Implant-associated infections are among the most serious post-surgical complications of medical device implants, including prosthetic joints (e.g., hip, knee and shoulder) and fracture fixation hardware.[1,2] Infection rates related to orthopedic implants have been reduced to below 5% owing to strict hygienic protocols and intraoperative systemic prophylactic treatment.[3] However, the overall number of such infections has been continuously increasing with growing demands for surgical implantation as a result of population aging and increasing participation in recreational activities.[4] Timely diagnosis of implant-associated infections is a significant challenge, and established infections may not be effectively treated with long-term systemic antibiotic therapy.[2] Therefore, postsurgical infections often require complex additional surgical procedures such as debridement, prosthesis removal and re-implantation. Even systemic antibiotic treatment raises several concerns, such as systemic toxicity, low efficiency and need for hospitalization. Given this context, extended delivery of antimicrobial agents at the site of implantation is highly desirable to offer high local antibiotic concentration without systemic toxicity and thereby prevent postoperative, implant-associated infections.
Calcium phosphate (CaP) mineral is the main inorganic component of vertebrate bone and teeth. Hydroxyapatite (HAP), β-tricalcium phosphate (β-TCP) and biphasic calcium phosphate (BCP) represent typical examples of CaP minerals, which are known to integrate well with surrounding bone tissue and facilitate bone tissue growth.[5] Therefore, CaP biomaterials are commonly used in orthopedic and dental surgery as bone void fillers[6] or as a coating material on metallic implants.[7] Although various technologies have been developed to produce CaP coatings, considerable attention has recently focused on “biomimetic” coating processes using simulated body fluids.[8] These solution-based processes are performed in conditions that mimic body fluids, and therefore can be applied to three dimensional substrates with complex geometry and a broad range of materials including polymer,[9,10] ceramic[11,12] as well as metal.[13] Notably, recent studies demonstrated that biomimetic CaP coatings can also deliver a range of therapeutic molecules including biologically active proteins[9,14] and plasmid DNA[15] in a controlled manner. In view of the existing and emerging clinical prevalence of CaP coatings, we hypothesized that local, sustained release of an antimicrobial agent from CaP-coated implants could prevent the formation of biofilm and serve as an efficient anti-infection strategy.
Silver has proven to possess excellent antimicrobial activity against a broad spectrum of pathogens, including gram-positive and gram-negative bacteria.[16] This antimicrobial activity is exerted mainly by deactivating respiratory enzymes, and in part by interfering with DNA replication and disrupting the cell membrane. Silver nanoparticles and silver salts are included within commercially available burn ointments,[17] wound dressings[18] and medical devices such as catheters,[19] vascular grafts[20] and endotracheal tubes[21] to effectively prevent infections. We reasoned that nanoparticulate silver crystals created on the surface of CaP minerals would provide a broadly applicable strategy to prevent orthopedic infections. Here we present a facile method to grow antibacterial silver particles on CaP coatings and subsequently release silver species for a controllable timing and dosage. The approach involves incubating CaP materials in silver nitrate solution with or without citric acid pretreatment. We hypothesized that silver particles would be grown on the CaP surface by reacting with phosphate and carbonate ions dissolved from the coating, and that synthesized silver particles would be subsequently released in a sustained manner. In addition, the non-toxic citric acid would suppress initial burst release of silver species by strongly binding to the surface and reducing the dissolution rate of CaP coating.[22]
We created CaP coatings on poly(d,l-lactide-co-glycolide) (PLG) films by incubating in modified simulated body fluid (mSBF) at pH 6.8, 37 °C for 7 days. The CaP coatings were composed of spherical clusters with nanoporous plate-like structure (Figure 1a). The EDS reveals that the obtained coatings consisted of calcium and phosphate with their ratio being 1.81 ± 0.04 (Figure 1b). The FT-IR and XRD spectra of the coatings showed peaks corresponding to carbonate and phosphate and characteristic peaks from hydroxyapatite, respectively (Figure 1c,d). Collectively, the coatings created on PLG films comprised carbonated hydroxyapatite with nanoporous structure.
Figure 1.
