Abstract
Objectives
Asbestos describes a group of naturally occurring silicate mineral fibers that were widely used in industry during the 20th century due to their desirable physical properties. Although use in the United States has fallen over the last three decades, significant exposure in the developing world continues and the burden of disease is considerable. Asbestos is a known risk factor for several malignant diseases, including lung cancer and mesothelioma, and has more recently been implicated in pharyngeal and laryngeal cancer. However, studies of asbestos and cancers of the larynx or pharynx with adequate sample-size that control for major head and neck squamous cell carcinoma (HNSCC) risk factors remain relatively sparse.
Methods
We report findings from a case-control study of 674 incident male HNSCC cases from the greater Boston region and 857 population-based male controls, matched on age (+/− 3 years), sex, and town or neighborhood of residence. Multivariable logistic regression was used to assess the association between occupational asbestos exposure and HNSCC by primary tumor site.
Results
A total of 190 cases (28.2%) and 203 controls (23.7%) reported an occupational exposure to asbestos. Occupational asbestos exposure was associated with an elevated risk of pharyngeal carcinoma in men (OR = 1.41, 95% CI: 1.01–1.97), adjusted for age, race, smoking, alcohol consumption, education, income, and HPV16 serology, with borderline increasing risk for each decade at the exposed occupation (OR = 1.10, 95% CI: 0.99–1.23).
Conclusion
These observations are consistent with the mounting evidence that asbestos is a risk factor for pharyngeal cancer.
Keywords: HNSCC, head and neck cancer, epidemiology, RERI
INTRODUCTION
Asbestos describes a group of naturally occurring fibrous silicate mineral compounds. Its use dates back thousands of years but became wide-spread in the late 19th century through the late 20th century1 due to its favorable industrial properties, including its strength, flexibility and thermal properties, with peak use in the United States occurring in the 1970s. Asbestos has been associated with a number of chronic respiratory diseases2, including malignancy, and was first linked to mesothelioma3 and lung cancer4 in the early to mid part of the 20th century. Despite the known health risks, an estimated 125 million people are still occupationally exposed worldwide5. While asbestos is tightly regulated or banned in parts of the developed world, its unregulated use continues in many developing countries, with an annual global production currently estimated at 2 million metric tons6.
Asbestos has also been implicated as a risk factor for squamous cancers of the upper airway, in particular laryngeal and pharyngeal carcinoma, as indicated by recent meta-analyses7,8. While the association between asbestos exposure and laryngeal cancer is now fairly well established, the data associating pharyngeal cancer with asbestos exposure is somewhat sparse. Many, if not most, currently available studies suffer from relatively small sample-size, and proper control of potentially confounding factors has been somewhat inconsistent1,7. A number of cohort studies are available in the literature but generally offer a very limited number of cases with no adjustment for potential confounding. Case-control studies have been more successful in addressing confounding issues but are, to date, relatively few in number, particularly for pharyngeal cancer9–15; only one study presently exists that specifically examined the relationship with oral cancer10. Further, among the studies that do adjust for confounding, none to date have included past exposure to HPV16, a major risk factor for head and neck cancer16,17, in their analyses. These limitations of the current literature, along with the compelling public health implications, indicate a need for continued study of asbestos exposure to enhance the evidence pool and add to the precision of risk estimates for head and neck cancer. Thus, the objective of our study was to assess the risk of head and neck cancer associated with occupational asbestos exposure in a large, well-controlled, population-based case-control study of men from the greater Boston area.
