant or that another post-translational modification occurs in the periplasm that changes its electrophoretic mobility. The presence of a faint band in lane 2 of the mutant is likely explained by the slight cytoplasmic spill-age detected with the control NADH oxidase analysis rather than true secretion. The secretion of Agu2A′ into the periplasm via the tat system is a unique feature of this operon and suggested the purpose of agu2ABCA′ is not a duplication of the metabolic role of aguBA.
Fig. 4. Agu2A′ is secreted into the periplasm through the twin-arginine translocase system.
A. N-terminal amino acid sequence of Agu2A′ and its secretion mutant demonstrating the predicted twin arginine consensus sequence (critical amino acids are highlighted) and the proposed cleavage site in the wild type strain (arrow).
B. Western blot to C-terminal 6-His tag of protein fractions from Agu2A′ or Agu2A′ R3S, R4G expression vectors induced with arabinose in strain PAO1. Cell fractionation is designated as 1. Inner membrane, cytosol, 2. Outer membrane, periplasm, 3. Supernatant. Each lane was loaded with 15 μg of protein from the various fractions. See Results section for description of the enzymatic assays used to ensure proper fractionation.
Agu2ABCA′ complementation of AguBA
PAO1 and PA14 can use agmatine as their sole carbon and nitrogen source in liquid or solid media growth. The aguBA operon was shown to be critical for this growth in PAO1 with aguA absolutely required (Nakada et al., 2001). We sought to determine if the agu2ABCA′ operon could play a role in agmatine metabolism in a manner that could restore the growth of an aguA mutant grown in agmatine minimal media. We were generously given aguA transposon mutants of both PAO1 and PA14 as well as agu2A, and agu2A′ in PA14. We also introduced the entire agu2ABCA′ operon in an E. coli/P. aeruginosa shuttle vector into the PAO1 aguA mutant, which normally does not contain the agu2ABCA′ operon. We also generated agu2ABCA′ operon deletions in both PA14 and the aguA mutant of PA14 using a suicide vector as described in the Experimental procedures section. Table 2 shows the growth of these mutants on minimal media supplemented with agmatine with or without other carbon or nitrogen sources. This experiment demonstrated that aguA makes agmatine available as a carbon and nitrogen source in PAO1 and PA14. In the absence of aguA, agu2ABCA′ was capable of complementation when present on a multicopy plasmid as in the PAO1 aguA mutant, or in its genomic configuration as in the PA14 aguA mutant. When gluconate was supplied as a carbon source, agu2ABCA′ appeared to readily metabolize agmatine to provide a nitrogen source allowing growth. This is likely given that agu2A, agu2B and agu2A′ all liberate ammonia during their proposed enzymatic step. When no supplemental carbon source was provided, agu2ABCA′ weakly converted agmatine into a sufficient carbon source to allow for growth of very small colonies after 3 days. Loss of either agu2A or agu2A′ alone or together did not result in a phenotype different from wild-type PA14. When both aguA and agu2ABCA′ are removed from PA14, no growth was observed on any of the agmatine minimal media plates. When agmatine was not provided in this system, no growth occurred in any strains after 4 days and all strains grew at the same rate when grown in a rich media (data not shown). The presence of agu2ABCA′ on a multicopy shuttle vector allowed for a slightly faster utilization of agmatine as a carbon source then when present in a single copy on the chromosome. Re-streaking any of these colonies after growth on agmatine minimal media did not change the rate of growth on fresh agmatine minimal media plates, which shows these were not aguA mutant revertants (data not shown). These data suggest agu2ABCA′ is active and capable of metabolizing agmatine, however, at a different rate or possibly phase of growth than aguBA.
Table 2.
Complementation phenotype of AgDI mutants.
| Strain | AgDI genes present | Growtha on MM + Agmb | Growth on MM + NH4 + Agm | Growth on MM + gluconate + Agm |
|---|---|---|---|---|
| PAO1 | aguA | +++, 1 d | +++, 1 d | +++, 1 d |
| PAO5001 | – | – | – | – |
| PAO5001 + pBW110 | agu2A, agu2A′c | +, 3 d | +, 3 d | ++, 2 d |
| PA14 | aguA, agu2A, agu2A′ | +++, 1 d | +++, 1 d | +++, 1 d |
| PA14.27633 | aguA, agu2A′ | +++, 1 d | +++, 1 d | +++, 1 d |
| PA14.48017 | aguA, agu2A | +++, 1 d | +++, 1 d | +++, 1 d |
| PA14.521 | aguA | +++, 1 d | +++, 1 d | +++, 1 d |
| PA14.42664 | agu2A, agu2A′ | +/−, 4 d | +/−, 4 d | ++, 2 d |
| PA14.42664.521 | – | – | – | – |
Growth phenotype quantified after number of days indicated. +++ clearly visible growth with distinct colonies, ++ clearly visible growth with smaller colonies, + growth visible over areas of heavier streaking, no distinct colonies, +/− microcolonies visible over areas of heavy streaking when viewed under magnification, - no growth seen in any portion under magnification.
See Experimental procedures for description of minimal media composition.
AgDI genes located within multicopy plasmid.
Regulation of agu2ABCA′ expression
While the enzymatic analysis suggests the AgDI function of agu2ABCA′ is not grossly different than aguA, its lack of robust complementation of aguA mutants suggests its regulation may be different, supporting the notion that agu2ABCA′ may have evolved for a different role in agmatine metabolism. To better understand the regulation of agu2ABCA′, we cloned the putative promoter region between agu2R and agu2A into the mini-ctx-lacZ reporter vector and analysed its expression after integration into the PA14 and PAO1 chromosomes at the innocuous attB site (Becher and Schweizer, 2000). As shown in Fig. 5A and Fig. S1A we cloned the agu2ABCA′ promoter region with and without the orf for agu2R, a putative ‘lysR’ regulator. We also cloned the aguBA promoter with aguR, which encodes a tetR transcriptional regulator that was previously shown to constitutively bind the aguBA promoter until derepressed by agmatine (Nakada et al., 2001). While a similar growth curve was observed for bacteria containing aguBA and agu2ABCA′ promoter-reporter constructs (Fig. 5B) a differential expression pattern for these intact promoters was identified following the addition of agmatine (Fig. 5C). While aguBA was induced early during log phase, agu2ABCA′ had a delayed expression until the start of stationary phase. We hypothesized that this delay could be the result of agmatine depletion by AguA which is expressed earlier, so we also tested the agu2ABCA′ promoter-reporter in a aguA::Gm mutant of PA14. Mutation of aguA does increase the magnitude of agu2ABCA′ expression in expression but does not cause it to be expressed earlier. The delayed agmatine-induced expression of agu2ABCA′ was further examined by allowing the reporter strains to grow to stationary phase before exposure to agmatine. Figure 5D demonstrates a much faster expression of the agu2ABCA′ promoter when stimulated with agmatine after reaching stationary phase. In contrast, stimulation of reporter strains with glutamine at stationary phase resulted in little differences versus unstimulated cultures (Fig. 5E) which suggests the response to agmatine is specific and not simply one of nutrient repletion. We also tested the agu2ABCA′ promoter-reporter in PA14-based mutants of rpoS and lasR which are both well-known regulators of stationary phase genes in P. aeruginosa (Schuster et al., 2004; D’Argenio et al., 2007). Compared with WT PA14 we observed no differences in the β-gal response between any of these strains, suggesting neither rpoS nor lasR regulate agu2ABCA′ (data not shown).
