Abstract
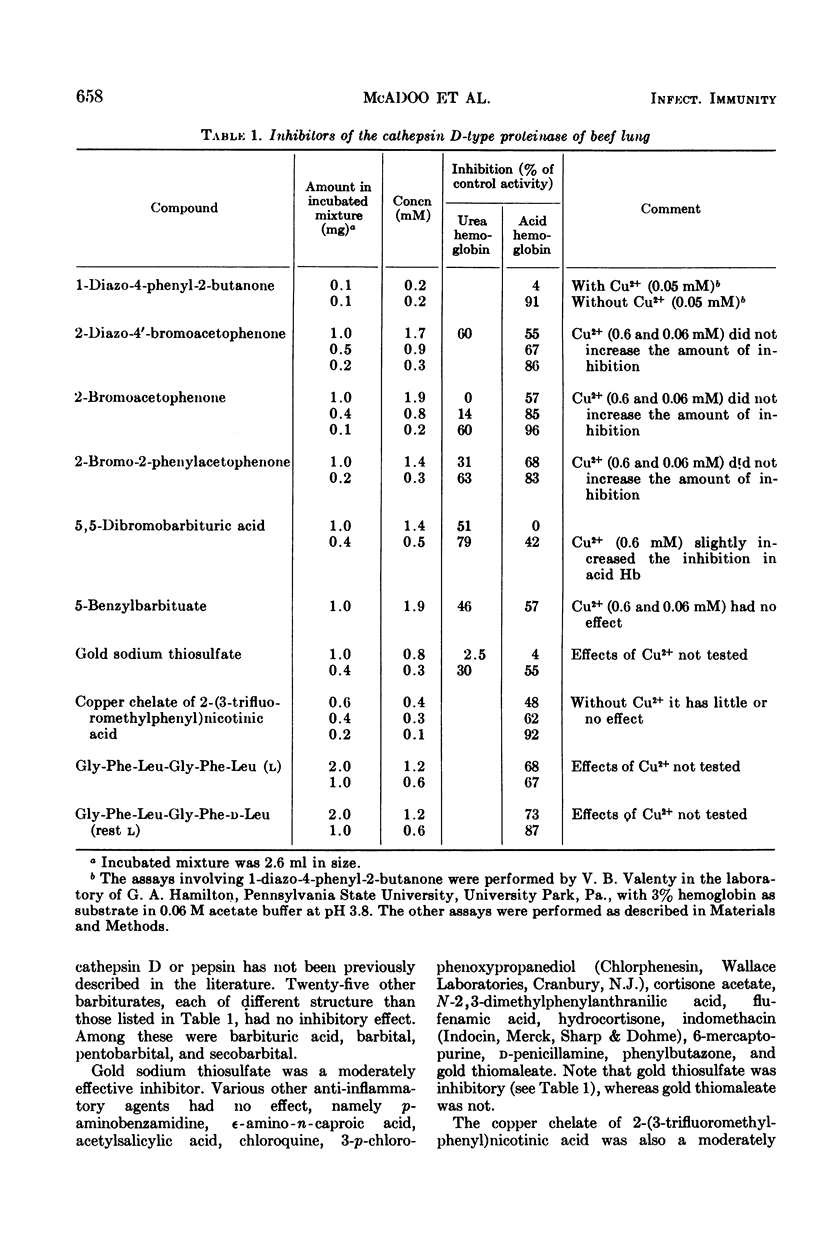
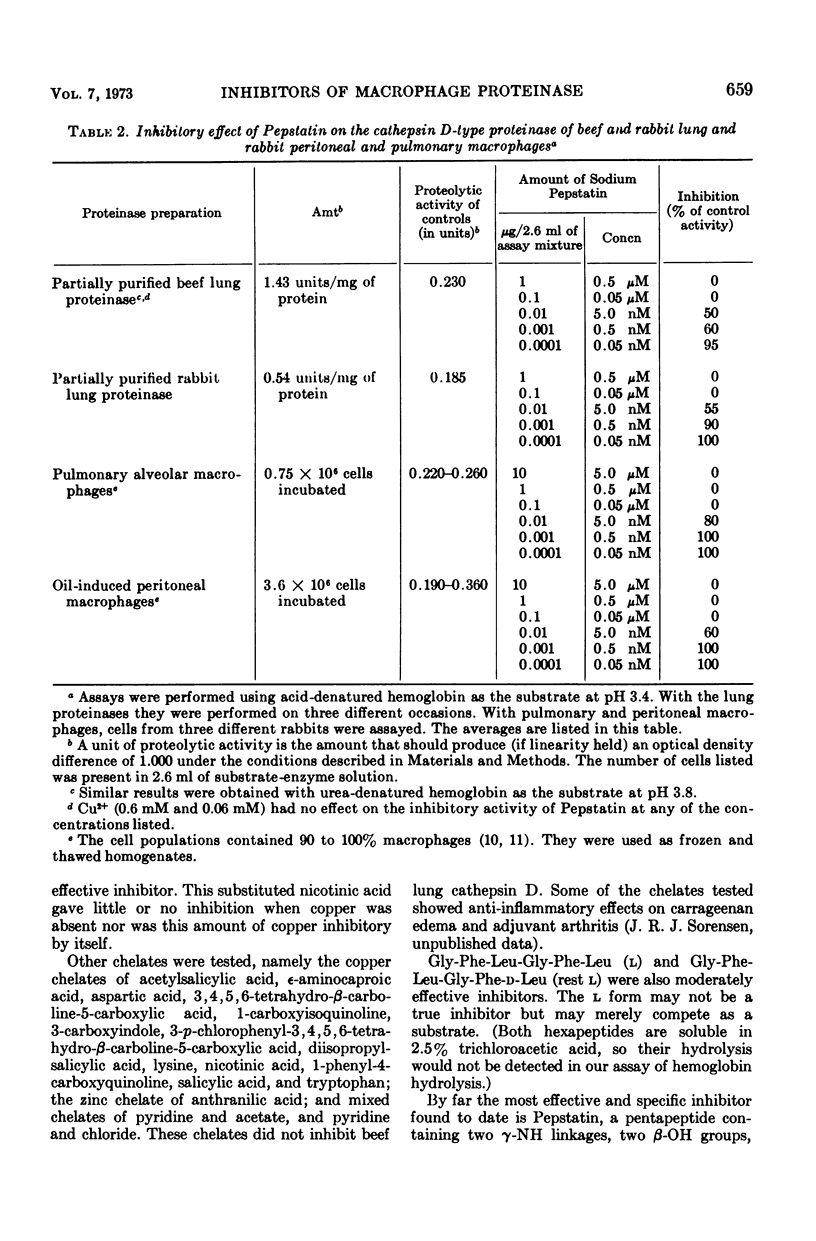
The macrophage is the main cell participating in chronic inflammation. It contains an acid-acting, cathepsin D-type proteinase with the specificity of pepsin, which may release mediators of the inflammatory process. To find new pharmaceutical inhibitors of this proteinase, we tested a variety of chemical compounds in vitro. For this survey, the possible inhibitor (at a concentration of 0.4 mg/ml) was assayed with partially purified cathepsin D-type proteinase from beef lung (a macrophage-rich tissue) and hemoglobin as the substrate. Diazophenylbutanone, three acetophenones, two barbiturates, a gold salt, a copper chelate of a substituted nicotinic acid, a hexapeptide containing a d-amino acid, and Pepstatin inhibited this enzyme; over 200 other potential inhibitors did not. By far the most active and specific inhibitor found to date is Pepstatin, a pentapeptide with two γ-NH linkages, two β-OH groups, and five branched aliphatic side chains. Banyu Pharmaceutical Co., Tokyo, Japan, produces this nontoxic compound for the treatment of peptic ulcers. In vitro, as little as 4 ng of Pepstatin inhibits the acid-acting cathepsin D-type proteinase purified from beef and rabbit lung as well as the similar proteinase of rabbit peritoneal and pulmonary macrophages.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyagi T., Kunimoto S., Morishima H., Takeuchi T., Umezawa H. Effect of pepstatin on acid proteases. J Antibiot (Tokyo) 1971 Oct;24(10):687–694. doi: 10.7164/antibiotics.24.687. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Dingle J. T. The inhibition of tissue acid proteinases by pepstatin. Biochem J. 1972 Apr;127(2):439–441. doi: 10.1042/bj1270439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Lysosomal acid proteinase of rabbit liver. Biochem J. 1967 Aug;104(2):601–608. doi: 10.1042/bj1040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss R. S., Knowles J. R., Wybrandt G. B. An aspartic acid residue at the active site of pepsin. The isolation and sequence of the heptapeptide. Biochem J. 1969 Jun;113(2):377–386. doi: 10.1042/bj1130377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheret A. M., Bonfils S. Pepsine et pepsinogène. Origine, propriétés, préparation. Pathol Biol (Paris) 1970 Mar;18(5):317–342. [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BENNETT W. E. HYDROLYTIC ENZYMES OF RABBIT MONONUCLEAR EXUDATE CELLS. I. QUANTITATIVE ASSAY AND PROPERTIES OF CERTAIN PROTEASES, NON-SPECIFIC ESTERASES, AND LIPASES OF MONONUCLEAR AND POLYMORPHONUCLEAR CELLS AND ERYTHROCYTES. J Cell Biol. 1964 Apr;21:1–13. doi: 10.1083/jcb.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BURSTONE M. S., WALTER P. C., KINSLEY J. W. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963 Jun;17:465–486. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, SMITH E. L. Action of proteinase I of bovine lung; hydrolysis of the oxidized B chain of insulin; polymer formation from amino acid esters. J Biol Chem. 1955 Jul;215(1):55–66. [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, SMITH E. L. Proteolytic enzymes of lung. J Biol Chem. 1955 Jul;215(1):45–54. [PubMed] [Google Scholar]
- Delpierre G. R., Fruton J. S. Inactivation of pepsin by diphenyldiazomethane. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1161–1167. doi: 10.1073/pnas.54.4.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre G. R., Fruton J. S. Specific inactivation of pepsin by a diazo ketone. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1817–1822. doi: 10.1073/pnas.56.6.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Poole A. R., Stovin P. Inhibition by pepstatin of human cartilage degradation. Biochem J. 1972 Apr;127(2):443–444. doi: 10.1042/bj1270443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingle J. T., Barrett A. J., Weston P. D. Cathepsin D. Characteristics of immunoinhibition and the confirmation of a role in cartilage breakdown. Biochem J. 1971 Jun;123(1):1–13. doi: 10.1042/bj1230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlanger B. F., Vratsanos S. M., Wassermann N., Cooper A. G. Stereochemical investigation of the active center of pepsin using a new inactivator. Biochem Biophys Res Commun. 1967 Jul 21;28(2):203–208. doi: 10.1016/0006-291x(67)90430-5. [DOI] [PubMed] [Google Scholar]
- Fruton J. S. The specificity and mechanism of pepsin action. Adv Enzymol Relat Areas Mol Biol. 1970;33:401–443. doi: 10.1002/9780470122785.ch9. [DOI] [PubMed] [Google Scholar]
- Fry K. T., Kim O. K., Kettering C. F., Spona J., Hamilton G. A. A reactive aspartyl residue of pepsin. Biochem Biophys Res Commun. 1968 Mar 12;30(5):489–495. doi: 10.1016/0006-291x(68)90078-8. [DOI] [PubMed] [Google Scholar]
- Greenwell P., Knowles J. R., Sharp H. The inhibition of pepsin-catalysed reactions by products and product analogues. Kinetic evidence for ordered release of products. Biochem J. 1969 Jun;113(2):363–368. doi: 10.1042/bj1130363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E., Morell J. L. Evidence for an active carboxyl group in pepsin. J Biol Chem. 1966 Aug 10;241(15):3638–3639. [PubMed] [Google Scholar]
- Gross F., Lazar J., Orth H. Inhibition of the renin-angiotensinogen reaction by pepstatin. Science. 1972 Feb 11;175(4022):656–656. doi: 10.1126/science.175.4022.656. [DOI] [PubMed] [Google Scholar]
- HERRIOTT R. M. Pepsinogen and pepsin. J Gen Physiol. 1962 Mar;45(4):57–76. [PMC free article] [PubMed] [Google Scholar]