METHODS
Study population
Incident cases of head and neck squamous cell carcinoma (HNSCC; ICD-9 codes 141, 143–146, 148, 149, 161) were enrolled through major teaching hospitals located in Boston, Massachusetts (Brigham and Women's Hospital, Beth Israel Deaconess Medical Center, Boston Medical Center, Dana-Farber Cancer Institute, Massachusetts Eye and Ear Infirmary, Massachusetts General Hospital, and New England Medical Center; which together see the vast majority of HNSCC cases in the region) as part of a population-based case-control study of head and neck cancer in the greater-Boston area18,19. For inclusion in the study, cases were required to reside in Boston or any of 162 contiguous cities and towns within approximately one hour drive from Boston at the time of diagnosis. Control subjects with no prior history of HNSCC were matched to each index case on age (within +/− 3 years), gender, and neighborhood/town of residence (determined by zip code) through town records (Massachusetts towns/municipalities maintain publicly available annual residential records). Due to minimal occupational asbestos exposure among female participants, we have restricted the present interrogation to male cases and controls. The study includes data collected from two periods of recruitment from the same population: Phase I was conducted between December 1999 and December 2003 (381 male cases and 493 male controls) and Phase II was conducted between October 2006 and June 2011 (373 male cases and 420 male controls). Men who did not provide a response for occupational asbestos exposure in the questionnaire were excluded (80 cases and 56 controls), leaving 674 cases and 857 controls for analysis. Participation rates for cases and controls were 78% and 47%, respectively. All cases and controls enrolled in the study provided written informed consent as approved by the Institutional Review Boards of the participating institutions.
Data collection
Subjects completed a self-administered, interviewer reviewed questionnaire that provided detailed data on sociodemographics and personal characteristics, alcohol and tobacco use, personal and family cancer history, occupational history of asbestos exposure, and other relevant dietary, health behavior, occupational, residential and medical history. In depth occupational history was collected for each different occupation held by each study subject, including start and end dates (available for 99.5% of subjects reporting occupational asbestos exposure), job titles and industries, and self-reported exposure to occupational dusts, exhausts/fumes, and asbestos. Duration of work in an asbestos-exposed occupation was calculated for each subject by subtracting the start from end date for each occupation for which asbestos exposure was reported and summing up the total duration across all occupations.
HPV16 serology
Serologic HPV16 testing for L1 viral protein antibodies was performed on study subjects as a measure of past HPV16 exposure. Sandwich ELISA assays were used for detection of HPV16 antibodies as previously described20,21. Positive HPV16 L1 serology is considered to be a proxy for past exposure to the virus.
Statistical analysis
Crude odds ratios (ORs) were generated for the association between each covariate and case-status (adjusted for age, which was a matching factor). Univariate statistics for normally distributed continuous covariates (i.e. age) were assessed by two-way ANOVA for differences by primary tumor site among cases and by the non-parametric Kruskal-Wallis test when not normally distributed, with normality determined by the Skewness-Kurtosis test22. Categorical differences by site were assessed by Fisher’s exact test. All tests were 2-sided and significance was considered where p ≤ 0.05.
Unconditional multivariable logistic regression was applied to estimate HNSCC risk for each respective primary tumor site (i.e. oral cavity, pharynx, larynx) associated with self-reported occupational asbestos exposure, adjusted for age (continuous, centered at the median), race (White vs. non-White), cigarette smoking (modeled both as a binary ever/never smoking term and continuously as pack-years, considered additively), alcohol consumption (categorized as: non-drinker, ≤ 14 drinks/week, and > 14 drinks/week), highest level of education (high school or less vs. greater than high school), annual household income (categorized as: < $25,000, $25,000–$79,999, and ≥ $80,000) and HPV16 L1 serology (negative vs. positive). For the purpose of quantifying alcohol consumption, an alcoholic beverage was defined as a 12 oz. beer, 5 oz. glass of wine, or 1.5 oz. of liquor. Occupational asbestos exposure, the primary independent variable in these analyses, was separately modeled as both a binary variable (ever vs. never occupationally exposed) and continuously, by total years at an occupation(s) with asbestos exposure. Functional form of continuous covariates included in the model was assessed in the multivariable setting using fractional polynomial analysis23, where any polynomial term offering a significantly improved fit over the linear term considered (p ≤ 0.05).
There were missing values for race (1 case, 1 control), alcohol consumption (2 cases, 2 controls), education (1 control), annual household income (81 cases, 92 controls) and HPV16 L1 serology (106 cases, 77 controls); data were complete for age and smoking. To compensate for the missing values in the logistic regression models, multiple imputation (5 imputations) was employed using multivariate normal regression, based on age and smoking data (ever/never + pack-years); multiple imputation results in less biased findings when dealing with missing covariate data24.