Fig. 5. Expression profile of agmatine operons during planktonic growth.
PA14 or its aguA::Gm mutant harbouring the reporter constructs shown in (A) were grown in liquid culture with or without supplements and samples were measured for β-gal activity at various times during the growth curve. For B–E ⬇ indicates agmatine addition at inoculation and
 indicates agmatine or glutamine addition at entry to stationary phase (agmatine or glutamine added to 20 mM final concentration; LB media used in all experiments). Dashed lines represent growth with glutamine.
indicates agmatine or glutamine addition at entry to stationary phase (agmatine or glutamine added to 20 mM final concentration; LB media used in all experiments). Dashed lines represent growth with glutamine.
B. Growth curve of the reporters used in studies shown in parts C–E.
C. β-gal expression with agmatine supplementation at inoculation.
D. β-gal expression with agmatine supplementation at stationary phase.
E. β-gal expression with and without glutamine supplementation at stationary phase. ‘Empty’ reporter constructs express < 200 Miller units regardless of supplement or growth condition (data not shown). β-gal assays performed at each timepoint from same culture in triplicate and SEM < 1% for most points (data not shown).
To better understand the nature of the putative ‘lysR’ regulator Agu2R, we generated a reporter construct that did not include the entire coding sequence of Agu2R but preserved the putative promoter region between agu2R and agu2A as shown in Fig. S1A and tested the constructs in PAO1 which does not harbour a copy of agu2ABCA′. With the laboratory domestication of PAO1, it is believed to have acquired mutations in its genetic regulatory networks as it displays different phenotypes between various laboratories (Friedman and Kolter, 2004; Tremblay and Deziel, 2008). Thus we repeated the growth curves and expression assays in PAO1 performed in Fig. 5 assuming they would show a different baseline than PA14. Fig. S1C–F shows that the agu2ABCA′ promoter requires both the agu2R orf and agmatine to be activated, and maximal expression occurs in stationary phase. This experiment also shows that the agu2ABCA′ promoter has much more activity in PAO1, especially in stationary phase where its activity is almost 10-fold higher than the aguBA promoter.
AgDI influence on intracellular agmatine
As the AgDI enzymes of P. aeruginosa are expressed during different phases of growth and appear to function in different cellular locations, we sought to determine if the agmatine concentration within the cell was influenced by AgDI activity. The complementation data suggest aguBA is the dominant AgDI operon within the cell. To determine if agu2ABCA′ could influence the intracellular agmatine pools, we used mass spectrometry to measure the intra-cellular agmatine of cultures that were grown specifically to induce agu2ABCA′. After 18 h of growth in LB, PA14 and select AgDI mutants were stimulated with either agmatine or glutamine and allowed to grow for 3 more hours. Table 3 shows the agmatine concentration within the lysed cell pellets. These data demonstrate that PA14 with intact AgDI enzymes metabolizes intracellular agmatine to levels below the sensitivity of our assay; however, removal of aguA or agu2A′ results in agmatine accumulation. The presence of intracellular agmatine in the agu2A′ mutant suggests a small portion of agmatine is not effectively eliminated by AguA, but is accessible to Agu2A′ most likely within the periplasm.
Table 3.
Intracellular agmatine concentration in AgDI mutants.
| Strain/mutant and supplementa | Intracellular agmatineb ± SEM |
|---|---|
| PA14 + Gln | < 0.001c |
| PA14 + Agm | < 0.001c |
| PA14 aguA::Gm + ln | 0.922 ± 0.076 |
| PA14 aguA::Gm + gm | 12.41 ± 0.22 |
| PA14 agu2A′::Gm + Gln | 0.0321 ± 0.0026 |
| PA14 agu2A′::Gm + Agm | 0.0300 ± 0.0185 |
Glutamine or agmatine was added to a final concentration of 20 mM after cultures grown in supplement free LB for 18 h. Cell pellets were collected for agmatine extraction after 3 h growth in supplement.
Value shown is average of three samples from same culture processed independently and expressed as μmol agmatine g−1 wet cell pellet. This experiment was repeated once with similar results.
Value below assay detection limit.
The high agmatine concentrations within the aguA mutant grown without agmatine suggests high activity of arginine decarboxylase to generate agmatine, or efficient acquisition from LB media which contains approximately 3 μM agmatine (determined in data not shown). Addition of agmatine to the media of the aguA mutant results in a large increase in intracellular agmatine. This latter finding is not seen with the agu2A′ mutant where the unmetabolized agmatine pool is essentially unchanged. This suggests the periplasmic agmatine supply is relatively stable compared with the cytosol.
Agu2ABCA′ enhances biofilm development in PA14
The clear delineation between the expression profiles of aguBA and agu2ABCA′ suggested a separate biological purpose for agu2ABCA′ such as providing polyamines or free ammonia to non-rapidly dividing Pseudomonads, or those in high cell densities or biofilms. To determine if AgDI genes contribute to biofilm formation in Pseudomonas, we tested PA14 AgDI mutants in a well-described 96-well microtiter plate biofilm assay with crystal violet staining of adherent bacteria (O’Toole and Kolter, 1998). When grown in rich media without supplemental agmatine, deletion of aguA augmented biofilm development, and the biofilm was further increased by combined deletion of aguA and agu2ABCA′ (Fig. 6). In addition, WT PA14 grown in the presence of increasing agmatine concentrations show a dose-dependent increase in biofilm development (Fig. 6 and Fig. S2). Together, these data suggested that agmatine augments the biofilm of PA14 and its metabolism reduces it. However, further analysis of the individual mutants grown in the presence of agmatine revealed a more complicated regulation. When aguA alone was deleted, the response to agmatine was similar to WT, but when agu2A′ or the entire agu2ABCA′ operon was mutated, agmatine inhibited the biofilm in a dose-dependent manner (Fig. 6 and Fig. S2). This finding indicates that agu2ABCA′, specifically the periplasmic AgDI Agu2A′, is responsible for the agmatine-induced augmentation of the PA14 biofilm.
Fig. 6.
Agmatine metabolism regulates the PA14 biofilm. PA14 and its AgDI mutants were grown overnight in LB media in 96-well polystyrene plates with or without supplemental agmatine (10 mM shown here). Crystal violet staining of the biofilm and quantification at A595nm was performed as described in the Experimental procedures. Each data point represents the average of eight wells and error bars are ± SEM. Comparison groups were all grown on the same plate and strains grown at 10 mM Agm were compared with the same strain grown at 0 mM Agm for statistical analysis (see Fig. S2 for Agm dose–response of each mutant). These results were confirmed with > 3 repeat assays. *P < 0.0001 compared with WT grown without Agm. †P < 0.0001 compared with same strain grown without Agm.
We further addressed this finding by testing our promoter-constructs during biofilm growth in PA14 (Fig. 7). We disrupted the bacteria containing reporter constructs remaining on the walls of plastic dishes with sonication and chemical lysis and measured the liberated β-galactosidase compared with the total liberated protein as a surrogate measure to the Miller assay (Sakuragi and Kolter, 2007). This experiment revealed an agmatine-induced expression of both aguBA and agu2ABCA′ during biofilm growth; however, agu2ABCA′ was preferentially expressed when agmatine was added to already-established biofilms.
