Abstract
Purpose
To assess the relationship between donor and recipient factors and corneal allograft rejection in eyes that underwent penetrating keratoplasty (PK) in the Cornea Donor Study.
Methods
1090 subjects undergoing corneal transplantation for a moderate risk condition (principally Fuchs’ dystrophy or pseudophakic corneal edema) were followed for up to 5 years. Associations of baseline recipient and donor factors with the occurrence of a probable or definite rejection event were assessed in univariate and multivariate proportional hazards models.
Results
Eyes with pseudophakic or aphakic corneal edema (N=369) were more likely to experience a rejection event than eyes with Fuchs’ dystrophy (N=676) (34% ± 6% versus 22% ± 4%; hazard ratio = 1.56; 95% confidence interval 1.21 to 2.03). Among eyes with Fuchs’dystrophy, a higher probability of a rejection event was observed in phakic post-transplant eyes compared with eyes that underwent cataract extraction with or without intraocular lens implantation during PK (29% vs. 19%; hazard ratio = 0.54; 95% confidence interval 0.36 to 0.82). Female recipients had a higher probability of a rejection event than males (29% vs. 21%; hazard ratio=1.42; 95% confidence interval 1.08 to 1.87), after controlling for the effect of preoperative diagnosis and lens status. Donor age and donor recipient ABO compatibility were not associated with rejection.
Conclusions
There was a substantially higher graft rejection rate in eyes with pseudophakic or aphakic corneal edema compared with eyes with Fuchs’ dystrophy. Female recipients were more likely to have a rejection event than males. Graft rejection was not associated with donor age.
Keywords: corneal allograft rejection, corneal transplantation, graft failure
Introduction
The Cornea Donor Study (CDS) was initiated for the principal purpose of determining the effect of donor age on the outcome of penetrating keratoplasty in moderate-risk recipients. The primary study led the investigators to conclude that donor age does not influence the 5-year success of corneal transplantation when corneas from donors up to 75 years of age are screened for adequate endothelial cell density.1 The prospective nature of the study and the consistent postoperative follow up of the CDS subjects provide a unique opportunity to evaluate the influence of other donor and recipient factors which may impact the outcome of penetrating keratoplasty. We utilized the CDS dataset to assess the effect of donor and recipient factors on corneal allograft rejection.
Materials and Methods
Study Protocol
Earlier publications1–3 provide a detailed description of the CDS study protocol, including the Specular Microscopy Ancillary Study. Institutional review boards at each participating site approved the study protocol, and written consent was obtained from each subject. Subjects were eligible for the study if they were 40 to 80 years of age and had corneal disease producing endothelial cell dysfunction (primarily Fuchs’ dystrophy and pseudophakic corneal edema). Surgeons reported the primary indication for transplant. Donors were 10 to 75 years of age with an endothelial cell density (ECD) of 2300 to 3300 cells/mm2. Surgeons and subjects were masked to age and ECD of the donors. Corneal tissue assignment was made without regard to age or any other subject characteristics. Preoperative care, surgical technique, and postoperative care (including prescription of medications), were provided according to each surgeon’s customary routine. The number and timing of visits for the first 6 months following penetrating keratoplasty were left to each investigator’s discretion. Thereafter, the minimum follow-up visit schedule included visits at 6 months, 1 year and then annually for 5 years.
Graft clarity was assessed, and signs of graft rejection, if present, were recorded at each follow-up examination. Graft failure was defined as a regraft or loss of central graft clarity sufficient to compromise vision for a minimum of three consecutive months. Graft rejection was classified as definite when an endothelial rejection line was present in a graft that was previously clear and probable when there was inflammation (stromal infiltrate, keratic precipitates, cells in the anterior chamber, or ciliary injection) without an endothelial rejection line in a graft that was previously clear. Treatment of graft rejection was left to the discretion of each investigator.