To explore the possibility of biological interaction between major HNSCC risk factors, we generated joint effects models for heavy smoking and/or drinking with asbestos and HPV16 serology with asbestos and then estimated the relative excess risk due to interaction (RERI), a measure of biological interaction as determined by departure from additivity25 ; separate models including multiplicative terms between history of occupational asbestos exposure and heavy smoking/drinking and HPV16 L1 serology were also generated to assess potential multiplicative interaction. The joint-effects models generated for the RERI estimates exclude subjects missing the covariate data included in the models. A very high number of imputations (i.e. in the hundreds) are required to obtain reliable variance-covariance matrices required for the RERI calculations, making it computationally prohibitive. For the purpose of the joint-effect models, heavy drinking was defined as consumption of more than 14 alcoholic beverages in a typical week. Heavy smokers were defined as subjects smoking more than 18.3 total pack-years; this cut-point was based on the top two tertiles of pack-years among ever-smoking control subjects.
Confidence intervals for the RERI analyses were estimated using the biological interaction tool available through EpiNET (http://www.epinet.se). All other statistical analyses were conducted in Stata 11 (College Station, TX).
RESULTS
A total of 190 cases (28.2%) and 203 controls (23.7%) reported an occupational exposure to asbestos. Cases were more likely than controls to be ever-smokers and smoked more, were more likely to be heavy drinkers, were less educated, had a lower annual household income, and were more likely to have positive HPV16 L1 serology. Among cases, there were significant differences across primary tumor sites (oral cavity, pharynx, and larynx) by smoking habit (p < 0.001), alcohol consumption (p = 0.003), education level (p = 0.01), annual household income (p < 0.001), and HPV16 serology (p < 0.001). A detailed description of the study population is provided in Table 1. Study subjects missing occupational asbestos data did not significantly differ from those reporting this data with respect to the covariates considered in Table 1 (data not shown).
Table 1.
Description of the study population by case-control status and primary tumor site.
| HNSCC by Site | |||||||
|---|---|---|---|---|---|---|---|
| Controls (n= 857) |
HNSCC (n= 674) |
Crude ORa (95% CI) |
Oral Cavity (n = 223) |
Pharynx (n = 333) |
Larynx (n = 118) |
pdifference | |
| Age, mean years (σ) | 61.0 (10.5) | 59.1 (10.5) | --- | 59.0 (12.3) | 58.4 (9.3) | 61.1 (9.8) | 0.06b |
| Race, n (%) | |||||||
| White | 783 (91.5%) | 612 (90.9%) | reference | 198 (88.8%) | 308 (92.8%) | 106 (89.8%) | 0.23c |