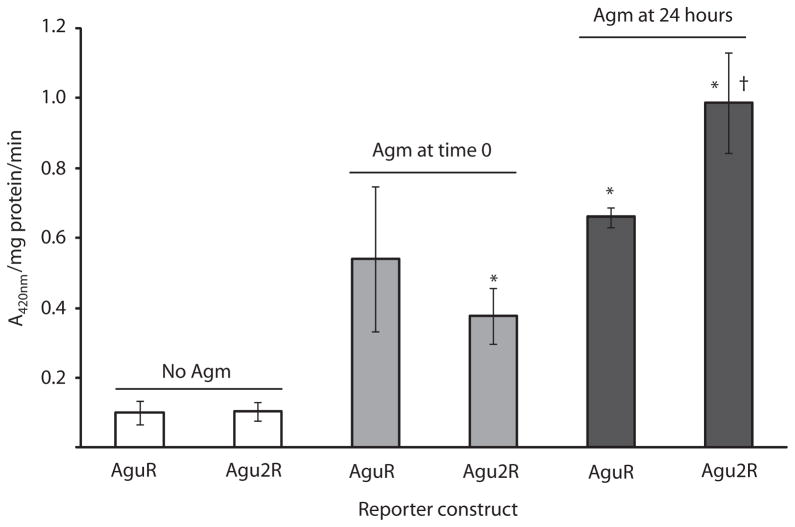
Fig. 7.
Expression profile of agmatine operons during biofilm growth. PA14 Biofilms were grown as described in Fig. 6 except 24-well polystyrene plates were used. Reporter configuration shown in Fig. 5. Biofilm growth was harvested as described in Experimental procedures at 27 h growth and analysed for β-gal activity. Assays were done in triplicate and error bars are ± SEM. Agmatine was added to a final concentration of 10 mM either at inoculation (time 0) or after 24 h of growth without agmatine. *P < 0.05 compared with the same reporter grown without agmatine. †P < 0.05 compared with same reporter grown with agmatine from time 0.
The biofilm of PA14 is fairly well described and has been shown to differ in composition from PAO1 (Wozniak et al., 2003; Friedman and Kolter, 2004). We tested PAO1 harbouring a plasmid borne copy of agu2ABCA′ in these assays but were not able to elucidate any differences in the biofilm phenotype (data not shown). This suggests other PA14 specific genes are necessary for this agmatine-induced biofilm phenotype. We also tested the effect of putrescine in this system to determine if polyamine addition would complement some of the mutant phenotypes (Fig. S2B). Unlike agmatine titration, putrescine titration had no effect on biofilm formation in any mutant and became toxic at concentrations above 1 mM.
Our working model of these data is presented in Fig. 8. In summary, these data suggest that cytosolic AgDI metabolism inhibits biofilm development, whereas peri-plasmic AgDI metabolism enhances biofilm development. As the periplasmic AgDI is specifically induced during biofilm growth, and appears responsible for periplasmic agmatine removal, it is possible that agu2ABCA′ evolved to regulate the biofilm response to exogenous agmatine in P. aeruginosa.
Fig. 8. Agmatine regulation of biofilm development.
A. During rapid growth agmatine (Agm) is created in the cytoplasm by arginine (Arg) decarboxylase or traverses the cell wall if exogenous agmatine is available. AguBA is upregulated and agmatine is metabolized to N-carbamoylputrescine (N-CP) and ultimately putrescine by the successive actions of AguA and AguB. Putrescine is used for a number of processes involved in cell division and can be used for energy production.
B. During biofilm growth, exogenous agmatine traverses the cell wall and distributes to both the cytosol and periplasm. In the cytosol agmatine preferentially stimulates transcription of agu2ABCA′ which results in Agu2A′ production and secretion to the periplasm, as well as production the cytosolic enzymes Agu2A and Agu2B. Exogenous agmatine stimulates the PA14 biofilm through its periplasmic metabolism, and this effect is dominant to the inhibitory effect of cytosolic agmatine metabolism. As agu2ABCA′ weakly complements the aguA mutant, it can metabolize agmatine for use as a sole carbon and nitrogen source, presumably through the actions of the cytosolic Agu2A and Agu2B.
Discussion
We have discovered a second putative agmatine operon in P. aeruginosa and have experimental evidence that two of the genes code for proteins that catalyse the deimination of agmatine to the polyamine precursor N-carbamoylputrescine. While polyamines have a myriad of purposes in bacteria, we investigated the P. aeruginosa biofilm and determined that agu2ABCA′ enhances its development. Several characteristics of the agu2ABCA′ operon suggested it may play a role in biofilm development. First, there is a precedence for polyamines in the biofilm development of Y. pestis, which shares similar polyamine enzymatic pathways with P. aeruginosa (Patel et al., 2006). Second, the secretion of Agu2A′ suggests the enzymatic step may be occurring in the periplasm or cell surface where a number of important processes in biofilm development occur (Mah et al., 2003; Campisano et al., 2006). Third, the twin-arginine translocation system responsible for the secretion of Agu2A′ is upregulated in P. aeruginosa grown as a biofilm (Whiteley et al., 2001). Finally, the preferred expression in stationary phase suggested agu2ABCA′ may be providing polyamines for a maintenance or reparative process over one of energy creation or stabilization of DNA synthesis.
More work is necessary to unravel the contribution of agu2ABCA′ to the chemical composition of the PA14 biofilm; however, it is possible that the periplasmic conversion of agmatine to N-carbamoylputrescine influences the synthesis or secretion of polysaccharides either through alkalinization from the deimination of agmatine or through an effect mediated by the accumulation of N-carbamoylputrescine. Addition of exogenous putrescine did not appear to have an effect on the biofilm phenotype. This could be expected as putrescine does not inhibit the AgDI enzymatic step (Nakada and Itoh, 2003). As the mechanism of biofilm enhancement may occur in the periplasm, it is not clear if exogenous putrescine addition would result in a periplasmic accumulation. Ideally, measurement of agmatine and N-carbamoylputrescine within the periplasm of cells, grown planktonically and as a biofilm, would begin to unravel this mechanism. We attempted to measure periplasmic agmatine for this study however, the fractionation process used for protein localization was not effective on stationary phase bacteria judged by poor partitioning of our control enzymes (see Experimental procedures).
Agu2ABCA′ is clearly not essential for biofilm development in all P. aeruginosa as it is not present in PAO1, a known biofilm former, or more than half of the clinical strains we tested. Furthermore, agu2ABCA′ appears to have little to no impact on the PA14 biofilm when grown without exogenous agmatine. These studies show that agu2ABCA′ was probably adapted from a strictly metabolic role, to one of environmental adaptation likely brought about by a hostile environment containing agmatine. As agmatine does not appear to be directly toxic to P. eruginosa, it would suggest agmatine could be a marker of other stressful conditions. Macrophages secrete agmatine when stimulated with endotoxin (Regunathan and Piletz, 2003). We have measured agmatine concentrations in the sputum of patients with CF and have shown it is positively correlated with markers of inflammation including IL1β, IL8 and TNFα (unpubl. data). Agmatine may be a direct initiator of inflammation in mammals as both neutrophils and macrophages harbour α2-adrenoreceptors that have been implicated in endotoxin induced lung inflammation (Flierl et al., 2007). We have also observed that agmatine is capable of inducing TNFα release from macrophages in a α2-adrenoreceptor-dependent mechanism (unpubl. data). As biofilms are associated with persistent infection (Davies, 2002), we speculate that Pseudomonads harbouring agu2ABCA′ may have a survival advantage during infections characterized by marked inflammation, such as the chronic airways infection of CF.