Statistical Methods
The primary outcome for the analyses was the occurrence of probable or definite graft rejection (irreversible or reversible). There were too few definite rejection events for an analysis of these events alone to be meaningful, so definite and probable events were combined. The lack of a standardized protocol for treatment of rejection episodes made it impossible to determine systematically when one rejection episode ended and the eye became at risk for another episode. Therefore, for purposes of this analysis, eyes were classified as to whether they experienced no rejection or at least one rejection event. Life-table analysis was used to compute the probability of a first rejection event within intervals defined by the study exam schedule (6 months, 1 year and annually during the 5 years of follow up). Data were censored at the time of a non-rejection graft failure or at the last visit. Associations of baseline recipient and donor factors with the occurrence of a rejection event were assessed in univariate and multivariate proportional hazards models. Baseline corneal diagnosis and post-transplant lens status were combined into a single variable because these two parameters were not independent, as pseudophakic/aphakic corneal edema, by definition, could not be associated with a phakic state. Missing covariates were handled by including missing as a separate category for discrete covariates and adding an indicator for a missing value for continuous covariates. The final multivariate model was obtained through forward selection of covariates (p<0.05). The large number of statistical comparisons increases the likelihood of a false positive and no attempt was made to control the overall type I error probability in these exploratory analyses. The impact of a rejection event on graft failure from all causes was assessed by including the rejection event as a time-dependent variable in a proportional hazards regression model. Proportional hazards assumptions were checked using time-dependent variables with logarithmic transformation of time. No significant deviation from the proportional hazards assumption was detected for these models.
All reported p-values are two-sided. Statistical analyses were conducted using SAS version 9.2 software (SAS Institute Inc., Cary, NC).
Results
Subject Characteristics
The mean (±SD) age at the time of transplant of the 1,090 subjects included in this analysis was 70±9 years; 697 (64%) were female and 1,011 (93%) were white, non-Hispanic. Indications for corneal transplantation included Fuchs’ dystrophy in 676 (62%) eyes, pseudophakic/aphakic corneal edema in 369 (34%) eyes, and a variety of other causes in 45 (4%) eyes.
Rejection Events
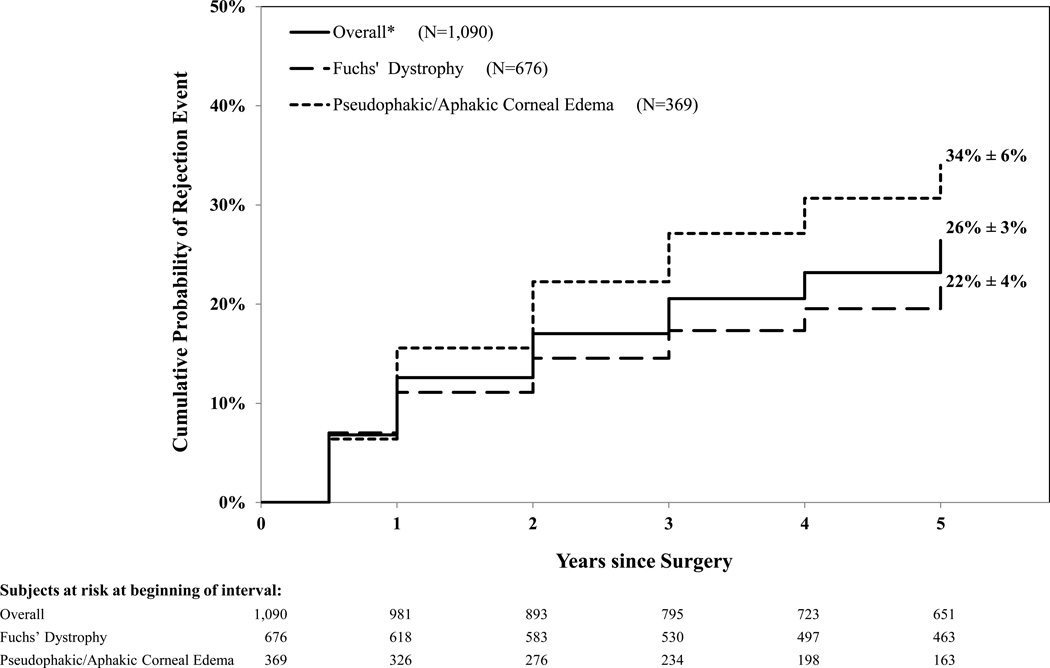
During the 5 years of follow-up, 247 (23%) subjects experienced at least one rejection event. Eighty (7%) subjects experienced a definite rejection event and an additional 167 (15%) experienced a probable rejection. The 5-year predicted probability (± 95% CI) of a probable or definite rejection was 26% ± 3%. The majority of these events occurred within the first 2 years postoperatively (2-year predicted probability =17% ± 2%; Figure 1).
Figure 1.
Life Table Plot of Cumulative Probability of Rejection Events (N=1,090)
* Includes 45 subjects with variety of diagnoses: 12 with interstitial keratitis, 7 with posterior polymorphous dystrophy, 6 with perforating corneal injury and 20 with other causes of endothelial failure
Factors Predictive of Rejection Events
Eyes with pseudophakic/aphakic corneal edema (N=369) were more likely than eyes with Fuchs’ dystrophy (N=676) to experience a rejection event (34% ± 6% versus 22% ± 4%; hazard ratio = 1.56; 95% confidence interval 1.21 to 2.03; p<0.001, Figure 1).