| Non-White | 73 (8.5%) | 61 (9.1%) | 1.07 (0.75–1.53) | 25 (11.2%) | 24 (7.2%) | 12 (10.2%) | |
| Cigarette smoking | |||||||
| Never-smoker, n (%) | 328 (38.3%) | 149 (22.1%) | reference | 50 (22.4%) | 91 (27.3%) | 8 (6.8%) | < 0.001c |
| Ever-smoker, n (%) | 529 (61.7%) | 525 (77.9%) | 2.40 (1.90–3.03) | 173 (77.6%) | 242 (72.7%) | 110 (93.2%) | |
| Pack-years, median (range) | 28.5 (0.1–200) | 36.0 (0.2–203) | 1.02 (1.01–1.02) | 36.8 (0.2–147) | 32.0 (0.4–203) | 45 (0.2–150) | < 0.001d |
| Alcohol consumption, n (%) | |||||||
| Non-drinker | 74 (8.7%) | 38 (5.7%) | reference | 15 (6.8%) | 18 (5.4%) | 5 (4.2%) | 0.003c |
| ≤ 2 drinks per day | 518 (60.6%) | 268 (39.9%) | 1.02 (0.67–1.55) | 80 (36.2%) | 155 (46.6%) | 33 (28.0%) | |
| > 2 drinks per day | 263 (30.8%) | 366 (54.5%) | 2.75 (1.80–4.20) | 126 (57.0%) | 160 (48.1%) | 80 (67.8%) | |
| Highest level of education, n (%) | |||||||
| High school or less | 225 (26.3%) | 273 (40.5%) | reference | 104 (46.6%) | 111 (33.3%) | 58 (49.2%) | 0.01c |
| Greater than high school | 631 (73.7%) | 401 (59.5%) | 0.51 (0.41–0.64) | 119 (53.4%) | 222 (66.7%) | 60 (50.9%) | |
| Annual household income, n (%) | |||||||
| < $25,000 | 121 (15.6%) | 135 (23.2%) | reference | 49 (26.1%) | 58 (19.5%) | 28 (28.9%) | < 0.001c |
| $25,000 – $79,999 | 323 (41.6%) | 230 (39.5%) | 0.61 (0.45–0.82) | 89 (47.3%) | 98 (33.0%) | 43 (44.3%) | |
| ≥ $80,000 | 332 (42.8%) | 217 (37.3%) | 0.51 (0.38–0.70) | 50 (26.6%) | 141 (47.5%) | 26 (26.8%) | |
| HPV16 L1 serostatus, n (%) | |||||||
| Negative | 704 (93.7%) | 408 (68.3%) | reference | 163 (85.3%) | 158 (51.8%) | 87 (86.1%) | < 0.001c |
| Positive | 47 (6.3%) | 189 (31.7%) | 6.71 (4.76–9.46) | 28 (14.7%) | 147 (48.2%) | 14 (13.9%) | |
Abbreviations: HNSCC = head and neck squamous cell carcinoma; σ = standard deviation
Adjusted for age (matching factor)
t-test for difference across primary tumor sites (cases only)
Fisher's exact test for difference across primary tumor sites (cases only)
Kruskal-Wallis test for difference across primary tumor sites (cases only)
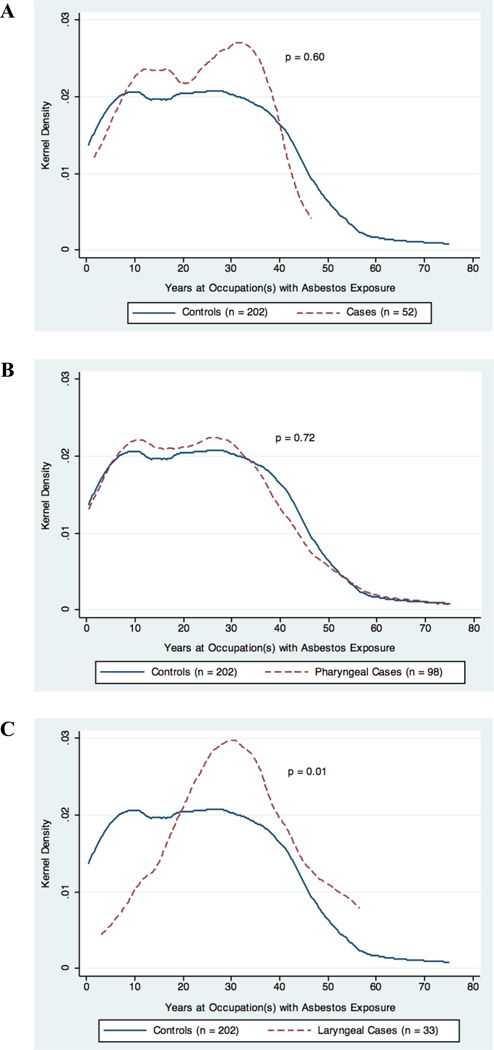
An elevated odds ratio was observed between occupational asbestos exposure and pharyngeal squamous cell carcinoma (OR = 1.41, 95% CI: 1.01–1.97), after adjusting for age, race, smoking, alcohol consumption, education, and HPV16 serology (Table 2). There was also a borderline dose-response observed for each decade working at an occupation with asbestos exposure and pharyngeal carcinoma (OR = 1.10, 95% CI: 0.99–1.23); the distribution of duration of occupational asbestos exposure by case-control status is provided in Figure 1. No significant associations were observed between occupational asbestos exposure and HNSCC originating in the oral cavity or larynx, although sample sizes may have limited our ability to examine these relationships, in particular for laryngeal carcinoma.