While the enzymatic properties of agu2ABCA′ initially suggested a duplication of the previously described aguBA operon of P. aeruginosa, a number of findings distinguish it. In addition to the genes for AgDI enzymes and N-carbamoylputrescine amidohydrolase, agu2ABCA′ also encodes a probable periplasmic polyamine binding protein. The likely purpose of this protein is to shuttle agmatine or N-carbamoylputrescine into the cell and putrescine or other downstream products out, as described for the Lactococcus brevis orthologue (Lucas et al., 2007). Agu2A′ is distinguished by its twin-arginine translocation sequence which allows for its transport to the periplasm in P. aeruginosa. The tat system is highly utilized by Pseudomonas species and most of the proteins it secretes are predicted to be involved in redox reactions; however, the tat system also appears important for bacterial virulence (Ochsner et al., 2002; Lee et al., 2006). Both Agu2A and Agu2A′ contain the highly conserved C-terminal cysteine active site motif found in all of the GME superfamily members. While crystal structures have been determined for other AgDI that contain this motif, our work shows that mutation of this site completely removes enzymatic function, reinforcing the excellent descriptive work done in this field to date (Tricot et al., 1990). Another important piece of evidence to suggest the lack of functional duplicity between the agmatine operons is their differing regulation. While both are clearly responsive to agmatine, agu2ABCA′ expression is delayed until early stationary phase whereas aguBA expression preferentially occurs during the rapid growth phase. This divergent regulation explains the slow growth of the aguA mutants complemented with agu2ABCA′. Their ability to enter stationary phase was reduced in agmatine minimal media as the log phase enzyme for its metabolism was absent. We presumed the genetic regulation of agu2ABCA′ would be under the control of rpoS or lasR which have been shown to control a number of genes expressed during the stationary phase or periods of high cell density (Schuster et al., 2004). We did not observe any decreased expression of the agu2ABCA′ promoter in rpoS or lasR mutants, thus the regulators of its delayed expression are still unclear. Analysis of agu2R expression, and its regulation of the promoter in future studies will help us resolve this remaining question.
Experimental procedures
Bacterial strains, media, growth conditions
Strains and plasmids used in this study are presented in the online Table S1. LB was used as the rich media for E. coli and P. aeruginosa with antibiotic concentrations for E. coli: ampicillin 100 μg ml−1, kanamycin 50 μg ml−1, tetracycline 20 μg ml−1, gentamicin 15 μg ml−1, and for P. aeruginosa: carbenicillin 200 μg ml−1, gentamicin 15 μg ml−1, tetracycline 100 μg ml−1. The minimal media for P. aeruginosa consists of 10.5 g of K2HPO4, 4.5 g of KH2PO4 and 48 mg of MgSO4 per liter of water. For carbon source supplementation sodium gluconate was added to 20 mM, and for nitrogen source supplementation ammonium sulfate was added to 7.5 mM. When indicated agmatine or glutamine was added at 20 mM (Fukuoka et al., 1991). All growth was carried out at 37°C except where indicated.
Plasmid construction and mobilization
Primer description and sequences are presented in the online Table S2. All PCR reactions generated for cloning were performed with the GC-Rich PCR System following manufacturer’s recommendations (Roche) in a Veriti Thermocycler (Applied Biosystems). The pCR-XL TOPO TA cloning system (Invitrogen) was used to clone the agu2ABCA′ PCR product generated from PA14 chromosomal DNA with primers 1F and 1R. This plasmid, pBW100, contains the entire agu2ABCA′ operon and ~2 kb of flanking chromosomal DNA. To mobilize this operon back into P. aeruginosa the EcoRI fragment of this plasmid containing the operon was subcloned into pUCP20 creating pBW110. The agu2A and agu2A′ genes were PCR amplified from PA14 genomic DNA and inserted into pBAD202 TOPO expression system (Invitrogen) per manufacturer’s protocol. Agu2A was amplified with primers 2F and 2R and agu2A′ was amplified with primers 3F and 3R. These primers were designed to allow removal of the thioredoxin fusion partner inherent in the pBAD202 system and the 3′ stop signal which permits linkage to the 6-His tag in pBAD202 and ultimately the C-terminus of the expressed protein. To create pBW4216, the proposed enzymatic active site (C364) of Agu2A′ was mutated in pBW421 using the Quick Change II mutagenesis kit (Stratagene) to change Cys to Gly, creating a NcoI site following the manufacturer’s protocol with primers 4F and 4R. To move pBW421 into P. aeruginosa, the NsiI-PmeI fragment containing the arabinose promoter, araC regulator, and the 6-His-labelled agu2A′ was cloned into the PstI-SmaI site of pUCP20 to create pBW42101. To create pBW42151, the twin-arginine translocation sequence of agu2A′ was mutated in pBW42101 using the Quick Change II mutagenesis kit to change the N-terminus Arg-Arg sequence to Gly-Ser with primers 5F and 5R.
To expand the usefulness of pPS856 we generated a ‘tetracycline cassette’ version by PCR amplifying the TetR cassette from pEX18Tet using primers 6F and 6R which contain MfeI sites. The pPS856 ‘backbone’ was amplified using primers 7F and 7R and the EcoRI digest of this product was ligated to the MfeI digested product above to generate pPS856Tet. This plasmid retains the flanking multiple cloning sites and FRT sites around the TetR cassette.
To make pBW5 we cloned the entire agu2ABCA′ operon and ~4 kb of flanking chromosomal DNA into the pCR-XL TOPO TA vector using primers 8F and 8R. To generate a suicide vector to delete the entire operon pBW5 was digested with AgeI and NruI, blunt ended with the NEB ‘Quick blunting’ enzyme kit, gel purified and ligated to the gentamicin cassette generated from pPS856 treated with SmaI. To generate pBW521 the NheI-HindIII fragment containing the deleted operon and gentamicin cassette was cloned into the HindIII-XbaI sites in pEX18Ap. To generate pBW521tet, the tetracycline cassette from pPS856tet was removed with BamHI and ligated into the BglII site within the gentamicin cassette of pBW521. To make the agu2ABCA′ chromosomal deletion mutants we moved pBW521 into PA14 and pBW521Tet into the aguA mutant of PA14 and isolated sucrose resistant colonies as described elsewhere (Hoang et al., 1998). Loss of agu2ABCA′ was verified by PCR of chromosomal DNA.
To make pBW71 we cloned the promoter and regulator of the PA14 aguBA operon using primers 9F and 9R. The EcoRI/HindIII digested product was inserted into the multiple cloning site of the mini-ctx-lacZ reporter plasmid (Becher and Schweizer, 2000). To clone the putative promoter of agu2ABCA′ we PCR amplified it with (pBW72) and without (pBW73) the agu2R orf which codes for the putative transcriptional regulator into the mini-ctx-lacZ reporter plasmid. Primers 10F1 and 10R were used for pBW72 and primers 10F2 and 10R were used for pBW73. The sequences of pBW321, 421, 42151 and 4216 were all verified by DNA sequencing at Vanderbilt’s DNA core facility.