In a multivariate analysis, a variable combining preoperative diagnosis and lens status (p<0.001) and recipient gender (p=0.01) demonstrated significant associations with a rejection event (Table 2). These relationships remained significant after the model was adjusted for donor age. Among eyes with Fuchs’ dystrophy, a higher probability of a rejection event was observed in the 153 eyes that were phakic post-transplant compared with the 307 eyes that had their natural lens removed or removed and replaced with an intraocular lens during the surgery (29% vs. 19%; hazard ratio = 0.54; 95% confidence interval 0.36 to 0.82; Table 2). The probability of rejection in the 216 eyes with Fuchs’ dystrophy that were pseudophakic or aphakic prior to transplant surgery was intermediary (23%).
Table 2.
Multivariate Analysis of Baseline Factors Predictive of a Rejection Event*
| Baseline Factors | N | Cumulative Incidence ± 95% CI |
Multivariate Proportional Hazard Model1 |
||
|---|---|---|---|---|---|
| HR | 95% CI | P-value | |||
| Overall | 1,090 | 26% ± 3% | |||
| Baseline diagnosis & Pre/Post-operative lens status | <0.001 | ||||
| Fuchs’: pre/post phakic | 153 | 29% ± 7% | 1.00 | ||
| Fuchs’: pre phakic/post pseudophakic/aphakic2 | 307 | 19% ± 5% | 0.54 | 0.36 – 0.82 | |
| Fuchs’: pre/post pseudophakic/aphakic3 | 216 | 23% ± 7% | 0.70 | 0.45 – 1.07 | |
| PACE: post pseudophakic/aphakic4 | 369 | 34% ± 6% | 1.12 | 0.78 – 1.61 | |
| Other diagnoses5 | 45 | 35% ± 19% | 1.02 | 0.52 – 1.98 | |
| Recipient Gender | 0.01 | ||||
| Male | 393 | 21% ± 4% | 1.00 | ||
| Female | 697 | 29% ± 4% | 1.42 | 1.08 – 1.87 | |
Includes both probable and/or definite rejection events.
PACE = Pseudophakic/Aphakic Corneal Edema
Multivariate proportional hazards model obtained through forward selection of variables, if p<0.05
Postoperatively, 299 subjects were pseudopahkic and 8 subjects were aphakic
Preoperatively 179 subjects were pseudophakic and 37 subjects were aphakic; postoperatively, 202 subjects were pseudopahkic and 14 subjects were aphakic
Preoperatively 345 subjects were pseudophakic and 24 subjects were aphakic; postoperatively, 361 subjects were pseudopahkic and 8 subjects were aphakic
Includes 45 subjects with variety of diagnoses: 12 with interstitial keratitis, 7 with posterior polymorphous dystrophy, 6 with perforating corneal injury and 20 with other causes of endothelial failure
A higher probability of a rejection event was observed among female recipients compared with male recipients (29% vs. 21%; hazard ratio=1.42; 95% confidence interval 1.08 to 1.87; Table 2), after controlling for the effect of baseline diagnosis and lens status. History of glaucoma, history of vitrectomy, and recipient race were significantly associated with rejection in univariate, but not in multivariate analysis after controlling for the confounding effect of preoperative diagnosis (pseudophakic or aphakic corneal edema versus Fuchs’ dystrophy). Donor age, ABO compatibility or any other donor factors (Table 1) were not associated with the occurrence of rejection.
Table 1.