- HERRIOTT R. M., VAN VUNAKIS H. Structural changes associated with the conversion of pepsinogen to pepsin. I. The N-terminal amino acid residue and amino acid composition of the pepsin inhibitor. Biochim Biophys Acta. 1956 Dec;22(3):537–543. doi: 10.1016/0006-3002(56)90064-6. [DOI] [PubMed] [Google Scholar]
- Hamilton G. A., Spona J., Crowell L. D. The inactivation of pepsin by an equimolar amount of 1-diazo-4-phenylbutanone-2. Biochem Biophys Res Commun. 1967 Jan 23;26(2):193–198. doi: 10.1016/0006-291x(67)90233-1. [DOI] [PubMed] [Google Scholar]
- Hollands T. R., Voynick I. M., Fruton J. S. Action of pepsin on cationic synthetic substrates. Biochemistry. 1969 Feb;8(2):575–585. doi: 10.1021/bi00830a017. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M., Heinze J. E., Mills J. N. Comparative studies on the effect of specific inactivators on human gastricsin and pepsin. Biochemistry. 1970 Jul 7;9(14):2897–2902. doi: 10.1021/bi00816a022. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Ferguson J. B., Fruton J. S. Bifunctional inhibitors of pepsin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2765–2768. doi: 10.1073/pnas.68.11.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa H., Aoyagi T., Takeuchi T., Umezawa H. Effect of protease inhibitors of actinomycetes on lysosomal peptide-hydrolases from swine liver. J Antibiot (Tokyo) 1971 Jul;24(7):488–490. doi: 10.7164/antibiotics.24.488. [DOI] [PubMed] [Google Scholar]
- Inouye K., Fruton J. S. Studies on the specificity of pepsin. Biochemistry. 1967 Jun;6(6):1765–1777. doi: 10.1021/bi00858a027. [DOI] [PubMed] [Google Scholar]
- Inouye K., Fruton J. S. The inhibition of pepsin action. Biochemistry. 1968 May;7(5):1611–1615. doi: 10.1021/bi00845a001. [DOI] [PubMed] [Google Scholar]
- KATCHALSKI E., BERGER A., NEUMANN H. Reversible inhibition of pepsin by polylysine. Nature. 1954 May 22;173(4412):998–999. doi: 10.1038/173998a0. [DOI] [PubMed] [Google Scholar]
- Keilová H., Bláha K., Keil B. Effect of steric factors on digestibility of peptides containing aromatic amino acids by cathepsin D and pepsin. Eur J Biochem. 1968 May;4(4):442–447. doi: 10.1111/j.1432-1033.1968.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Keilová H., Tomásek V. Effect of pepsin inhibitor from Ascaris lumbricoides on cathepsin D and E. Biochim Biophys Acta. 1972 Oct 12;284(2):461–464. doi: 10.1016/0005-2744(72)90143-x. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. On the mechanism of action of pepsin. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):135–146. doi: 10.1098/rstb.1970.0016. [DOI] [PubMed] [Google Scholar]