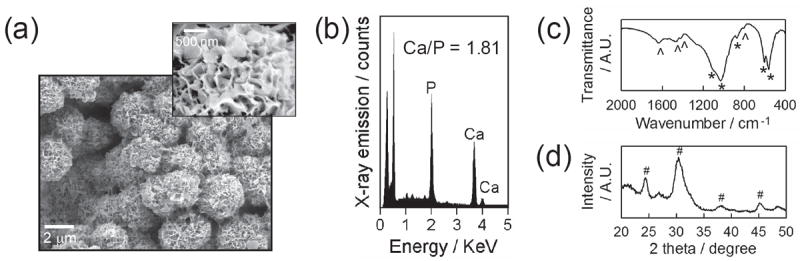
CaP coatings created on PLG films via biomimetic coating method. (a) SEM images of CaP coatings and (b) EDS, (c) FT-IR and (d) XRD spectra of CaP coatings. * and ˆ in FT-IR spectra represent characteristic peaks of phosphate and carbonate, respectively. # in XRD pattern denotes the characteristic peaks associated with hydroxyapatite.
We incubated CaP-coated PLG films sequentially in citric acid solution and silver nitrate solution to produce silver nanoparticles and microparticles on CaP coatings. We changed the concentration of solutions (1, 5 and 10 mm) and incubation time (0.5, 1 and 4 hour) to investigate the effect of each incubation step on silver particle formation and silver release kinetics. The FT-IR spectra of CaP coatings (Figure 2a) displayed the typical pattern associated with phosphate (600, 1050 cm−1) and carbonate (850, 1450 and 1650 cm−1) groups (Figure 2a, (i)). The spectra of CaP coatings after citric acid treatment showed an increased peak area at 1450 cm−1 and new peak appearance at 800 cm−1 and 1200 cm−1 (Figure 2a, (ii)-(iv)). Those peaks arose from carboxylic acid groups in citric acid molecules, indicating the adsorption of citric acid molecules on CaP coatings. The 4-hour citric acid incubation resulted in the appearance of another new peak at 1700 ~ 1800 cm−1 (Figure 2a, (iv)), which is likely due to the larger quantity of citric acid with extended incubation time. On the contrary, changing the citric acid concentration did not affect the FT-IR spectrum (data not shown), which implies that citric acid adsorption was not concentration-dependent over the range of concentrations tested in this study. This lack of concentration dependence may be attributed to the particularly strong interaction of citric acid molecules to CaP materials, which is mediated by the interaction between carboxylic groups in citric acid and calcium ions on the CaP surface.[22] The crystallinity index calculated from the FT-IR phosphate peaks (at 550 ~ 600 cm−1) was not influenced by citric acid treatment (Figure 2b), suggesting that the inherent solubility of CaP coatings was not influenced by citric acid treatment. However, the dissolution of CaP coatings, as measured by calcium dissolution, was dependent on the citric acid treatment. When citric acid was adsorbed, calcium ions were slowly released from CaP coatings with less “burst” release during day 1 (Figure 2c). It indicates that citric acid adsorbed on CaP surface could delay the dissolution of CaP coating, which concurs with the previous reports on the inhibitory effect of strongly adsorbed molecules including citric acid on hydroxyapatite dissolution.[23]
Figure 2.
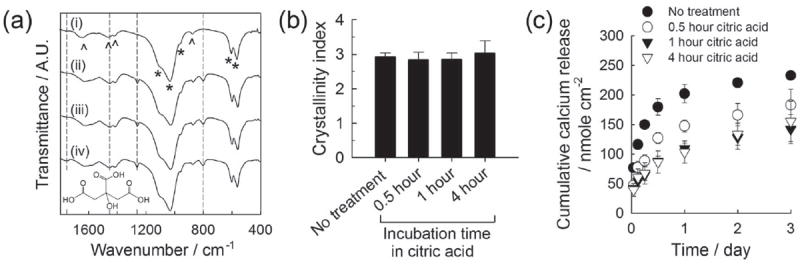
(a) FT-IR spectra of CaP coatings (i) before citric acid treatment and (ii-iv) after incubating in 5 mm citric acid solution for different time periods: (ii) 0.5, (iii) 1 and (iv) 4 hours. The structural formula of citric acid is shown on the bottom. (b) Crystallinity index calculated from FT-IR spectra based on Shemesh’s method. (c) Calcium release from CaP coatings treated differently with citric acid.
The citric acid-treated CaP coatings were then transferred in silver nitrate solution to grow silver particles. Silver particles covered the entire CaP substrate uniformly in all conditions tested (Figure 3a). SEM images showed that the size of silver particles was in the range of tens of nanometers (Figure 3b) to several micrometers (Figure 3c) depending on the preparation conditions. Specifically, the size of silver particles was increased with longer silver nitrate incubation time and higher silver nitrate concentrations, whereas citric acid incubation time and concentration did not influence the silver particle size. The appearance of a silver peak in EDS spectra after incubating in silver nitrate solution confirmed the formation of silver particles (Figure 3c). The XRD pattern of CaP coating after silver nitrate incubation revealed the characteristic peaks of silver phosphate[24] and silver carbonate[25] in addition to those from hydroxyapatite (Figure 3d). It should be noted that silver particles were synthesized on both citric acid-treated and non-treated CaP surfaces, and there was no noticeable difference in the size and morphology of the silver particles between these two groups. In addition, we could not observe any indication of silver particle formation in the bulk solution (e.g., color/turbidity change) during incubation in silver nitrate solution (Figure 3b, right inset). Collectively, the results indicate that silver carbonate and silver phosphate particles were created locally on CaP surface by the reaction of silver ions with carbonate or phosphate ions dissolved from CaP coating.