Univariate Analysis of Baseline Factors Predictive of a Rejection Event* by Baseline Diagnosis
| Total** | Fuchs’ Dystrophy |
Pseudophakic/Aphakic Corneal Edema |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Factors | N | Cumulative Incidence ± 95% CI |
Univariate Proportional Hazard Models |
N | Cumulative Incidence ± 95% CI |
Univariate p-value |
N | Cumulative Incidence ± 95% CI |
Univariate p-value |
||
| HR | 95% CI | p-value | |||||||||
| Overall | 1,090 | 26% ± 3% | 676 | 22% ± 4% | 36 | 34% ± 6% | |||||
| RECIPIENT FACTORS | |||||||||||
| Age | 0.59*** | 0.74*** | 0.89*** | ||||||||
| 40 – <50 yrs | 34 | 41% ± 21% | 1.00 | 22 | 42% ± 24% | 9 | 24% ± 29% | ||||
| 50 – <60 yrs | 128 | 26% ± 8% | 0.69 | 0.35 – 1.38 | 104 | 25% ± 9% | 20 | 35% ± 23% | |||
| 60 – <70 yrs | 284 | 21% ± 5% | 0.53 | 0.28 – 1.01 | 201 | 16% ± 5% | 70 | 33% ± 13% | |||
| 70 – <80 yrs | 594 | 28% ± 4% | 0.68 | 0.37 – 1.26 | 329 | 23% ± 5% | 242 | 35% ± 7% | |||
| 80 – 86 yrs | 50 | 39% ± 19% | 0.82 | 0.37 – 1.84 | 20 | 49% ± 25% | 28 | 35% ± 32% | |||
| Race1 | 0.04 | 0.04 | 0.93 | ||||||||
| White (non-Hispanic) | 1,011 | 25% ± 3% | 1.00 | 651 | 22% ± 4% | 322 | 33% ± 6% | ||||
| African-American | 50 | 36% ± 14% | 1.71 | 1.03 – 2.84 | 19 | 41% ± 24% | 27 | 30% ± 19% | |||
| Gender | 0.06 | 0.10 | 0.08 | ||||||||
| Male | 393 | 21% ± 4% | 1.00 | 210 | 17% ± 5% | 158 | 26% ± 7% | ||||
| Female | 697 | 29% ± 4% | 1.30 | 0.99 – 1.71 | 466 | 25% ± 4% | 211 | 40% ± 8% | |||
| History of diabetes2 | 0.86 | 0.82 | 0.37 | ||||||||
| No | 899 | 26% ± 3% | 1.00 | 587 | 22% ± 4% | 276 | 35% ± 7% | ||||
| Yes | 141 | 24% ± 8% | 0.97 | 0.66 – 1.42 | 67 | 22% ± 10% | 69 | 26% ± 12% | |||
| Smoking status at time of surgery | 0.07 | 0.10 | 0.87 | ||||||||
| No | 988 | 26% ± 3% | 1.00 | 628 | 21% ± 4% | 325 | 34% ± 6% | ||||
| Yes | 102 | 35% ± 11% | 1.43 | 0.97 – 2.09 | 48 | 33% ± 15% | 44 | 31% ± 16% | |||
| Prior use of glaucoma medications/surgery | 0.005 | 0.42 | 0.35 | ||||||||
| No medications and no surgery | 920 | 24% ± 3% | 1.00 | 627 | 22% ± 4% | 259 | 31% ± 7% | ||||
| Medications and no surgery | 99 | 34% ± 10% | 1.55 | 1.06 – 2.28 | 34 | 32% ± 17% | 61 | 34% ± 13% | |||
| No medications and surgery | 26 | 31% ± 24% | 1.14 | 0.51 – 2.57 | 8 | 13% ± 23% | 15 | 30% ± 25% | |||
| Medications and surgery | 45 | 54% ± 21% | 2.16 | 1.31 – 3.55 | 7 | 33% ± 39% | 34 | 61% ± 26% | |||
| Recipient Bed size3 | 0.61*** | 0.05*** | 0.64*** | ||||||||
| ≤7.5 | 309 | 26% ± 6% | 1.00 | 148 | 16% ± 7% | 144 | 38% ± 10% | ||||
| 7.6 – <8.0 | 155 | 30% ± 8% | 1.16 | 0.78 – 1.72 | 92 | 30% ± 10% | 54 | 29% ± 15% | |||
| =8.0 | 523 | 25% ± 4% | 0.99 | 0.73 – 1.34 | 365 | 21% ± 5% | 144 | 31% ± 8% | |||
| >8.0 | 102 | 31% ± 10% | 1.34 | 0.87 – 2.08 | 70 | 29% ± 12% | 27 | 39% ± 21% | |||
| OPERATIVE FACTORS | |||||||||||