- Knowles J. R., Sharp H., Greenwell P. The pH-dependence of the binding of competitive inhibitors to pepsin. Biochem J. 1969 Jun;113(2):343–351. doi: 10.1042/bj1130343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto S., Aoyagi T., Morishima H., Takeuchi T., Umezawa H. Mechanism of inhibition of pepsin by pepstatin. J Antibiot (Tokyo) 1972 Apr;25(4):251–255. doi: 10.7164/antibiotics.25.251. [DOI] [PubMed] [Google Scholar]
- Lundblad R. L., Stein W. H. On the reaction of diazoacetyl compounds with pepsin. J Biol Chem. 1969 Jan 10;244(1):154–160. [PubMed] [Google Scholar]
- Lurie M. B., Dannenberg A. M. Macrophage Function in Infectious Disease with Inbred Rabbits. Bacteriol Rev. 1965 Dec;29(4):466–476. doi: 10.1128/br.29.4.466-476.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Miller R. P., Poper C. H., Wilson C. W., DeVito E. Renin inhibition by pepstatin. Biochem Pharmacol. 1972 Nov 1;21(21):2941–2944. doi: 10.1016/0006-2952(72)90221-3. [DOI] [PubMed] [Google Scholar]
- Morishima H., Takita T., Aoyagi T., Takeuchi T., Umezawa H. The structure of pepstatin. J Antibiot (Tokyo) 1970 May;23(5):263–265. doi: 10.7164/antibiotics.23.263. [DOI] [PubMed] [Google Scholar]
- NEUMANN H., LEVIN Y., BERGER A., KATCHALSKI E. Pepsincatalysed transpeptidation of the amino-transfer type. Biochem J. 1959 Sep;73:33–41. doi: 10.1042/bj0730033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong E. B., Perlmann G. E. Specific inactivation of pepsin by benzyloxycarbonyl-L-phenylalanyldiazomethane. Nature. 1967 Sep 30;215(5109):1492–1494. doi: 10.1038/2151492b0. [DOI] [PubMed] [Google Scholar]
- PRESS E. M., PORTER R. R., CEBRA J. The isolation and properties of a proteolytic enzyme, cathepsin D, from bovine spleen. Biochem J. 1960 Mar;74:501–514. doi: 10.1042/bj0740501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Dingle J. T., Barrett A. J. The immunocytochemical demonstration of cathepsin D. J Histochem Cytochem. 1972 Apr;20(4):261–265. doi: 10.1177/20.4.261. [DOI] [PubMed] [Google Scholar]
- Rajagopalan T. G., Stein W. H., Moore S. The inactivation of pepsin by diazoacetylnorleucine methyl ester. J Biol Chem. 1966 Sep 25;241(18):4295–4297. [PubMed] [Google Scholar]
- SCHLAMOWITZ M., VARANDANI P. T., WISSLER F. C. Pepsinogen and pepsin: conformational relations, studied by iodination, immunochemical precipitation, and the influence of pepain inhibitor. Biochemistry. 1963 Mar-Apr;2:238–246. doi: 10.1021/bi00902a006. [DOI] [PubMed] [Google Scholar]
- Schlamowitz M., Shaw A., Jackson W. T. The nature of the binding of inhibitors to pepsin and the kinetics of inhibited peptic hydrolysis of N-acetyl-L-phenylalanyl-L-tyrosine. J Biol Chem. 1968 May 25;243(10):2821–2828. [PubMed] [Google Scholar]