Figure 3.
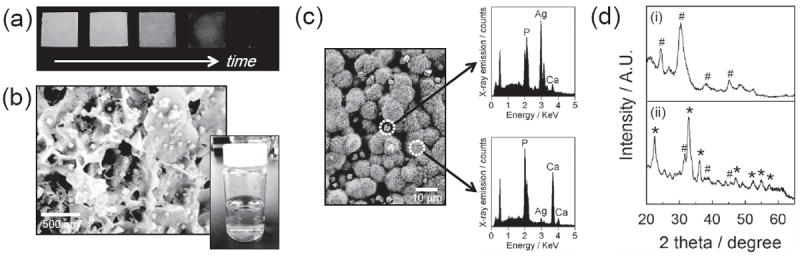
(a) Photographs of silver-incorporated, CaP-coated PLG films during the course of incubation in silver nitrate solution. The CaP-coated films were incubated in 5 mm citric acid solution for 1 hour and subsequently in 5 mm silver nitrate solution for 0, 0.5, 1, 2 and 4 hours (from left to right). (b) SEM image of nano-sized silver particles synthesized on CaP coating. Silver nanoparticles were synthesized by sequentially incubating in 5 mm citric acid solution for 1 hour and 5 mm silver nitrate solution for 0.5 hour. Lower right inset is a photograph of silver nitrate solution where a citric acid-treated CaP-coated PLG film was incubated on the bottom. (c) SEM image of micrometer-scale particles on CaP coating (left) and EDS spectra of distinct regions (right). They were created from 5 mm citric acid solution for 1 hour and 5 mm silver nitrate solution for 4 hours. (d) XRD spectra of (i) untreated CaP coating and (ii) CaP coating after silver particle formation. # and * in spectra denote the characteristic peaks from hydroxyapatite and silver phosphate or silver carbonate, respectively.
Once we confirmed the formation of silver salt particles on CaP coatings, we measured the time course release of silver which was prepared from different incubating conditions (Figure 4a). The silver release from CaP coatings continued for time periods ranging from 3 days to over 30 days, and the total quantity of released silver species ranged from 0.7 μg to 75.4 μg per cm2 of sample surface (Figure 4). Interestingly, the silver release occurred with nearly linear release kinetics in all groups treated with citric acid, while the groups not treated with citric acid showed an initial burst release during the first two days of release (Figure 4b and 4c). These release kinetics are likely dictated by the different dissolution rates of CaP coatings in presence of adsorbed citric acid molecules. Burst release poses a significant practical problem in the application of drug delivery systems, because it often happens in an unpredictable manner and may cause negative side effects due to overdose of the released drug.[26] Our results here suggest that citric acid treatment can help avoid burst release of silver species, and thereby prevent any complications related to silver overdose. The 4-hour incubation in citric acid solution led to more rapid release kinetics compared to 0.5 and 1 hour incubations (Figure 4b). On the other hand, the release behavior of silver was not influenced by the citric acid concentration (Figure 4c). This trend was in good accordance with the FT-IR data and dissolution results for citric acid-treated CaP coatings (Figure 2), and suggested that the amount of released silver was dictated by the adsorbed citric acid and thus dissolution of CaP coatings. As the incubation time in silver nitrate solution was extended from 0.5 to 4 hours, a larger amount of silver was released for a prolonged time period (Figure 4d). Similarly, higher concentrations of silver nitrate solution during growth of silver particles led to larger quantities of silver released over longer release periods (Figure 4e). Taken together, these results indicate that the dosage and timeframe of silver release could be readily controlled by varying the conditions during growth of silver salt particles on the CaP surface.
Figure 4.
