Abstract
Objective
17β-Estradiol (E2) offers cardiovascular protection in young female animals and postmenopausal women. In contrast, randomized trials of menopausal hormones carried out in older women have shown harm or no cardiovascular benefit. We hypothesize that E2 effects on vascular inflammation are age-dependent.
Approach and Results
Young (10-wk) and aged (52-wk) female C57BL/6 mice were used as source for primary cultures of bone marrow-derived macrophages (BMMs) and vascular smooth muscle cells (VSMCs). E2 pre-treatment of cells derived from young mice attenuated C-reactive protein (CRP)-induced expression of inflammatory mediators. In contrast, E2 pre-treatment of cells from aged mice did not alter (BMMs) or paradoxically exaggerated (VSMCs) inflammatory mediator response to CRP. Using E2 receptor (ER)-knockout mice, we demonstrated that E2 regulates inflammatory response to CRP in BMMs via ERα and in VSMCs via ERβ. BMMs derived from aged (vs. young) mice expressed significantly less ERα mRNA and protein. A selective ligand of the novel ER GPR30 reproduced the E2 effects in BMMs and VSMCs. Unlike in young mice, E2 did not reduce neointima formation in ligated carotid arteries of aged CRP transgenic mice.
Conclusions
E2 attenuates inflammatory response to CRP in BMMs and VSMCs derived from young but not aged mice and reduces neointima formation in injured carotid arteries of young but not aged CRP transgenic mice. ERα expression in BMMs is greatly diminished with aging. These data suggest that vasoprotective effects of E2 are age-dependent and may explain the vasotoxic effects of E2 seen in clinical trials of postmenopausal women.
Keywords: Estrogen, vascular inflammation, vascular injury, c-reactive protein, aging
Introduction
Inflammation plays a major role in the pathogenesis of vascular disease, including atherosclerosis and the response to acute vascular injury.1 In mechanistic studies, 17β-estradiol (E2), via engagement of E2 receptors (ERs), attenuates the inflammatory response to vascular injury.2-5 Further, observational studies indicate that post-menopausal women who choose to use hormone replacement therapy experience a significantly lower rate of cardiovascular disease (CVD) events than those who do not.6 Clinical trials such as the Women’s Health Initiative (WHI) however, reported an increase in CVD events in women treated with menopausal hormone therapy compared to placebo, calling into question the concept of estrogenic vasoprotection.7 Importantly, the WHI enrolled older women than the observational studies. It has been hypothesized that E2 signaling pathways are altered with aging such that the anti-inflammatory/vasoprotective effects seen in pre-menopausal women are converted to proinflammatory/vasotoxic effects in women who are many years post-menopausal.8-10
C-reactive protein (CRP) is a widely accepted blood marker for CVD risk in women.11 Using young ovariectomized (OVX) CRP transgenic mice (CRPtg) that express human CRP in a manner that closely resemble the human condition, we have shown that CRP exacerbates the inflammatory response to vascular injury,12,13 and that E2 attenuates the inflammatory response and neointima formation induced by CRP in this model.14 The role of age-related alterations in ER signaling in this model is not known. E2 exerts its anti-inflammatory effects in the vasculature via the traditional nuclear ERs, ERα and ERβ, and the more recently discovered G-protein coupled receptor 30 (GPR30).15-17 These ERs have distinct tissue distributions and functions. Since injury-induced vascular inflammation and remodeling are mediated by both activated macrophages and vascular smooth muscle cells (VSMC),15 we tested the hypothesis that E2 regulates CRP-induced inflammation in these cell-types in an age-dependent manner and via different ERs.