All E. coli transformations occurred via commercially available chemically competent cells supplied with the cloning vectors or mutagenesis kits (TOP10 or XL1 blue, respectively) and following manufacturer specifications. All P. aeruginosa transformations occurred via electroporation as previously described (Choi et al., 2006).
Colony PCR screen
Sixty-four clinical P. aeruginosa isolates were obtained from the Vanderbilt Medical Center Microbiology laboratory in a de-identified fashion. This collection received an exemption from Vanderbilt’s Institutional Review Board. Each sample was regrown on MacConkey agar then saved as a frozen stock for future analysis. The cultures were regrown in 500 μl of LB broth, pelleted, resuspended in 100 μl of sterile water and boiled for 10 min. The samples were centrifuged and 1 μl was used per 25 μl of PCR reaction. The primers to detect aguA (11F and 11R) were designed to amplify a 350 bp region in either PAO1 or PA14. Primers 12F and 12R were used to detect agu2A. PCR was performed using the GC Rich PCR system (Roche) and modified annealing temperatures of 60°C for five cycles, reduced by 0.2°C per cycle for the remaining 25 cycles.
Gene expression and protein isolation
Protein expression in E. coli occurred using the arabinose inducible promoter in pBAD202 following the manufacturer’s protocol. To induce agu2A, the culture was grown overnight at 30°C to overcome solubility issues. Agu2A′ and its mutant derivatives could be induced at either 30°C overnight or 37°C for 4 h in either the pBAD202 or pUCP20 derivatives. Arabinose induction conditions were determined by titration of arabinose at subjective evaluation of protein yield as monitored by Western blotting. The lowest concentration of arabinose for which protein yield appeared to no longer increase was 0.002% for E. coli and 0.2% for P. aeruginosa.
To harvest total cell protein for analysis or further purification E. coli cell pellets were resuspended in 10X Bug Buster (Novagen) that was reconstituted in 50 mM NaCl, 50 mM Na2PO4, 0.02% sodium azide with a pH of 8.0 at a ratio of 1.5 ml per 50 ml of overnight culture for E. coli or 400 μl per 10 ml of overnight culture of P. aeruginosa. This solution was supplemented with 1/200 v/v Bacterial Protease Inhibitor (Sigma) and 1/1000 v/v Benzonase (Novagen). The cell pellet was gently rocked at RT for 20 min, and centrifuged at 16 000 g for 20 min in a microfuge. The supernatant was used directly for enzyme and electrophoretic analysis or loaded onto an imidazole equilibrated nickel resin column. Nickel resin purification of Agu2A and Agu2A′ was performed per the pBAD202 TOPO expression system manufacturer specifications. Protein quantification was performed with the Bradford assay (Bio-Rad) for imidazole containing fractions and the BCA assay (Pierce) for all other samples.
Carbamido detection assay
The assay to detect the products of deimination of the guanidino groups of agmatine and related compounds is derived from the assay for citrulline from Boyd and Rahmatullah (Boyde and Rahmatullah, 1980). Briefly, 100 μl of sample containing enzyme is mixed with 100 μl of reaction cocktail containing 10 μl of 1 M Tris Buffer pH 9, 20 μl of 500 mM NaCl, 20 μl of 10 mg ml−1 BSA in water, 10 μl of 20 mM EDTA, 20 μl of 50 mM substrate in water, 20 μl of 10 mg ml−1 Type III Urease (Sigma) in 100 mM phosphate buffer pH 7.0 and 0.2 μl of 10 mM flavin adenine dinucleotide. The substrates for AgDI, PADI and ADI are agmatine sulfate, benzoyl-arginine ethyl ester, and arginine respectively. To account for background urea, citrulline and other carbamido compounds in the sample formed independent of AgDI activity a 100 μl sample is incubated with an identical reagent solution replacing the substrate component with water. When crude cell lysates are analysed urease is added to the reaction to remove the urea component which gives a colorimetric product in the final step. For screening purposes these reactions are incubated in a microfuge tube at 37°C for Agu2A and all P. aeruginosa extracts and 45°C for Agu2A′ and its mutants for 1 h and stopped with the addition of 40 μl of 100% TCA. The reaction is centrifuged at 16 000 g for 5 min to remove precipitate and 200 μl of this mixture is added to 800 μl of acidified diacetyl monoxime (DAMO). This solution contains a mixture of 5 mg ml−1 DAMO with 0.1 mg ml−1 of thiosemicarbazide mixed one part to two parts of a acid ferric solution which contains 550 ml of water, 250 ml of concentrated sulfuric acid, 200 ml of concentrated phosphoric acid and 250 mg l−1 ferric chloride. The sample and acidified DAMO are incubated for 5 min at 100°C, cooled, centrifuged as before to remove remaining precipitates and transferred in triplicate to a 96-well plate. Carbamido products were measured spectrophotometrically at A540nm using a Bio-Rad 680 plate reader. Citrulline was used to make standard curves in the same buffers used for sample incubation. This assay uses citrulline for the standard curve which has been demonstrated to be less chromogenic than carbamoylputrescine which is not commercially available (Llacer et al., 2007). All specific activities are reported in nanomole of citrulline produced per hour per μg of protein. The kinetic analysis of purified Agu2A and Agu2A′ was carried out with agmatine concentrations from 30 mM to 100 μM and time points from 5 min to 2 h. While Agu2A was analysed at 37°C, Agu2A′ was analysed at 25°C to avoid enzymatic degradation which was apparent as early as 5 min at 37°C. Initial velocities were calculated from linear rates usually achieved before 15 min. The Kcat calculation for Agu2A′ was determined using the estimated molecular weight of the enzyme after cleavage of the secretory N-terminal leader. Graph Pad Prism 5 software (Graph Pad software Inc.) was used to perform non-linear regression analysis of the velocity versus substrate graph to determine the Km and Kcat. All chemicals for this assay are from Sigma.
Western blotting
The Nu-Page system by Invitrogen was used for all protein electrophoresis experiments. Manufacturer protocols were used for all steps through transfer to nitrocellulose. The C-terminal 6-His tag antibody from Invitrogen was used as the primary antibody and the anti-mouse IR680 secondary antibody from LiCor was used for detection on the Odyssey digital imaging system (LiCor). The Western blotting technique followed the protocol suggested by the manufacturers of the Odyssey system to minimize background.