| Vitrectomy | 0.003 | 0.05 | 0.33 | ||||||||
| No | 931 | 25% ± 3% | 1.00 | 645 | 22% ± 4% | 242 | 33% ± 7% | ||||
| Yes | 159 | 36% ± 9% | 1.61 | 1.17 – 2.20 | 31 | 38% ± 21% | 127 | 36% ± 10% | |||
| Post-operative Intraocular Pressure4(mmHg) | 0.98*** | 0.46** | 0.10*** | ||||||||
| ≤ 25 | 953 | 26% ± 3% | 1.00 | 608 | 21% ± 4% | 301 | 35% ± 7% | ||||
| >25 | 130 | 30% ± 10% | 1.11 | 0.76 – 1.62 | 63 | 31% ± 14% | 66 | 27% ± 12% | |||
| DONOR FACTORS | |||||||||||
| Age | 0.40*** | 0.23*** | 0.80*** | ||||||||
| 12 – <40 years | 114 | 31% ± 10% | 1.00 | 76 | 30% ± 12% | 35 | 34% ± 17% | ||||
| 40 – <50 years | 122 | 26% ± 9% | 0.85 | 0.51 – 1.41 | 65 | 26% ± 11% | 49 | 34% ± 18% | |||
| 50 – <60 years | 272 | 25% ± 6% | 0.79 | 0.51 – 1.21 | 175 | 21% ± 7% | 88 | 32% ± 10% | |||
| 60 – <70 years | 365 | 27% ± 5% | 0.78 | 0.52 – 1.18 | 223 | 20% ± 6% | 126 | 36% ± 10% | |||
| 70 – 76 years | 217 | 24% ± 7% | 0.73 | 0.46 – 1.15 | 137 | 21% ± 8% | 71 | 33% ± 14% | |||
| Race5 | 0.10 | 0.03 | 1.00 | ||||||||
| White (non-Hispanic) | 1,024 | 26% ± 3% | 1.00 | 632 | 21% ± 4% | 351 | 34% ± 6% | ||||
| African-American | 41 | 39% ± 18% | 1.57 | 0.92 – 2.70 | 28 | 41% ± 22% | 10 | 33% ± 32% | |||
| Gender | 0.90 | 0.69 | 0.55 | ||||||||
| Male | 716 | 27% ± 4% | 1.00 | 451 | 22% ± 4% | 238 | 36% ± 8% | ||||
| Female | 374 | 26% ± 5% | 1.02 | 0.78 – 1.32 | 225 | 23% ± 6% | 131 | 30% ± 9% | |||
| Cause of death | 0.81 | 0.56 | 0.57 | ||||||||
| Cardio/Stroke | 659 | 25% ± 4% | 1.00 | 418 | 22% ± 4% | 214 | 30% ± 7% | ||||
| Cancer | 207 | 28% ± 7% | 1.10 | 0.79 – 1.52 | 118 | 18% ± 8% | 79 | 43% ± 13% | |||
| Trauma | 96 | 27% ± 10% | 1.08 | 0.69 – 1.69 | 57 | 25% ± 13% | 35 | 33% ± 17% | |||
| Respiratory | 78 | 31% ±11% | 1.32 | 0.84 – 2.07 | 54 | 29% ±14% | 21 | 36% ±22% | |||
| Other | 50 | 26% ± 15% | 1.01 | 0.55 – 1.87 | 29 | 21% ± 15% | 20 | 36% ± 30% | |||
| History of diabetes | 0.62 | 0.39 | 0.74 | ||||||||
| No | 891 | 26% ± 3% | 1.00 | 552 | 22% ± 4% | 298 | 34% ± 6% | ||||
| Yes | 199 | 28% ± 7% | 1.08 | 0.79 – 1.48 | 124 | 25% ± 8% | 71 | 33% ± 14% | |||
| Baseline ECD (cells/mm2) | 0.43*** | 0.55*** | 0.44*** | ||||||||
| ≤2500 | 324 | 24% ± 5% | 1.00 | 215 | 20% ± 5% | 101 | 32% ± 10% | ||||
| 2501 – 2999 | 625 | 27% ± 4% | 1.06 | 0.80 – 1.41 | 370 | 22% ± 5% | 227 | 35% ± 8% | |||
| ≥3000 | 141 | 29% ± 9% | 1.18 | 0.79 – 1.77 | 91 | 27% ± 10% | 41 | 35% ± 15% | |||
| Tissue retrieval | 0.51 | 0.90 | 0.59 | ||||||||
| Enucleation | 218 | 27% ± 7% | 1.00 | 152 | 24% ± 8% | 57 | 33% ± 15% | ||||
| In situ | 872 | 26% ± 3% | 1.11 | 0.81 – 1.53 | 524 | 22% ± 4% | 312 | 34% ± 6% | |||
| Tissue refrigerated | 0.82 | 0.63 | 0.55 | ||||||||
| No | 255 | 26% ± 6% | 1.00 | 157 | 24% ± 8% | 91 | 30% ± 11% | ||||
| Yes | 835 | 27% ± 3% | 1.04 | 0.77 – 1.39 | 519 | 22% ± 4% | 278 | 35% ± 7% | |||