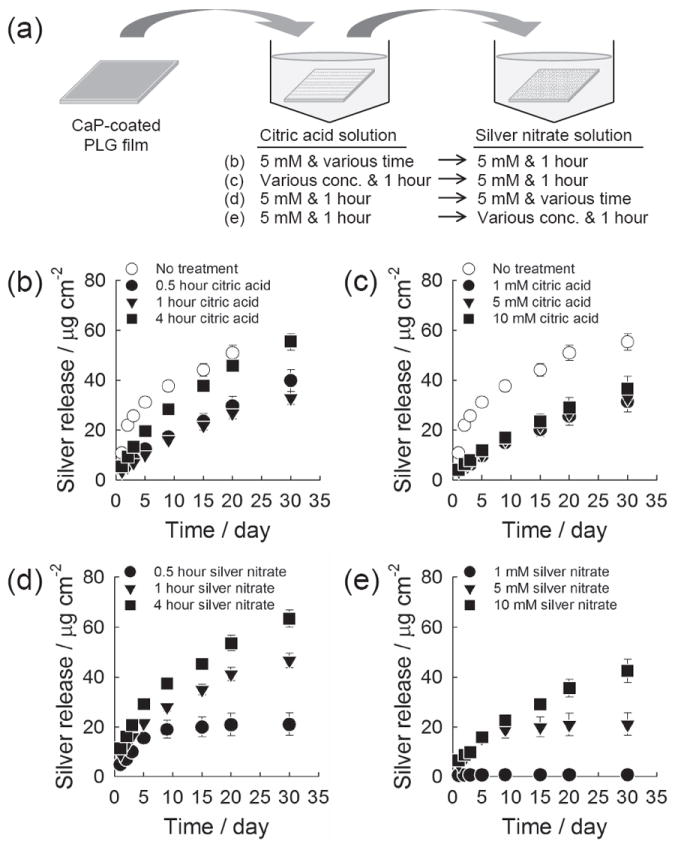
Cumulative silver release from CaP coating. (a) Schematic of experiment. (b,c) The CaP coatings were incubated (b) in 5 mm citric acid solution for different time periods or (c) in citric acid solution of different concentrations for 1 hour. The coatings were then incubated in 5 mm silver nitrate solution for 1 hour. (d,e) The CaP coatings were pretreated in 5 mm citric acid solution for 1 hour, and subsequently incubated (d) in 5 mm silver nitrate solution for different time periods or (e) in silver nitrate solution of different concentrations for 1 hour.
The antibacterial activity of released silver was evaluated against gram-positive Staphylococcus aureus (Figure 5b,c) and gram-negative Escherichia coli (Figure 5d,e). The majority of orthopedic infections are attributed to gram-positive Staphylococci, of which S. aureus is the leading pathogen causing implant-related infections.[27] We chose both gram-positive and gram-negative bacteria to demonstrate the broad antibacterial activity of silver delivered from CaP coatings. Briefly, we added media from silver-releasing CaP coatings to bacterial suspensions in their exponential growth phase, and monitored bacterial growth by measuring optical density at 600 nm (Figure 5a). We tested release media from a very early time point, day-1 (Figure 5b,d) as well as a later time point, day-30 (Figure 5c,e). When release media were added to bacteria suspension, the bacteria growth was delayed to an extent which was proportional to the amount of silver present in the added release media (Figure 5b,c). Importantly, these data demonstrate that the antibacterial activity of released silver was similar against S. aureus and E. coli, and the silver released at a later time point remained antimicrobially active. In addition, previous studies reported that citric acid is effective to treat chronic wound infection by preventing colony formation of microorganism, which is also beneficial to our purpose.[28]
Figure 5.