Table 2.
Self-reported occupational exposure of men to asbestos and head and neck squamous cell carcinoma (HNSCC) by primary tumor site, for any occupational asbestos exposure and by duration spent at an asbestos-exposed occupation.
| Primary Tumor Site | ||||||
|---|---|---|---|---|---|---|
| Oral Cavity | Pharynx | Larynx | ||||
| Occupational Asbestos Exposure |
ncases/ncontrol | ORa (95% CI) | ncases/ncontrol | ORa (95% CI) | ncases/ncontrol | ORa (95% CI) |
| Never occupationally exposed | 168/654 | reference | 233/654 | reference | 83/654 | reference |
| Ever occupationally exposed | 55/203 | 0.86 (0.59–1.24) | 100/203 | 1.41 (1.01–1.97) | 35/203 | 1.04 (0.64–1.67) |
| Per decade of exposure | 52/202 | 0.92 (0.80–1.05) | 98/202 | 1.10 (0.99–1.23) | 33/202 | 1.07 (0.93–1.24) |
Abbreviations: OR = odds ratio; CI: confidence interval
Adjusted for age, race, smoking, alcohol consumption, education, income, and HPV16 serology
Figure 1.
Distribution of duration at an occupation(s) with asbestos exposure in years by case-control status among study subjects reporting ever having been exposed. The plots represent the kernel density of duration of exposure for controls and A) oral cavity carcinoma cases, B) pharyngeal carcinoma cases, C) and laryngeal carcinoma cases. The p-value for difference between cases and controls was determined by Wilcoxon rank sum test and is presented on each respective corresponding plot.
Since unconditional logistic regression can potentially result in overestimation of the OR, we performed a sensitivity analysis using conditional logistic regression, in which we were able to pair 94% of our cases to controls. The conditional analyses yielded nearly identical point estimates (in fact the magnitude of the point estimate for the association of asbestos exposure and pharyngeal SCC is slightly higher for the conditional model), alleviating concern that estimates were inflated (data not shown).
The models examining asbestos and heavy smoking and/or drinking did not distinguish risk patterns of biological interdependence for any HNSCC site (neither on the additive nor multiplicative scale). The joint effects models and RERI estimates of biological interaction between major risk factors for HNSCC and occupational asbestos exposure are presented in Table 3.
Table 3.
Self-reported occupational exposure of men to asbestos and HNSCC risk stratified by heavy smoking and/or heavy drinking status and by HPV16 serology. The RERI estimates (and corresponding 95% CIs) represent the magnitude and direction of departure from additivity between asbestos and the effect modifier.
| Primary Tumor Site | ||||||
|---|---|---|---|---|---|---|
| Oral Cavity | Pharynx | Larynx | ||||
| Asbestos Exposure by HNSCC Risk Factors | ncases/ncontrols | OR (95% CI) | ncases/ncontrols | OR (95% CI) | ncases/ncontrols | OR (95% CI) |
| Heavy smoking and/or drinkinga,b | ||||||
| Neither | ||||||
| Never occupationally exposed | 35/242 | Reference | 67/242 | Reference | 12/242 | Reference |
| Ever occupationally exposed | 8/65 | 0.78 (0.34–1.80) | 30/65 | 2.01 (1.09–3.71) | 2/65 | 0.59 (0.13–2.71) |
| Either | ||||||
| Never occupationally exposed | 87/275 | 2.30 (1.44–3.67) | 123/275 | 2.52 (1.64–3.87) | 48/275 | 3.24 (1.70–6.57) |
| Ever occupationally exposed | 30/97 | 1.87 (1.04–3.35) | 54/97 | 3.22 (1.89–5.47) | 22/97 | 4.02 (1.86–8.70) |
| RERI = −0.22 (−1.45, 1.02) | RERI = −0.31 (−2.08, 1.45) | RERI= 1.09 (−1.24, 3.43) | ||||
| HPV16 serologyc | ||||||
| Negative (L1, E6 and E7) | ||||||
| Never occupationally exposed | 101/480 | Reference | 93/480 | Reference | 51/480 | Reference |
| Ever occupationally exposed | 35/153 | 0.75 (0.47–1.18) | 49/153 | 1.45 (0.96–2.18) | 20/153 | 0.86 (0.48–1.57) |
| Positive (L1, E6 or E7) | ||||||
| Never occupationally exposed | 20/37 | 2.89 (1.54–5.40) | 37/97 | 16.73 (10.41–26.91) | 9/37 | 2.25 (0.96–5.25) |
| Ever occupationally exposed | 3/9 | 1.73 (0.43–6.97) | 35/9 | 22.21 (9.93–49.68) | 4/9 | 3.99 (1.03–15.41) |
| RERI = −0.90 (−3.87, 2.07) | RERI = 5.03 (−13.20, 23.25) | RERI = 1.89 (−3.68, 7.45) | ||||
Note Joint-effects models for the RERI calculations exclude subjects with any missing covariate data considered in the model. Variance-covariance matrices required for RERI calculations cannot be accurately estimated through multiple imputation in a computationally efficient manner.