| Time from death to preservation | 0.87 | 0.74 | 0.74 | ||||||||
| 0–4 hrs | 206 | 29% ± 7% | 1.00 | 120 | 28% ± 10% | 76 | 29% ± 11% | ||||
| 5–8 hrs | 577 | 26% ± 4% | 0.86 | 0.62 – 1.19 | 364 | 20% ± 4% | 187 | 36% ± 8% | |||
| 9–10 hrs | 165 | 26% ± 8% | 0.90 | 0.59 – 1.37 | 108 | 21% ± 8% | 51 | 39% ± 16% | |||
| 11–12 hrs | 113 | 25% ± 9% | 0.83 | 0.50 – 1.35 | 67 | 25% ± 12% | 43 | 23% ± 15% | |||
| >12 hrs | 29 | 30% ± 18% | 1.07 | 0.51 – 2.25 | 17 | 24% ± 21% | 12 | 41% ± 32% | |||
| Time from death to surgery | 0.38 | 0.65 | 0.54 | ||||||||
| 0–2 days | 146 | 33% ± 9% | 1.00 | 85 | 28% ± 11% | 56 | 44% ± 17% | ||||
| 3–4 days | 597 | 26% ± 4% | 0.78 | 0.55 – 1.11 | 358 | 22% ± 5% | 205 | 32% ± 8% | |||
| 5–8 days | 347 | 24% ± 5% | 0.81 | 0.56 – 1.19 | 233 | 21% ± 6% | 108 | 32% ± 10% | |||
| RECIPIEN/DONOR FACTORS | |||||||||||
| ABO Compatible6 | 0.32 | 0.52 | 0.39 | ||||||||
| No | 364 | 25% ± 5% | 1.00 | 229 | 21% ± 6% | 122 | 31% ± 11% | ||||
| Yes | 638 | 27% ± 4% | 1.15 | 0.87 – 1.52 | 390 | 22% ± 5% | 220 | 36% ± 8% | |||
| Rh Compatible7 | 0.53 | 0.69 | 0.27 | ||||||||
| No | 125 | 28% ± 9% | 1.00 | 84 | 18% ± 8% | 36 | 50% ± 24% | ||||
| Yes | 832 | 25% ± 3% | 0.89 | 0.61 – 1.30 | 517 | 22% ± 4% | 280 | 32% ± 6% | |||
| ABO/Rh Compatible 8 | 0.56 | 0.81 | 0.48 | ||||||||
| ABO and Rh Compatible | 494 | 25% ± 4% | 1.00 | 300 | 22% ± 5% | 171 | 33% ± 8% | ||||
| ABO compatible, Rh incompatible | 82 | 30% ± 11% | 1.27 | 0.81 – 1.99 | 54 | 20% ± 11% | 24 | 51% ± 27% | |||
| ABO incompatible, Rh compatible | 294 | 25% ± 6% | 0.91 | 0.67 – 1.25 | 188 | 21% ± 7% | 95 | 33% ± 11% | |||
| Neither ABO nor Rh compatible | 43 | 23% ± 16% | 0.82 | 0.40 – 1.69 | 30 | 14% ± 12% | 12 | 50% ± 52% | |||
Includes both probable and/or definite rejection events
Includes 45 subjects with variety of diagnoses: 12 with interstitial keratitis, 7 with posterior polymorphous dystrophy, 6 with perforating corneal injury and 20 with other causes of endothelial failure
P-value from model fit with continuous factor
Excludes 13 Hispanics, 8 Asian and 8 other race recipients, because groups were too small to analyze separately
Unknown for 50 subjects
One subject with missing value for bed size
7 subjects with missing value for post-operative IOP
Excludes 11 Hispanics, 3 Asian and 11 other race donors, because groups were too small to analyze separately
88 subjects with missing ABO type
133 subjects with missing Rh blood type
177 subjects with missing ABO/Rh type
Association of Rejection Events and Graft Failure
By 5 years postoperatively, 37% (92/247) of the eyes with a rejection event experienced graft failure compared with 5% (43/843) of eyes without a rejection event (time dependent hazard ratio (HR) = 15.03; 95% CI [10.34, 21.83]; p<0.001).