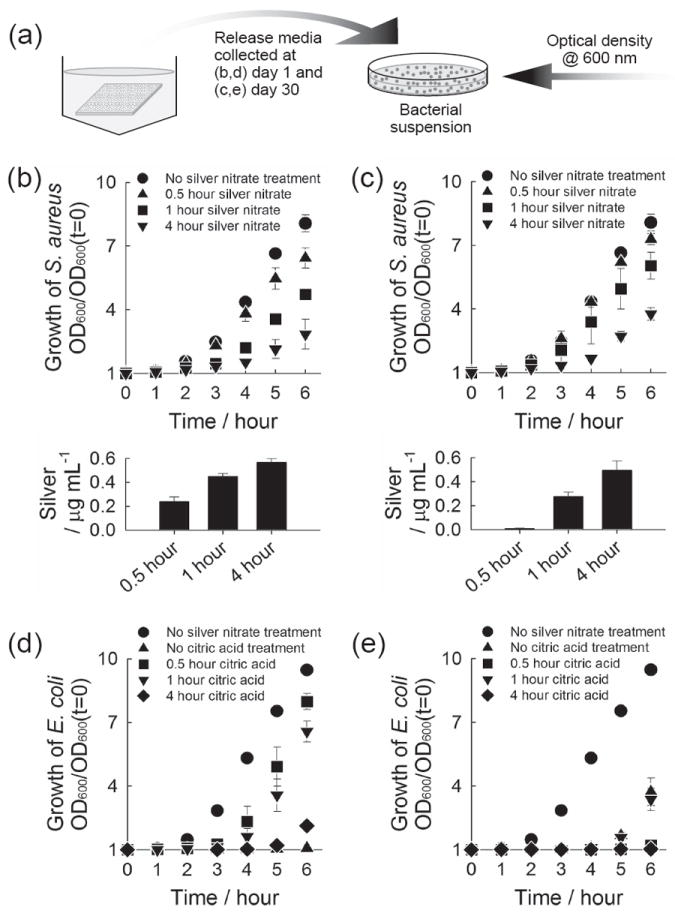
Antibacterial activity of released silver. (a) Schematic of experiment. (b-e) Growth curve of S. aureus (b,c) and E. coli (d,e) upon the addition of release media. The release media were collected from CaP coatings which were incubated sequentially in (b,c) 5 mm citric acid solution for 1 hour and 5 mm silver nitrate solution for different time periods, and (d,e) 5 mm citric acid solution for different time periods and 5 mm silver nitrate solution for 1 hour. The release media collected (b,d) at day 1 where CaP coatings were incubated for the first day and (c,e) at day 30 where the coatings were incubated from day 20 to day 30, were added to S. aureus (b,c) and E. coli (d,e). Lower plots in (b) and (c) show the concentration of silver in the bacteria solution tested.
Silver wound dressings currently used in clinical applications have been reported to contain silver species with the range of tens to hundreds of μg cm−2, and to release up to ~ 30 μg cm−2 in cell culture medium and ~ 100 μg cm−2 in water during the first day.[29] In contrast, the first day silver release from CaP coatings in our study was less than 15 μg cm−2. Therefore, the silver amounts released are likely to be within the clinically acceptable range. Here we examined toxicity of released silver to mammalian cells in a “worst case scenario” by incubating the most silver-loaded CaP coating, which eluted 14.3 μg cm−2 during the first day in PBS, in a small volume (0.5 mL) of Dulbecco’s modified Eagle’s medium (DMEM) for one day. The release medium was then used to culture NIH 3T3 mouse fibroblasts after diluting to varying extents with fresh DMEM. When cells were both seeded and cultured in DMEM with released silver, significant cytotoxicity was observed only from 4-fold diluted DMEM and further diluted solutions did not affect cell number at 24 or 48 hours (Figure 6a). When cells were seeded in fresh DMEM then exposed to silver-containing DMEM, some limited cytotoxicity (78% cell viability) was observed only from the 8-fold diluted DMEM (Figure 6b), with no cytotoxicity observed for any further dilution. These results provide further evidence that silver species eluted from mineral coatings are unlikely to cause significant cytotoxicity in clinical scenarios, as released silver species are likely to be rapidly diluted in an implant site due to simple diffusion and fluid transport.
Figure 6.
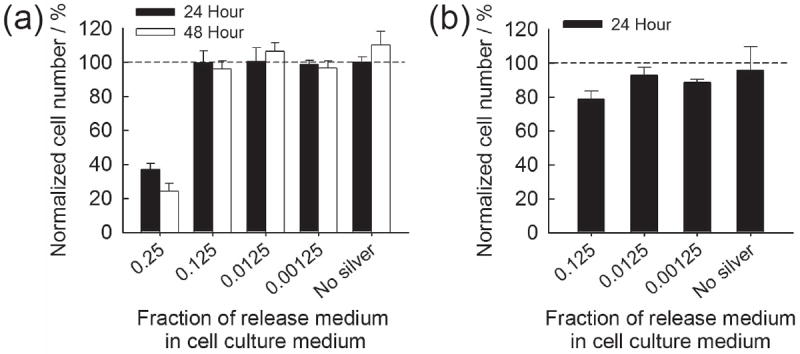
Normalized NIH 3T3 cell number when exposed to silver-containing cell culture medium. (a) Cells were seeded and cultured in silver-containing DMEM and (b) attached cells were exposed to silver-containing DMEM.
It is noteworthy that the method described here may be applicable to a range of CaP biomaterials such as sintered HAP, β-TCP and BCP, which are also commonly used in current orthopedic procedures. To demonstrate broader applicability of silver-releasing coatings, we used our approach to form silver salt particles on sintered HAP and β-TCP biomaterials. Results were similar to those observed for silver particle growth on CaP coatings and the rate of particle growth was likely dependent on the dissolution rate of CaP materials (Figure 7). Therefore, we can conclude that controlled release of antibacterial silver can be achieved from a range of CaP-based devices used in current clinical settings, which may efficiently suppress implant-associated infections.