Material and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
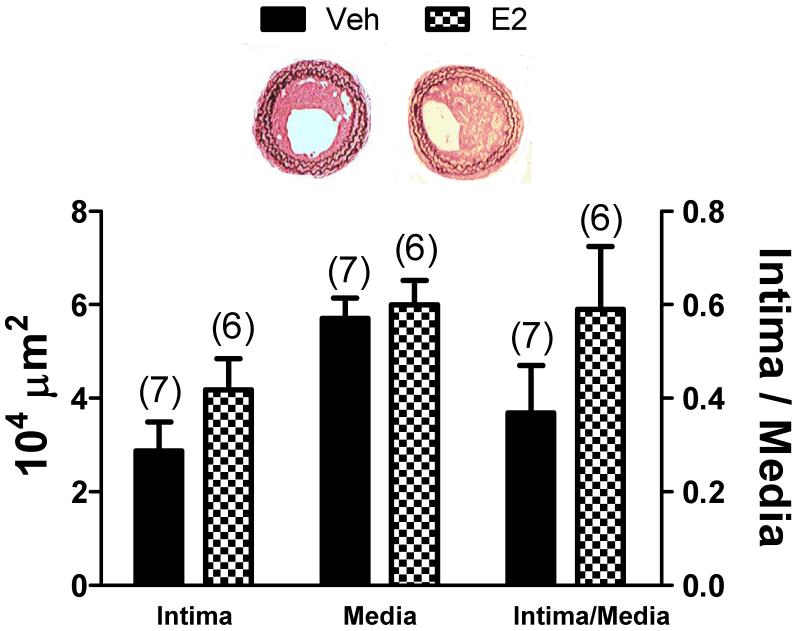
To test the hypothesis that the vasoprotective effects of E2 observed in young OVX CRPtg subjected to carotid artery ligation14 are lost in older mice, aged (50-54 wk) CRPtg underwent OVX and subcutaneous implantation of pellets containing E2 or vehicle followed 1 wk later by carotid artery ligation. In aged CRPtg there was an ~40% increase in intima formation in the E2 treated animals compared with vehicle controls (Figure 1). Although the comparison did not reach the level of statistical significance, the E2 effect is opposite what we observed earlier for similarly treated young OVX CRPtg, wherein E2 treatment resulted in ~85% reduction in neointima formation.14
Figure 1.
17β-estradiol (E2) treatment in aged C-reactive protein transgenic mice (CRPtg) enhances neointima formation. The bar graphs show the cross-sectional areas of the neointima and media (and the neointima/media cross-section ratios) of carotid arteries harvested from 50-54 wk old CRPtg mice 28 days after carotid artery ligation. Mice underwent OVX 1 wk prior to carotid artery ligation and were implanted with subcutaneous E2 or vehicle pellets prior to artery ligation. The bars and whiskers are means±SEMs. The number of mice is indicated above the bar. Representative light micrographs of injured arteries from vehicle and E2 treated mice are shown.
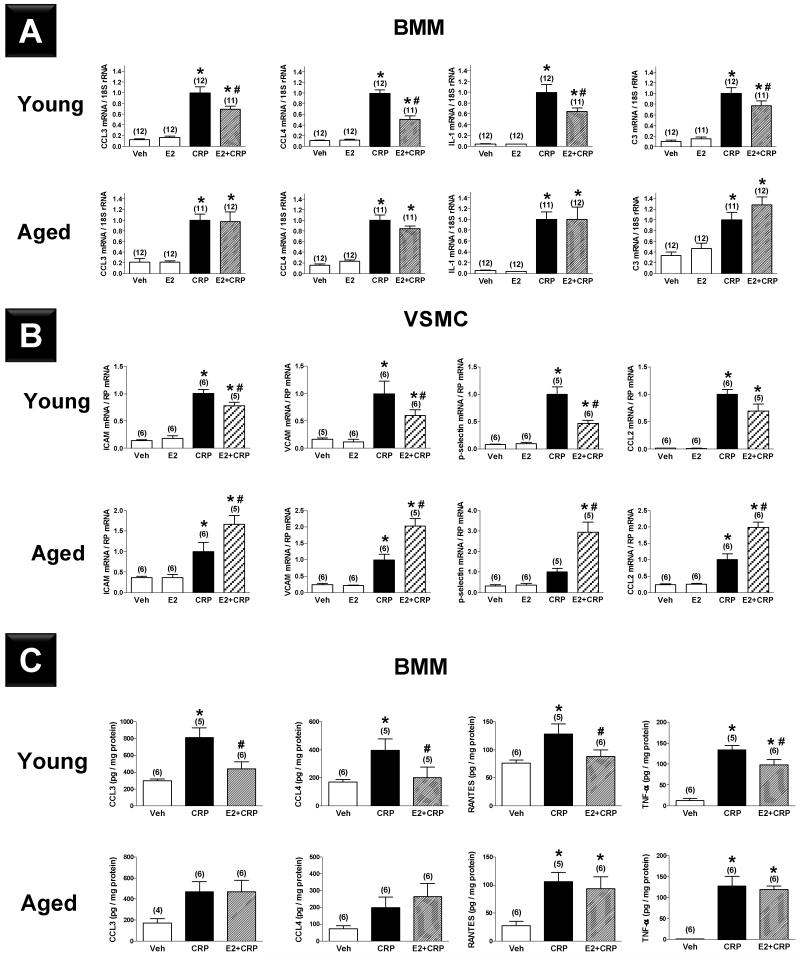
Real-time quantitative RT-PCR analysis of bone marrow macrophages (BMMs) and VSMCs derived from young and aged C57BL/6 mice showed that proinflammatory mediators (CCL3, CCL4, IL-1, and C3 for BMMs; ICAM, VCAM, p-selectin, CCL-2 and IL-8 for VSMCs) were expressed at low levels in the vehicle treated groups, and that mRNA levels increased markedly (5- to 20-fold for BMMs and 4- to 150-fold for VSMCs) in response to CRP treatment (Figure 2A-B). In the absence of CRP, E2 did not affect the expression of inflammatory mediators in either group. E2 pre-treatment significantly attenuated the CRP-induced increased expression of inflammatory mediators in BMMs (Figure 2A) and VSMCs (Figure 2B) derived from young mice. In contrast, E2 pre-treatment of BMMs from aged mice did not significantly change the CRP-induced