Discussion
Immunologic graft rejection after corneal transplantation is less likely than it is after solid organ transplantation. This is attributable to the naturally avascular corneal anatomy, the immunosuppressive ocular microenvironment, and the phenomenon of anterior chamber associated immune deviation. The latter produces tolerance, rather than the induction of immunity to antigens introduced into the anterior chamber in experimental animal models.4, 5 However, in eyes with corneal neovascularization and previous graft rejection, the prognosis for corneal graft survival is reduced and the incidence of rejection is high, similar to that of solid organ transplants.
Histocompatibility antigens are expressed by corneal epithelial, stromal, and endothelial cells. Their density is greatest on epithelial cells, and histocompatibility antigen expression is greater on corneas of younger individuals when compared to older individuals.6 These laboratory data have led to the hypothesis that rejection of corneal tissue from younger donors might be more likely than rejection of tissue from older donors. This hypothesis is supported by the case-control study of Palay et al.7, who found a greater likelihood of allograft rejection when younger donor tissue is transplanted into adult recipients.
Traditionally, eye banks and surgeons tend to match donors and recipients for age. Thus, it often is difficult to study effects of donor and recipient age independently in any retrospective analysis of corneal graft failure. Many previously published studies have focused on prognostic factors for the success of transplants into high-risk recipients, which represent a minority of corneal grafts, and the results of these studies may not be applicable to low risk recipients. In contrast, the CDS excluded eyes with ocular surface disease, neovascularization, and previous corneal grafts. Donor tissue was randomly assigned to recipients without regard to age. Thus, the CDS provides an excellent opportunity to study prospectively the impact of donor and recipient age, and other factors, on corneal graft rejection in a moderate-risk population. In addition, the CDS provides an opportunity to further explore the findings of the Collaborative Corneal Transplant Studies (CCTS) that ABO compatibility between donor and recipient is a good prognostic factor for corneal transplant survival.8, 9
In the current study, we found that the likelihood of rejection events was significantly higher in eyes with pseudophakic or aphakic corneal edema than in eyes with Fuchs’ dystrophy. One possible explanation would be that the presence of an intraocular lens causes inflammation, that may, in turn, promote rejection.10 A curious finding was the fact that among the eyes with Fuchs’ dystrophy, graft rejection occurred less frequently in eyes that were pseudophakic or aphakic after the transplant surgery compared with the eyes that were phakic. This seems counter-intuitive particularly since the rejection rate was higher in eyes with pseudophakic or aphakic corneal edema than in eyes with Fuchs’ dystrophy. This may be a chance finding due to the large number of statistical comparisons performed in this exploratory analysis. Alternately, it could reflect the possibility that the eyes that had the lens removed at the time of transplant were treated more intensively or longer with corticosteroids compared with the phakic eyes, and this treatment reduced the rate of rejection. Unfortunately, we did not collect detailed treatment data to be able to address this issue.
We also found that female recipients were more likely to have a rejection event than male recipients. This finding may also be a spurious result of analysis with multiple comparisons. Review of the literature shows conflicting results for the effect of gender. Jonas et al.11 reported that graft rejections were more frequent in males than females, while Kuchle et al.12 reported no correlation of gender with rejection episodes. Anshu et al. found that female gender was predictive of failure13, while Bachmann et al.14 reported the opposite. Interestingly, even though females were more likely than males to have a rejection episode in the CCTS, female gender was not predictive of graft failure. We have been unable to reconcile these apparently conflicting findings.
We did not find an association between graft rejection and donor age. Thus, the present study fails to confirm the results of Palay et al.7, who found a greater likelihood of allograft rejection when younger donor tissue was transplanted into adult recipients using a case-control experimental design. However, donors included in the Palay study were less than 6 years old, whereas in CDS only corneas from donors ≥10 years old were used. Laboratory investigations have reported a significantly higher histocompatibility antigen expression only in donors less than 2 years of age. Thus, failure of the CDS to confirm the greater likelihood of rejection of younger donor tissue may be attributable to the exclusion of very young donors from the CDS.
The results of this study also did not confirm the finding of the CCTS, that ABO incompatibility reduces corneal transplant survival.8 This could be explained by the fact that the CCTS conclusion is based on an observation that is attributable to chance, that the sample size of the CDS was too small to demonstrate statistical significance, or that factors operative in the CCTS high-risk population are not the same as those operative in the CDS population which excluded high-risk recipients.
In summary, the CDS found a substantially higher graft rejection rate in eyes with pseudophakic or aphakic corneal edema compared with eyes with Fuchs’ dystrophy. Further studies would be useful to address whether anti-inflammatory postoperative treatment affects the rate of graft rejection.