Figure 7.
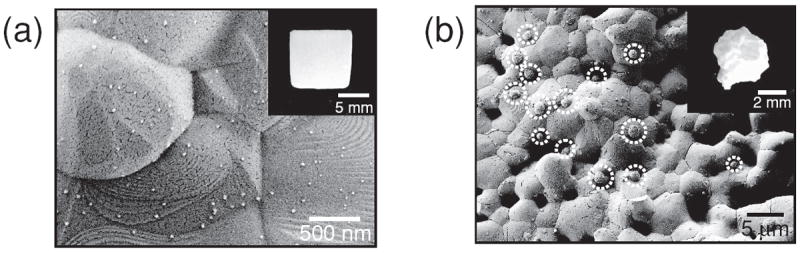
SEM images of silver particles created on sintered (a) hydroxyapatite and (b) β-tricalcium phosphate. The hydroxyapatite and β-tricalcium phosphate were incubated in 5 mm citric acid solution for 1 hour and subsequently in 5 mm silver nitrate solution for 1 hour.
Experimental Section
Calcium Phosphate (CaP) Coating Formation
Poly(d,l-lactic-co-glycolide) (PLG, lactide:glycolide = 85:15) films were prepared by solvent-casting PLG-dissolving chloroform solution on polytetrafluoroethylene plates. The PLG films were cut into 1cm × 1cm and subsequently coated with CaP minerals. The CaP coatings were created by incubating PLG films in modified simulated body fluid (mSBF; 141 mm NaCl, 4 mm KCl, 0.5 mm MgSO4, 1.0 mm MgCl2, 4.2 mm NaHCO3, 5.0 mm CaCl2, 2.0 mm KH2PO4, 20 mm Tris base) as described elsewhere [9,11]. The incubation was continued at 37 °C, pH 6.8 for 7 days under mild agitation, and mSBF was refreshed daily. The resulting PLG films were rinsed with deionized water and lyophilized. The CaP coatings on PLG films were characterized using field emission scanning electron microscopy (FE-SEM; LEO 1530, Zeiss, Germany) equipped with energy dispersive X-ray spectroscopy (EDS). The chemical and phase characteristics of the CaP coating scraped from PLG film were identified by Fourier transform infrared spectroscopy (FT-IR; Equinox 55, Bruker AXS, Germany) and X-ray diffraction spectroscopy (XRD; Hi-Star 2-D, Bruker AXS, Germany) with CuKα radiation, respectively.
Synthesis and Characterization of Silver Particles on CaP Coating
CaP coatings were incubated in silver nitrate solution in deionized water (pH 7.0, 5mL) to synthesize silver particles on the surface of coating. In a set of experiments, CaP coatings were pre-treated by incubating in citric acid solution in deionized water (pH 7.0, 5mL) prior to silver nitrate incubation. The concentrations of both solutions were changed to 1, 5, and 10 mm, and incubation times were also varied to 0.5, 1 and 4 hour. After citric acid pre-treatment, the resultant CaP coatings were characterized using FT-IR, and crystallinity index was calculated from Shemesh’s method [30]. The calcium released from citric acid-treated CaP coating in PBS at 37 °C was quantified by colorimetric assay using Arsenazo III (MP Biocehmicals, USA). At predetermined time points, the release medium was collected to quantify calcium ions and fresh PBS was added for further incubation. The silver particles on CaP coatings were imaged using FE-SEM and elemental analysis was carried out using EDS. The silver particles scraped from CaP coatings were characterized using XRD.
Silver Release from CaP Coating
The treated CaP coating was incubated in 5 mL of PBS (pH 7.4) at 37 °C. At each time points (1, 2, 3, 5, 9, 15, 20 and 30 day), the release media were collected and CaP coatings were transferred in a fresh PBS for further incubation. The silver species in release media was quantified using inductively coupled plasma optical emission spectrometry (ICP-OES; Optima 4300DV, Perkin Elmer, USA) at 328.07 nm.
Antibacterial Activity of Released Silver
The release media collected at day 1 (where CaP coatings were incubated for day 0 to 1) and day 30 (where CaP coatings were incubated for day 20 to 30) were used to test the antibacterial activity of released silver against S. aureus (ATCC 25923) and E. coli (ATCC 25922). All bacterial culture was performed in Luria-Bertani (LB) broth. The 50 μL of release media were added to 150 μL of bacteria suspension (108 CFU mL−1) in exponential growth phase in a 96-well plate and incubated at 37 °C. The bacterial growth was monitored by measuring optical density of bacterial suspension at 600 nm.