Cell fractionation
The technique to separate the various fractions of the Pseudomonal cell wall was based on a prior protocol developed for this organism (Snyder et al., 2006). After induction of the bacterial culture as described above, the sample was centrifuged at 12 000 g at 4°C for 10 min. The supernatant was removed and proteins were precipitated with a final concentration of 13% TCA at 4°C overnight. The pellet was air-dried and resuspended in a minimal volume of PBS. The cell pellet was washed in ice cold 20% sucrose at a volume of 50% the original culture volume. The washed cells were weighed and resuspended in 18 ml of 20% ice cold sucrose per 1.5 g of cells. To this, per 1.5 g of cell pellet, the following were added: 9 ml of 2 M Sucrose, 10 ml of 0.1 M Tris (pH 7.8), 1.8 ml of fresh 0.5% (wt/vol) lysozyme and 10 μl of Benzonase. This solution was gently mixed and placed at 30°C for 30 min. At the 30 min mark 0.8 ml of 150 mM EDTA was added and the sample was returned to 30°C for another 30 min. After this incubation the sample was centrifuged for 20 min at 20 000 g at 4°C. The supernatant was removed and TCA precipitated as described for the culture supernatant. This fraction contains the outer membrane and periplasm. The remaining pellet contains spheroplasts which represents the inner membrane and cytosol. The spheroplasts were washed once in 20% ice cold sucrose, and recentrifuged as above. The spheroplasts were resuspended in a minimal volume of PBS and immediately boiled for 5 min before either freezing or preparing for protein electrophoresis as described above. Fraction purity was assessed with the nitrocefin and NADH oxidase assays as previously described (Snyder et al., 2006). 15 μg from each fraction was loaded per lane and analysed with Western blot to the C-terminal His tag as described above.
Promoter analysis
The TSS of the agu2ABCA′ mRNA was determined using a previously described protocol employing tobacco acid pyrophosphatase (TAP) (Epicentre) processing of mRNA with ligation of a synthetic RNA oligo (Bensing et al., 1996). The mRNA was harvested from stationary phase PA14 grown for 3 additional hours in the presence of 20 mM agmatine using the RNeasy system (Qiagen). After TAP treatment and RNA oligo ligation, the reverse transcription reaction was performed with primer 12R using the Transcriptor High Fidelity cDNA system (Roche). The PCR reaction was performed with primers P1 and 10R and the sequence of the subsequent PCR product was determined at the UMN sequencing facility.
To determine the expression patterns of the agu2ABCA′ and aguBA promoters the plasmids mini-ctx-lacZ, pBW71, pBW72 and pBW73 were transformed into PAO1 and PA14 (WT, rpoS::Gm, ΔlasR and aguA::Gm mutants) by triparental mating using HB101 as described before (Hoang et al., 1998). The TetR transconjugates were further subjected to transformation with the pFLP2 plasmid to remove the tetra-cycline resistance gene and int gene as described previously (Hoang et al., 1998). The pFLP2 plasmid was cured by growth on 5% sucrose and the final reporter strains were verified to have insertions into the genomic attB site by loss of the 270 bp PCR product of the Pser primers (Becher and Schweizer, 2000; Hoang et al., 2000).
Overnight cultures of each reporter strain were pelleted, resuspended in PBS and repelleted before suspension to an A600nm of 0.2. 50 μl of this suspension was used to inoculate 50 ml of LB broth with or without 20 mM agmatine in 125 ml of baffled flasks. These were grown at 37°C in a shaking incubator and 1 ml of aliquots were removed at the respective time points for A600nm measurement in a UVmini-1240 spectrophotometer (Shimadzu). The detection of β-galactosidase activity was based on a modified Miller assay and is described with some modification next (Zhang and Bremer, 1995; OpenWet-WAre, unpublished). 20 μl of culture from each timepoint was added to 80 μl of permeabilization solution (100 mM Na2HPO4, 20 mM KCl, 2 mM MgSO4, 0.8 mg ml−1 CTAB, 0.4 mg ml−1 Na deoxycholate, 5.4 μl ml−1 β-mercaptoethanol) before addition of 600 μl of substrate solution (60 mM Na2HPO4, 40 mM NaH2PO4, 1 mg ml−1 ONPG, 2.7 μl ml−1 β-mercaptoethanol). This was allowed to incubate at 37°C until yellow color development and the reaction was stopped with 700 μl of 1 M Na2CO3 and the reaction time recorded. The sample was centrifuged at 16 000 g for 5 min and the A420nm of the supernatant was measured. Miller units were calculated as 1 MU = 1000 × [A420nm/(A600nm × reaction time (min) × culture volume tested in ml)]. All ABS readings occurred in 1 cm cuvettes and measurement was taken after the measurement reading stabilized. Repeat measurements at either A600nm or A420nm typically varied by less than 0.005 ABS units. To analyse the reporter constructs after biofilm growth, the cultures were grown and washed as described below in the biofilm assay and 500 μl of permeablization solution was added to each well of the 24-well plates. These wells were sonicated with two 10 s pulses at 50% duty cycle on ice with a VibraCell sonicator (Sonics Materials). 100 μl of this solution was assayed for β-galactosidase activity as described above. The A420nm was normalized to the protein content (determined by Pierce Brad-ford Assay) of the analysed sample as published previously (Sakuragi and Kolter, 2007).
Intracellular agmatine determination
The strains were grown as described in the text and harvested by centrifugation. The cell pellets (~100 mg) were resuspended in PBS and sonicated on ice. The lysate was collected after centrifugation at 20 000 g for 20 min at 4°C and frozen for future use. Internal standard homoagmatine was synthesized from the reaction of cadaverine and cyanamide in boiling 12 N HCl. The crude product mixture was cooled to 0°C, brought to slightly alkaline pH by careful addition of 9 N NaOH, and washed three times with EtOAc. The organic fractions were combined and evaporated to dryness at 50°C under a gentle stream of N2 gas. The dried extracts were dissolved in H2O/MeCN/HCOOH (80:20:0.1) to a final concentration of 2.5 μM and used without further purification. 100 μL of thawed lysate was spiked with 10 μl of homoagmatine, lightly vortexed, allowed to stand at room temperature for 15–20 min and deproteinized with 200 μl of 2-propanol. Samples were then cooled to −20°C for 45–60 min, and precipitated proteins were removed by centrifugation (18 000 g, 30 min, 4°C). The clear supernatant of each sample was transferred to a membrane dialysis cartridge (3000 MWCO) and filtered at 10°C for 6–8 h (10 000 g). The filtrate was evaporated under a gentle stream of N2 gas. The residue was reconstituted in 75 μl of H2O/ CH3CN (1:1) containing 20 mM dansyl chloride (Sigma) and 100 mM NaHCO3 (pH 8.0) and heated to 50°C for 20 min. Following derivatization, samples were diluted with 120 μl of H2O and centrifuged to remove particulates (18 000 g, 22°C, 10 min). The clear supernatant was transferred to 200 μl of silanized autosampler vials equipped with Teflon-lined bonded rubber septa.
A Zorbax SB-C18 Rapid Resolution HT column (2.1 mm × 50 mm, 1.8 μm, Agilent Technologies, Palo Alto, CA) equipped with an Acquity UPLC in-line stainless steel filter unit (0.2 μm, Waters) was used for all chromatographic separations. Separations were carried out at room temperature; the autosampler tray temperature was set to 10°C. Mobile phases were made up of 0.5% (v/v) formic acid in (A) H2O/CH3CN (95:5) and in (B) H2O/2-PrOH/CH3CN (5:10:85). Gradient conditions were as follows: 0–3 min, B = 0%; 3–6 min, B = 0–90%; 6–9 min, B = 90%; 9–10 min, B = 90-0%; 10–15 min, B = 0%. The flow rate was maintained at 350 μl min−1. A software-controlled divert valve was used to transfer eluent from 0 to 4.7 min and from 6 to 15 min of each chromatographic run to waste. The total chromatographic run time was 15 min. The sample injection volume was 20 μl.