Acknowledgments
Funding/Support: Supported by cooperative agreements with the National Eye Institute, National Institutes of Health, Department of Health and Human Services EY12728 and EY12358. Additional support provided by: Eye Bank Association of America, Bausch & Lomb, Inc., Tissue Banks International, Vision Share, Inc., San Diego Eye Bank, The Cornea Society, Katena Products, Inc., ViroMed Laboratories, Inc., Midwest Eye-Banks (Michigan Eye-Bank, Illinois Eye-Bank), Konan Medical Corp., Eye Bank for Sight Restoration, SightLife, Sight Society of Northeastern New York (Lions Eye Bank of Albany), Lions Eye Bank of Oregon
APPENDIX
A listing of the Cornea Donor Study Investigator Group, including clinical site investigators, eye bank staff, coordinating center staff, specular microscopy reading center staff, and committees, has been previously published online.
The following CDS Publications Committee members independently reviewed and approved this manuscript for submission: Jonathan I. Macy, MD, Christopher J. Rapuano, MD, Patricia W. Smith, MD.
References
- 1.Cornea Donor Study Investigator Group. The effect of donor age on corneal transplantation outcome: results of the cornea donor study. Ophthalmology. 2008;115:620–626. doi: 10.1016/j.ophtha.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benetz BA, Gal RL, Ruedy KJ, et al. Specular Microscopy Ancillary Study methods for donor endothelial cell density determination of Cornea Donor Study images. Curr Eye Res. 2006;31:319–327. doi: 10.1080/02713680500536738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornea Donor Study Investigator Group. Donor age and corneal endothelial cell loss five years after successful cornea transplantation: specular microscopy ancillary study results. Ophthalmology. 2008;115:627–632. doi: 10.1016/j.ophtha.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hori J. Mechanisms of immune privilege in the anterior segment of the eye: what we learn from corneal transplantation. J Ocul Bio Dis Inform. 2008;1:94–100. doi: 10.1007/s12177-008-9010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niederkorn JY, Mellon J. Anterior chamber-associated immune deviation promotes corneal allograft survival. Invest Ophthalmol Vis Sci. 1996;37:2700–2707. [PubMed] [Google Scholar]
- 6.Whitsett CF, Stulting RD. The distribution of HLA antigens on human corneal tissue. Invest Ophthalmol Vis Sci. 1984;25:519–524. [PubMed] [Google Scholar]
- 7.Palay DA, Kangas TA, Stulting RD, et al. The effects of donor age on the outcome of penetrating keratoplasty in adults. Ophthalmology. 1997;104:1576–1579. doi: 10.1016/s0161-6420(97)30094-3. [DOI] [PubMed] [Google Scholar]
- 8.Collaborative Corneal Transplantation Studies Research Group. The Collaborative Corneal Transplantation Studies (CCTS): effectiveness of histocompatibility matching in high-risk corneal transplantation. Arch Ophthalmol. 1992;110:1392–1403. [PubMed] [Google Scholar]
- 9.Cornea Donor Study Investigator Group. The effect of ABO blood incompatibility on corneal transplant failure in conditions with low-risk of graft rejection. Am J Ophthalmol. 2009;147(3):432–438. doi: 10.1016/j.ajo.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornea Donor Study Investigator Group. Recipient risk factors for graft failure in the cornea donor study. Ophthalmology. 2009;116(6):1023–1028. doi: 10.1016/j.ophtha.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonas JB, Rank RM, Budde WM. Immunologic graft reactions after allogenic penetrating keratoplasty. Am J Ophthalmol. 2002;133(4):437–443. doi: 10.1016/s0002-9394(01)01426-x. [DOI] [PubMed] [Google Scholar]
- 12.Kuchle M, Cursiefen C, Nguyen NX, et al. Risk factors for corneal allograft rejection: intermediate results of a prospective normal-risk keratoplasty study. Graefes Arch Clin Exp Ophthalmol. 2002;240(7):580–584. doi: 10.1007/s00417-002-0496-5. [DOI] [PubMed] [Google Scholar]
- 13.Anshu A, Lim LS, Htoon HM, Tan DTH. Postoperative risk factors influencing corneal graft survival in the Singapore Corneal Transplant Study. Am J Ophthalmol. 2011;151:442–448. doi: 10.1016/j.ajo.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Bachmann B, Taylor RS, Cursiefen C. Corneal neovascularization as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta analysis. Ophthalmology. 2010;117:1300–1305. doi: 10.1016/j.ophtha.2010.01.039. [DOI] [PubMed] [Google Scholar]