Abbreviations HNSCC = head and neck squamous cell carcinoma; OR = odds ratio; CI: confidence interval
Adjusted for age, race, education, income, and HPV16 serostatus
Heavy smoker was defined as the 2nd and 3rd tertiles of pack-years among smokers (> 18.3 pack-years) and heavy drinker was defined as consumption of more than 14 alcoholic drinks per week
Adjusted for age, race, smoking, alcohol consumption, education, and income
DISCUSSION
The International Agency for Research of Cancer considers asbestos to be carcinogenic to humans in all forms1, with a well established link to mesothelioma and cancers of the lung, larynx, and ovary. Although mounting, the evidence is not as clear for an association with pharyngeal cancer and little has been done to examine the risk (if any) that is specifically associated with oral cancer. Here, we bolster this evidence with our observed association between occupational asbestos exposure and pharyngeal squamous cell carcinoma in men from a large, population-based case-control study of the greater Boston area, with an apparent dose-response. Further, to our knowledge, this is the first study of asbestos and head and neck cancer that accounts for past HPV exposure, a major risk factor for the disease, particularly for cancers originating in the pharynx.
Our findings are in-line with other case-control studies in the literature regarding asbestos exposure and pharyngeal cancer risk9,13,15, with point estimates similar in magnitude to the summary estimates reported in recent meta-analyses7,8, although the meta-analyses did not show a clear dose-response8. While we did not observe a significant relationship between asbestos and laryngeal squamous cell carcinoma, we do not believe that this finding draws into question the validity of our pharyngeal cancer results, as it is improbable that differential misclassification or bias would occur for pharyngeal (the lesser established association) and not for laryngeal carcinoma (the better established association). It should be noted that our statistical power to detect such a relationship was limited by our sample-size (118 laryngeal cancers with only 35 men reporting an occupational asbestos exposure) and that our estimated confidence interval for asbestos and laryngeal carcinoma overlaps the summary estimate obtained in a meta-analysis of ever-exposure by the Institute of Medicine (metaOR = 1.43, 95% CI: 1.15–1.78)7. We also observed no association with oral squamous cell carcinoma, which is consistent with the published literature10,26.
Case-control studies, although subject to certain limitations, offer considerable advantages over occupational cohort studies in the assessment of asbestos and head and neck cancer risk. Our use of a case-control study design enabled us to enrich our study population with a large number of cases of head and neck cancer, a relatively rare disease, allowing us to overcome the limitation of a cohort study design, for which few cases would be observed, even for comparatively large cohorts. Additionally, the relatively large sample-size of our study enhanced our ability to detect an association with pharyngeal cancer, while the population-based nature of the study provided us with a generalizable risk estimate. Further, the well-annotated subject-specific data on major risk factors for head and neck cancer, including HPV16 serology, enabled thorough control of potential confounding in the examination of HNSCC risk associated with occupational asbestos exposure. A potential limitation of our study design was the use of self-reported occupational exposure. If the misclassification is non-differential between case and control subjects, we believe that this would result in underestimation of the true risk, as study subjects are more likely to under report asbestos exposure (i.e. not realize that they were exposed). However, we cannot rule out the potential for differential misclassification or recall bias due to the retrospective nature of the case-control study design. We were also limited by our inability to gather direct information on dose and type of asbestos that subjects were exposed to. Nonetheless, we were able to indirectly estimate dosage using duration of work at occupations where asbestos exposure occurred. Despite our inability to discriminate exposure by form of asbestos, it should be noted that IARC considers all types of asbestos to be carcinogenic and, moreover, we would likely have had insufficient statistical power to assess risk associated with individual subtypes. Regardless, future studies should take aim at quantifying the magnitude of risk associated with the various forms of asbestos with respect to pharyngeal and laryngeal cancer.