Sample analyses were carried out using an Accela UHP quaternary pump and a ThermoPal refrigerated autosampler (Thermo-Fisher Scientific, Waltham, MA). Tandem mass spectrometric detection was performed using a TSQ Quantum Access triple-stage quadrupole mass spectrometer (Thermo-Fisher) equipped with an electrospray ion source and a 100 μm ID deactivated fused silica capillary. The mass spectrometer was operated in positive ion mode. Quantification was based on multiple reaction monitoring (MRM) detection at a collision energy of 30 V (agmatine: m/z 364 → 170, homoagmatine: m/z 378 → 170).
Data acquisition and quantitative spectral analysis were done using Thermo-Fisher Xcalibur version 2.0.7 and Thermo-Fisher LCQuan version 2.5.6 respectively. Calibration curves were constructed by plotting the peak area ratio (agmatine/ homoagmatine) against the concentration of agmatine for a series of six aqueous buffered standards (0.122 μM–243 μM). A weighting factor of 1/C2 was applied in the linear least-squares regression analysis to maintain homogeneity of variance across the concentration range. The lower limit of quantification was 122.0 nM, defined as the lowest standard on the calibration curve where %RSD and %RE ≤ 20%.
Biofilm growth and microtiter assay
The biofilm assay used in this study has been described before by O’Toole and Kolter (1998). Briefly overnight cultures grown in LB were pelleted and washed in PBS then diluted to A600nm of 0.2. A 1:100 dilution was made into LB, or LB supplemented with agmatine or Putrescine, and 150 μl was added per well to a Costar 96-well or 24-well polystyrene plate. Samples were tested with eight replicates for 96-well assay or three wells for 24-well assays. For the 96-well crystal violet assay the plates were incubated for 24 h at RT, then washed by repeated dunking in warm tap water, tapped dry and to each well 175 μl of 1% crystal violet was added and placed on a rotating platform for 15 min. The crystal violet was washed out as before staining then 200 μl of DMSO was added to each well and the plate was placed back on the rotating platform for 30 min after which 150 μl of each sample was transferred to a new plate for A595nm measurement.
Statistical analysis
GraphPad InStat software was used to determine statistical significance of the experimental groups. When comparing two means, t-tests were used, and when comparing more than two means, one-way ANOVA was used.
Supplementary Material
Acknowledgments
We thank Drs H. P Schweizer, F. Ausubel, G. O’Toole, L. Hoffman and C. Lu for PA plasmids, strains and mutants. We also thank the Vanderbilt Clinical Microbiology lab for saving the clinical isolates used in this study and Dr E. Skaar for critical review of this work, and Dr G. Dunny, C. Johnson and A. Gilbertsen for helpful discussions.
This work was supported by Third and Fourth Year Cystic Fibrosis Foundation Research Fellowship awards to BJW. This work was also supported in part by NIH RO1 HL 61419 and NIH P30 HL 101311-01.
Footnotes
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Battaglia V, Rossi CA, Colombatto S, Grillo MA, Toninello A. Different behavior of agmatine in liver mitochondria: inducer of oxidative stress or scavenger of reactive oxygen species? Biochim Biophys Acta. 2007;1768:1147–1153. doi: 10.1016/j.bbamem.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Baumann S, Sander A, Gurnon JR, Yanai-Balser GM, Van Etten JL, Piotrowski M. Chlorella viruses contain genes encoding a complete polyamine biosynthetic pathway. Virology. 2007;360:209–217. doi: 10.1016/j.virol.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becher A, Schweizer HP. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. Biotechniques. 2000;29:948–950. 952. doi: 10.2144/00295bm04. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, Widdick D, Palmer T, Brunak S. Prediction of twin-arginine signal peptides. BMC Bioinformatics. 2005;6:167. doi: 10.1186/1471-2105-6-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensing BA, Meyer BJ, Dunny GM. Sensitive detection of bacterial transcription initiation sites and differentiation from RNA processing sites in the pheromone-induced plasmid transfer system of Enterococcus faecalis. Proc Natl Acad Sci USA. 1996;93:7794–7799. doi: 10.1073/pnas.93.15.7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyde TR, Rahmatullah M. Optimization of conditions for the colorimetric determination of citrulline, using diacetyl monoxime. Anal Biochem. 1980;107:424–431. doi: 10.1016/0003-2697(80)90404-2. [DOI] [PubMed] [Google Scholar]
- Campisano A, Schroeder C, Schemionek M, Overhage J, Rehm BH. PslD is a secreted protein required for biofilm formation by Pseudomonas aeruginosa. Appl Environ Microbiol. 2006;72:3066–3068. doi: 10.1128/AEM.72.4.3066-3068.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KH, Kumar A, Schweizer HP. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods. 2006;64:391–397. doi: 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- D’Argenio DA, Wu M, Hoffman LR, Kulasekara HD, Deziel E, Smith EE, et al. Growth phenotypes of Pseudomonas aeruginosa lasR mutants adapted to the airways of cystic fibrosis patients. Mol Microbiol. 2007;64:512–533. doi: 10.1111/j.1365-2958.2007.05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JC. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev. 2002;3:128–134. doi: 10.1016/s1526-0550(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Demady DR, Jianmongkol S, Vuletich JL, Bender AT, Osawa Y. Agmatine enhances the NADPH oxidase activity of neuronal NO synthase and leads to oxidative inactivation of the enzyme. Mol Pharmacol. 2001;59:24–29. doi: 10.1124/mol.59.1.24. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- Friedman L, Kolter R. Genes involved in matrix formation in Pseudomonas aeruginosa PA14 biofilms. Mol Microbiol. 2004;51:675–690. doi: 10.1046/j.1365-2958.2003.03877.x. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Masuda N, Takenouchi T, Sekine N, Iijima M, Ohya S. Increase in susceptibility of Pseudomonas aeruginosa to carbapenem antibiotics in low-amino-acid media. Antimicrob Agents Chemother. 1991;35:529–532. doi: 10.1128/aac.35.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Hoang TT, Kutchma AJ, Becher A, Schweizer HP. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid. 2000;43:59–72. doi: 10.1006/plas.1999.1441. [DOI] [PubMed] [Google Scholar]