In Vitro Cytotoxicity of Released Silver
Mouse fibroblast NIH 3T3 cells were used to assess the cytotoxicity of silver released from silver-incorporated CaP coating prepared by incubating in 5 mm citric acid solution for 1 hour and subsequently in 10 mm nitric acid solution for 4 hours. The cells were cultured in cell culture Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% cosmic calf serum and a mixture of penicillin (100 U mL−1) and streptomycin (100 μg mL−1) at 37°C in with 5% CO2. The release medium was collected after silver-incorporated CaP coating was incubated in 0.5 mL of cell culture medium for 24 hours at 4 °C. After serially diluted with fresh cell culture medium, 25 μL of silver-containing release medium was mixed with 75 μL of cell suspension and seeded in a 96-well plate (5000 cells/well). In another set of experiment, serially diluted silver-containing cell culture medium was added to the cells 24 hours after the cells were seeded in a 96-well plate (5000 cells/well). The cell viability was determined using CellTiter Blue cell viability assay (Promega, USA) and was normalized to the values from non-treated condition.
Supplementary Material
Acknowledgments
The authors acknowledge financial support from the AO Research Foundation (Exploratory Research Grant) and the National Institutes of Health (R01AR059916).
Footnotes
Supporting Information
Supporting Information is available from the Wiley online Library or from the author.
Contributor Information
Dr. Jae Sung Lee, Department of Biomedical Engineering, University of Wisconsin, 1550 Engineering Drive, Madison, WI 53706, USA
Prof. William L. Murphy, Email: wlmurphy@wisc.edu, Department of Biomedical Engineering, University of Wisconsin, 1550 Engineering Drive, Madison, WI 53706, USA; Department of Orthopedics and Rehabilitation, University of Wisconsin, 600 Highland Avenue, Madison, WI 53792, USA; Collaborative Research Center, AO Foundation, Davos, Switzerland.
References
- 1.Engemann JJ, Carmeli Y, Cosgrove SE, Fowler VG, Bronstein MZ, Trivette SL, Briggs JP, Sexton DJ, Kaye KS. Clin Infect Dis. 2003;36:592. doi: 10.1086/367653. [DOI] [PubMed] [Google Scholar]
- 2.Zimmerli W, Ochsner PE. Infection. 2003;31:99. doi: 10.1007/s15010-002-3079-9. [DOI] [PubMed] [Google Scholar]
- 3.a) Garvin KL, Hanssen AD. J Bone Joint Surg Am. 1995;77:1576. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]; b) Sperling JW, Kozak TKW, Hanssen AD, Cofield RH. Clin Orthop Rel Res. 2001:206. doi: 10.1097/00003086-200101000-00028. [DOI] [PubMed] [Google Scholar]
- 4.Deyo RA, Nachemson A, Mirza SK. New Engl J Med. 2004;350:722. doi: 10.1056/NEJMsb031771. [DOI] [PubMed] [Google Scholar]
- 5.a) Dorozhkin SV. Biomaterials. 2010;31:1465. doi: 10.1016/j.biomaterials.2009.11.050. [DOI] [PubMed] [Google Scholar]; b) LeGeros RZ. Clin Orthop Rel Res. 2002:81. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 6.a) Daculsi G. Biomaterials. 1998;19:1473. doi: 10.1016/s0142-9612(98)00061-1. [DOI] [PubMed] [Google Scholar]; b) Larsson S, Bauer TW. Clin Orthop Rel Res. 2002:23. doi: 10.1097/00003086-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 7.a) Geesink RGT. Clin Orthop Rel Res. 2002:53. doi: 10.1097/00003086-200202000-00007. [DOI] [PubMed] [Google Scholar]; b) Junker R, Manders PJD, Wolke J, Borisov Y, Jansen JA. Clin Oral Implant Res. 2010;21:189. doi: 10.1111/j.1600-0501.2009.01819.x. [DOI] [PubMed] [Google Scholar]
- 8.Liu YL, Wu G, de Groot K. J R Soc Interface. 2010;7:S631. doi: 10.1098/rsif.2010.0115.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JS, Lu Y, Baer GS, Markel MD, Murphy WL. J Mater Chem. 2010;20:8894. [Google Scholar]
- 10.Tanahashi M, Yao T, Kokubo T, Minoda M, Miyamoto T, Nakamura T, Yamamuro T. J Am Ceram Soc. 1994;77:2805. [Google Scholar]