These observations are consistent with the mounting evidence that asbestos is a risk factor for pharyngeal cancer. Despite its known toxicity, no wholesale ban on asbestos exists, and it is still in use, particularly in developing countries. Continued efforts at studying the health effects of asbestos are still indicated and have clear implications toward policy and risk assessment.
What this paper adds.
There is evidence in the literature of an association between occupational asbestos exposure and pharyngeal and laryngeal cancer, although studies are limited and few adequately control for major risk factors for these diseases, particularly for pharyngeal cancer.
Additional studies are needed to better establish asbestos as a risk factor for pharyngeal and laryngeal cancer and increase the precision of the estimated risk.
We observed an increasedrisk of pharyngeal carcinoma associated with occupational asbestos exposure in a large case-control study of men from the greater Boston area.
This study is the first of its kind to include adjustment for HPV16 exposure, along with control for other major head and neck cancer risk factors.
Our observations are consistent with the mounting evidence that asbestos is a risk factor for pharyngeal cancer and have important implications onoccupational risk assessment and policy.
Acknowledgments
FUNDING SOURCES
This work was supported by the National Cancer Institute [R01CA121147, R01CA100679, and R01CA078609 to K.T.K.]; National Institute of Environmental Health Sciences [T32ES07272 to S.M.L.]; and National Institute of Occupational Safety and Health grant [K01OH009390 to K.M.A].
Footnotes
COMPETING INTERESTS
We have no competing interests to declare.
REFERENCES
- 1.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: International Agency for Research on Cancer (IARC); 2012. Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolyte, and anthophylite) pp. 219–309. [Google Scholar]
- 2.Jamrozik E, de Klerk N, Musk AW. Asbestos-related disease. Intern Med J. 2011;41:372–380. doi: 10.1111/j.1445-5994.2011.02451.x. [DOI] [PubMed] [Google Scholar]
- 3.Gloyne S. Two cases of squamous cell carcinoma of the lung occuring in asbestosis. Tubercle. 1935;17:5–10. [Google Scholar]
- 4.Doll R. Mortality from lung cancer in asbestos workers. Br J Ind Med. 1955;12:81–86. doi: 10.1136/oem.12.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. Asbestos: elimination of asbestos-related diseases. 2010 Fact sheet: http://www.who.int/mediacentre/factsheets/fs343/en/index.html.