- Shin H. S., Snyderman R., Friedman E., Mellors A., Mayer M. M. Chemotactic and anaphylatoxic fragment cleaved from the fifth component of guinea pig complement. Science. 1968 Oct 18;162(3851):361–363. doi: 10.1126/science.162.3851.361. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Shin H. S., Hausman M. H. A chemotactic factor for mononuclear leukocytes. Proc Soc Exp Biol Med. 1971 Nov;138(2):387–390. doi: 10.3181/00379727-138-35903. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Shin H., Dannenberg A. M., Jr Macrophage proteinase and inflammation: the production of chemotactic activity from the fifth complement by macrophage proteinase. J Immunol. 1972 Oct;109(4):896–898. [PubMed] [Google Scholar]
- Stepanov V. M., Lobareva L. S., Mal'tsev N. I. Coloured inhibitors of pepsin. Biochim Biophys Acta. 1968 Mar 25;151(3):719–721. doi: 10.1016/0005-2744(68)90030-2. [DOI] [PubMed] [Google Scholar]
- Stepanov V. M., Vaganova T. I. Identification of the carboxyl group of pepsin reacting with diazoacetamide derivatives. Biochem Biophys Res Commun. 1968 Jun 10;31(5):825–830. doi: 10.1016/0006-291x(68)90637-2. [DOI] [PubMed] [Google Scholar]
- Suda H., Aoyagi T., Hamada M., Takeuchi T., Umezawa H. Antipain, a new protease inhibitor isolated from actinomycetes. J Antibiot (Tokyo) 1972 Apr;25(4):263–266. doi: 10.7164/antibiotics.25.263. [DOI] [PubMed] [Google Scholar]
- TANG J. SPECIFICITY OF PEPSIN AND ITS DEPENDENCE ON A POSSIBLE 'HYDROPHOBICBINDING SITE'. Nature. 1963 Sep 14;199:1094–1095. doi: 10.1038/1991094a0. [DOI] [PubMed] [Google Scholar]
- Tappel A. L., Dillard C. J. Retinol inhibition of some proteolytic enzymes. Lipids. 1968 May;3(3):221–224. doi: 10.1007/BF02531190. [DOI] [PubMed] [Google Scholar]
- Trout G. E., Fruton J. S. The side-chain specificity of pepsin. Biochemistry. 1969 Oct;8(10):4183–4190. doi: 10.1021/bi00838a041. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Aoyagi T., Morishima H., Matsuzaki M., Hamada M. Pepstatin, a new pepsin inhibitor produced by Actinomycetes. J Antibiot (Tokyo) 1970 May;23(5):259–262. doi: 10.7164/antibiotics.23.259. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Newman L. J. A neutrophil chemotactic factor from human C'5. J Immunol. 1969 Jan;102(1):93–99. [PubMed] [Google Scholar]
- Weston P. D. A specific antiserum to lysosomal cathepsin D. Immunology. 1969 Sep;17(3):421–428. [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Pepstatin inhibits the digestion of hemoglobin and protein-polysaccharide complex by cathepsin D. Biochem Biophys Res Commun. 1972 May 26;47(4):965–970. doi: 10.1016/0006-291x(72)90587-6. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr, Shamberger R. J., Jr Purification and properties of cathepsin D from bovine utrus. J Biol Chem. 1971 Apr 10;246(7):1951–1960. [PubMed] [Google Scholar]