- 11.Lee JS, Suarez-Gonzalez D, Murphy WL. Adv Mater. 2011;23:4279. doi: 10.1002/adma.201100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suarez-Gonzalez D, Lee JS, Levengood SKL, Vanderby R, Jr, Murphy WL. Acta Biomater. 2012;8:1117. doi: 10.1016/j.actbio.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Abe Y, Kokubo T, Yamamuro T. J Mater Sci Mater Med. 1990;1:233. [Google Scholar]; b) Habibovic P, Barrere F, van Blitterswijk CA, de Groot K, Layrolle P. J Am Ceram Soc. 2002;85:517. [Google Scholar]
- 14.a) Liu Y, Li JP, Hunziker EB, De Groot K. Philos Trans A Math Phys Eng Sci. 2006;364:233. doi: 10.1098/rsta.2005.1685. [DOI] [PubMed] [Google Scholar]; b) Lu Y, Lee JS, Nemke B, Baer G, Graf BK, Murphy WL, Markel MD. Curr Orthop Pract. 2011;22:425. [Google Scholar]
- 15.a) Choi S, Murphy WL. Acta Biomater. 2010;6:3426. doi: 10.1016/j.actbio.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Luong LN, McFalls KM, Kohn DH. Biomaterials. 2009;30:6996. doi: 10.1016/j.biomaterials.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rai M, Yadav A, Gade A. Biotechnol Adv. 2009;27:76. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Klasen HJ. Burns. 2000;26:131. doi: 10.1016/s0305-4179(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 18.Silver S, Phung LT, Silver G. J Ind Microbiol Biotechnol. 2006;33:627. doi: 10.1007/s10295-006-0139-7. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JR, Kuskowski MA, Wilt TJ. Ann Intern Med. 2006;144:116. doi: 10.7326/0003-4819-144-2-200601170-00009. [DOI] [PubMed] [Google Scholar]
- 20.Batt M, Magne JL, Alric P, Muzj A, Ruotolo C, Ljungstrom KG, Garcia-Casas R, Simms M. J Vasc Surg. 2003;38:983. doi: 10.1016/s0741-5214(03)00554-8. [DOI] [PubMed] [Google Scholar]
- 21.Kollef MH, Afessa B, Anzueto A, Veremakis C, Kerr KM, Margolis BD, Craven DE, Roberts PR, Arroliga AC, Hubmayr RD, Restrepo MI, Auger WR, Schinner R. JAMA-J Am Med Assoc. 2008;300:805. doi: 10.1001/jama.300.7.805. [DOI] [PubMed] [Google Scholar]
- 22.a) Filgueiras MRT, Mkhonto D, de Leeuw NH. J Cryst Growth. 2006;294:60. [Google Scholar]; b) Hu YY, Rawal A, Schmidt-Rohr K. Proc Natl Acad Sci U S A. 2010;107:22425. doi: 10.1073/pnas.1009219107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.a) Johnsson M, Richardson CF, Sallis JD, Nancollas GH. Calcif Tissue Int. 1991;49:134. doi: 10.1007/BF02565136. [DOI] [PubMed] [Google Scholar]; Christoffersen J, Christoffersen MR. J Cryst Growth. 1981;53:42. [Google Scholar]
- 24.Suwanprateeb J, Thammarakcharoen F, Wasoontararat K, Chokevivat W, Phanphiriya P. Mater Sci Eng C Mater Biol Appl. 2012;32:2122. doi: 10.1016/j.msec.2012.05.051. [DOI] [PubMed] [Google Scholar]
- 25.Xu C, Liu Y, Huang B, Li H, Qin X, Zhang X, Dai Y. Appl Surf Sci. 2011;257:8732. [Google Scholar]
- 26.Huang X, Brazel CS. J Control Release. 2001;73:121. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 27.Arciola CR, An YH, Campoccia D, Donati ME, Montanaro L. Int J Artif Organs. 2005;28:1091. doi: 10.1177/039139880502801106. [DOI] [PubMed] [Google Scholar]
- 28.a) Daly CG. J Clin Periodontol. 1982;9:386. doi: 10.1111/j.1600-051x.1982.tb02049.x. [DOI] [PubMed] [Google Scholar]; b) Nagoba BS, Punpale AS, Ayachit R, Gandhi RC, Wadher BJ. Int Wound J. 2011;8:425. doi: 10.1111/j.1742-481X.2011.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burd A, Kwok CH, Hung SC, Chan HS, Gu H, Lam WK, Huang L. Wound Repair Regen. 2007;15:94. doi: 10.1111/j.1524-475X.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 30.Farlay D, Panczer G, Rey C, Delmas PD, Boivin G. J Bone Miner Metab. 2010;28:433. doi: 10.1007/s00774-009-0146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