- 6.Weiss SH. A call to action: epidemiologists assert themselves with scientific data. Int J Occup Environ Health. 2012;18:167–170. doi: 10.1179/1077352512Z.00000000039. [DOI] [PubMed] [Google Scholar]
- 7.Asbestos: Selected Cancers. Committee on Asbestos: Selected Health Effects: Institute of Medicine of the National Academies. 2006:159–192. [Google Scholar]
- 8.Paget-Bailly S, Cyr D, Luce D. Occupational exposures to asbestos, polycyclic aromatic hydrocarbons and solvents, and cancers of the oral cavity and pharynx: a quantitative literature review. Int Arch Occup Environ Health. 2012;85:341–351. doi: 10.1007/s00420-011-0683-y. [DOI] [PubMed] [Google Scholar]
- 9.Berrino F, Richiardi L, Boffetta P, et al. Occupation and larynx and hypopharynx cancer: a job-exposure matrix approach in an international case-control study in France, Italy, Spain and Switzerland. Cancer Causes Control. 2003;14:213–223. doi: 10.1023/a:1023661206177. [DOI] [PubMed] [Google Scholar]
- 10.Gustavsson P, Jakobsson R, Johansson H, Lewin F, Norell S, Rutkvist LE. Occupational exposures and squamous cell carcinoma of the oral cavity, pharynx, larynx, and oesophagus: a case-control study in Sweden. Occup Environ Med. 1998;55:393–400. doi: 10.1136/oem.55.6.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huebner WW, Schoenberg JB, Kelsey JL, et al. Oral and pharyngeal cancer and occupation: a case-control study. Epidemiology. 1992;3:300–309. doi: 10.1097/00001648-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Luce D, Bugel I, Goldberg P, et al. Environmental exposure to tremolite and respiratory cancer in New Caledonia: a case-control study. Am J Epidemiol. 2000;151:259–265. doi: 10.1093/oxfordjournals.aje.a010201. [DOI] [PubMed] [Google Scholar]
- 13.Marchand JL, Luce D, Leclerc A, et al. Laryngeal and hypopharyngeal cancer and occupational exposure to asbestos and man-made vitreous fibers: results of a case-control study. Am J Ind Med. 2000;37:581–589. doi: 10.1002/(sici)1097-0274(200006)37:6<581::aid-ajim2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Merletti F, Boffetta P, Ferro G, Pisani P, Terracini B. Occupation and cancer of the oral cavity or oropharynx in Turin, Italy. Scand J Work Environ Health. 1991;17:248–254. doi: 10.5271/sjweh.1706. [DOI] [PubMed] [Google Scholar]
- 15.Zheng W, Blot WJ, Shu XO, et al. Risk factors for oral and pharyngeal cancer in Shanghai, with emphasis on diet. Cancer Epidemiol Biomarkers Prev. 1992;1:441–448. [PubMed] [Google Scholar]
- 16.Gillison ML, Alemany L, Snijders PJ, et al. Human papillomavirus and diseases of the upper airway: head and neck cancer and respiratory papillomatosis. Vaccine. 2012;30(Suppl 5):F34–F54. doi: 10.1016/j.vaccine.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 17.Furniss CS, McClean MD, Smith JF, et al. Human papillomavirus 16 and head and neck squamous cell carcinoma. Int J Cancer. 2007;120:2386–2392. doi: 10.1002/ijc.22633. [DOI] [PubMed] [Google Scholar]
- 18.Applebaum KM, McClean MD, Nelson HH, Marsit CJ, Christensen BC, Kelsey KT. Smoking modifies the relationship between XRCC1 haplotypes and HPV16-negative head and neck squamous cell carcinoma. Int J Cancer. 2009;124:2690–2696. doi: 10.1002/ijc.24256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang C, McClean MD, Marsit C, et al. A population-based case-control study of marijuana use and head and neck squamous cell carcinoma. Cancer Prev Res (Phila) 2009;2:759–768. doi: 10.1158/1940-6207.CAPR-09-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meschede W, Zumbach K, Braspenning J, et al. Antibodies against early proteins of human papillomaviruses as diagnostic markers for invasive cervical cancer. J Clin Microbiol. 1998;36:475–480. doi: 10.1128/jcm.36.2.475-480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sehr P, Muller M, Hopfl R, Widschwendter A, Pawlita M. HPV antibody detection by ELISA with capsid protein L1 fused to glutathione S-transferase. J Virol Methods. 2002;106:61–70. doi: 10.1016/s0166-0934(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 22.D'Agostino RB, Belanger A, D'Agostino RBj. A suggestion for using powerful and informative tests of normality. Amer Statistician. 1990;44:316–321. [Google Scholar]
- 23.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S, Finkle WD. A critical look at methods for handling missing covariates in epidemiologic regression analyses. Am J Epidemiol. 1995;142:1255–1264. doi: 10.1093/oxfordjournals.aje.a117592. [DOI] [PubMed] [Google Scholar]
- 25.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 26.Purdue MP, Jarvholm B, Bergdahl IA, Hayes RB, Baris D. Occupational exposures and head and neck cancers among Swedish construction workers. Scand J Work Environ Health. 2006;32:270–275. doi: 10.5271/sjweh.1010. [DOI] [PubMed] [Google Scholar]