- Kwon DH, Lu CD. Polyamine effects on antibiotic susceptibility in bacteria. Antimicrob Agents Chemother. 2007;51:2070–2077. doi: 10.1128/AAC.01472-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PA, Tullman-Ercek D, Georgiou G. The bacterial twin-arginine translocation pathway. Annu Rev Microbiol. 2006;60:373–395. doi: 10.1146/annurev.micro.60.080805.142212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, et al. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci USA. 2006;103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llacer JL, Polo LM, Tavarez S, Alarcon B, Hilario R, Rubio V. The gene cluster for agmatine catabolism of Enterococcus faecalis: study of recombinant putrescine transcarbamylase and agmatine deiminase and a snapshot of agmatine deiminase catalyzing its reaction. J Bacteriol. 2007;189:1254–1265. doi: 10.1128/JB.01216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CD, Itoh Y, Nakada Y, Jiang Y. Functional analysis and regulation of the divergent spuABCDEFGH-spuI operons for polyamine uptake and utilization in Pseudomonas aeruginosa PAO1. J Bacteriol. 2002;184:3765–3773. doi: 10.1128/JB.184.14.3765-3773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Li L, Wu R, Feng X, Li Z, Yang H, et al. Kinetic analysis of Pseudomonas aeruginosa arginine deiminase mutants and alternate substrates provides insight into structural determinants of function. Biochemistry. 2006;45:1162–1172. doi: 10.1021/bi051591e. [DOI] [PubMed] [Google Scholar]
- Lucas PM, Blancato VS, Claisse O, Magni C, Lolkema JS, Lonvaud-Funel A. Agmatine deiminase pathway genes in Lactobacillus brevis are linked to the tyrosine decarboxylation operon in a putative acid resistance locus. Microbiology. 2007;153:2221–2230. doi: 10.1099/mic.0.2007/006320-0. [DOI] [PubMed] [Google Scholar]
- Maeda T, Wakasawa T, Shima Y, Tsuboi I, Aizawa S, Tamai I. Role of polyamines derived from arginine in differentiation and proliferation of human blood cells. Biol Pharm Bull. 2006;29:234–239. doi: 10.1248/bpb.29.234. [DOI] [PubMed] [Google Scholar]
- Mah TF, Pitts B, Pellock B, Walker GC, Stewart PS, O’Toole GA. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature. 2003;426:306–310. doi: 10.1038/nature02122. [DOI] [PubMed] [Google Scholar]
- Majumder S, Wirth JJ, Bitonti AJ, McCann PP, Kierszenbaum F. Biochemical evidence for the presence of arginine decarboxylase activity in Trypanosoma cruzi. J Parasitol. 1992;78:371–374. [PubMed] [Google Scholar]
- Mercenier A, Simon JP, Haas D, Stalon V. Catabolism of l-arginine by Pseudomonas aeruginosa. J Gen Microbiol. 1980;116:381–389. doi: 10.1099/00221287-116-2-381. [DOI] [PubMed] [Google Scholar]
- Minic Z, Herve G. Arginine metabolism in the deep sea tube worm Riftia pachyptila and its bacterial endosymbiont. J Biol Chem. 2003;278:40527–40533. doi: 10.1074/jbc.M307835200. [DOI] [PubMed] [Google Scholar]
- Nakada Y, Itoh Y. Identification of the putrescine biosynthetic genes in Pseudomonas aeruginosa and characterization of agmatine deiminase and N-carbamoylputrescine amidohydrolase of the arginine decarboxylase pathway. Microbiology. 2003;149:707–714. doi: 10.1099/mic.0.26009-0. [DOI] [PubMed] [Google Scholar]
- Nakada Y, Jiang Y, Nishijyo T, Itoh Y, Lu CD. Molecular characterization and regulation of the aguBA operon, responsible for agmatine utilization in Pseudomonas aeruginosa PAO1. J Bacteriol. 2001;183:6517–6524. doi: 10.1128/JB.183.22.6517-6524.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner UA, Snyder A, Vasil AI, Vasil ML. Effects of the twin-arginine translocase on secretion of virulence factors, stress response, and pathogenesis. Proc Natl Acad Sci USA. 2002;99:8312–8317. doi: 10.1073/pnas.082238299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- Patel CN, Wortham BW, Lines JL, Fetherston JD, Perry RD, Oliveira MA. Polyamines are essential for the formation of plague biofilm. J Bacteriol. 2006;188:2355–2363. doi: 10.1128/JB.188.7.2355-2363.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raasch W, Regunathan S, Li G, Reis DJ. Agmatine, the bacterial amine, is widely distributed in mammalian tissues. Life Sci. 1995;56:2319–2330. doi: 10.1016/0024-3205(95)00226-v. [DOI] [PubMed] [Google Scholar]
- Reese MG. Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput Chem. 2001;26:51–56. doi: 10.1016/s0097-8485(01)00099-7. [DOI] [PubMed] [Google Scholar]
- Regunathan S, Piletz JE. Regulation of inducible nitric oxide synthase and agmatine synthesis in macrophages and astrocytes. Ann N Y Acad Sci. 2003;1009:20–29. doi: 10.1196/annals.1304.002. [DOI] [PubMed] [Google Scholar]
- Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med. 1999;27:887–892. doi: 10.1097/00003246-199905000-00020. [DOI] [PubMed] [Google Scholar]
- Sakuragi Y, Kolter R. Quorum-sensing regulation of the biofilm matrix genes (pel) of Pseudomonas aeruginosa. J Bacteriol. 2007;189:5383–5386. doi: 10.1128/JB.00137-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M, Rodriguez R, Lopez N, Uribe E, Lopez V, Carvajal N. Insights into the reaction mechanism of Escherichia coli agmatinase by site-directed mutagenesis and molecular modelling. Eur J Biochem. 2002;269:5522–5526. doi: 10.1046/j.1432-1033.2002.03255.x. [DOI] [PubMed] [Google Scholar]
- Schuster M, Hawkins AC, Harwood CS, Greenberg EP. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol Microbiol. 2004;51:973–985. doi: 10.1046/j.1365-2958.2003.03886.x. [DOI] [PubMed] [Google Scholar]
- Shirai H, Blundell TL, Mizuguchi K. A novel superfamily of enzymes that catalyze the modification of guanidino groups. Trends Biochem Sci. 2001;26:465–468. doi: 10.1016/s0968-0004(01)01906-5. [DOI] [PubMed] [Google Scholar]
- Shirai H, Mokrab Y, Mizuguchi K. The guanidino-group modifying enzymes: structural basis for their diversity and commonality. Proteins. 2006;64:1010–1023. doi: 10.1002/prot.20863. [DOI] [PubMed] [Google Scholar]
- Snyder A, Vasil AI, Zajdowicz SL, Wilson ZR, Vasil ML. Role of the Pseudomonas aeruginosa PlcH Tat signal peptide in protein secretion, transcription, and cross-species Tat secretion system compatibility. J Bacteriol. 2006;188:1762–1774. doi: 10.1128/JB.188.5.1762-1774.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, et al. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Tremblay J, Deziel E. Improving the reproducibility of Pseudomonas aeruginosa swarming motility assays. J Basic Microbiol. 2008;48:509–515. doi: 10.1002/jobm.200800030. [DOI] [PubMed] [Google Scholar]
- Tricot C, Pierard A, Stalon V. Comparative studies on the degradation of guanidino and ureido compounds by Pseudomonas. J Gen Microbiol. 1990;136:2307–2317. doi: 10.1099/00221287-136-11-2307. [DOI] [PubMed] [Google Scholar]
- Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, Greenberg EP. Gene expression in Pseudomonas aeruginosa biofilms. Nature. 2001;413:860–864. doi: 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- Wozniak DJ, Wyckoff TJ, Starkey M, Keyser R, Azadi P, O’Toole GA, Parsek MR. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc Natl Acad Sci USA. 2003;100:7907–7912. doi: 10.1073/pnas.1231792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H. Agmatine deiminase from maize shoots: purification and properties. Phytochemistry. 2001;56:643–647. doi: 10.1016/s0031-9422(00)00491-x. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bremer H. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J Biol Chem. 1995;270:11181–11189. doi: 10.1074/jbc.270.19.11181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.