increase in inflammatory mediator expression (Figure 2A) and paradoxically, E2 enhanced rather than depressed CRP-induced upregulation of inflammatory mediators in VSMCs derived from aged mice (Figure 2B). Multiplex analysis confirmed that protein levels of inflammatory mediators increased in response to CRP in BMMs from both young and aged mice and that E2 pre-treatment significantly reduced these levels in BMMs derived from young but not aged mice (Figure 2C).
Figure 2.
Effects of C-reactive protein (CRP, 50 μg·mL−1) and 17β-estradiol (E2, 10−7 M) alone versus E2 pretreatment followed by CRP (E2+CRP) on mRNA expression of proinflammatory mediators in bone marrow macrophages (BMMs, A) and vascular smooth muscle cells (VSMCs, B) derived from young and aged female C57BL/6 mice. (C) Effects of CRP (50 μg·mL−1) alone and 17β-estradiol (E2, 10−7 M) pretreatment prior to CRP administration (E2+CRP) on protein expression of proinflammatory mediators in BMMs derived from young and aged female C57BL/6 mice. Results shown are mean±SEM. *; P<0.05 vs. respective vehicle group (Veh). #; P<0.05 vs. respective CRP group.
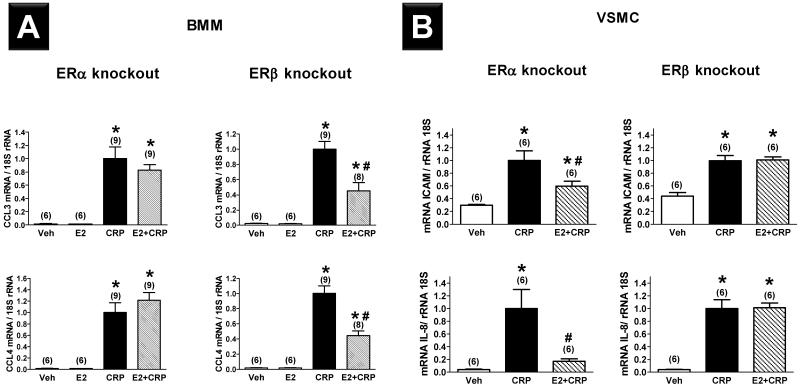
To test the hypothesis that the age-dependent influence of E2 on CRP-induced expression of proinflammatory mediators is ER-dependent, BMMs and VSMCs were cultured from young female ERα−/− and ERβ−/− mice. E2 had no effect on CRP-induced CCL3 and CCL4 mRNA expression in BMMs derived from ERα−/− mice, but it attenuated the CRP effect in BMMs derived from ERβ−/− mice (Figure 3A). The E2 effect in ERβ KO is comparable to that seen in BMMs cultured from young mice that express both ERα and ERβ (Figure 2A), indicating that the effect of E2 on CRP-induced mRNA expression of CCL3 and CCL4 in BMMs is mediated by ERα and not ERβ. Conversely, E2 attenuated CRP-induced ICAM and IL-8 mRNA expression in VSMCs derived from ERα−/− mice, but had no effect on CRP-driven inflammatory mediator expression in VSMCs derived from ERβ−/− mice (Figure 3B).
Figure 3.
Effects of C-reactive protein (CRP, 50 μg·mL−1) alone and 17β-estradiol (E2, 10−7 mol) pretreatment prior to CRP administration (E2+CRP) on mRNA expression of proinflammatory mediators in bone marrow macrophages (BMMs, A) and vascular smooth muscle cells (VSMCs, B) derived from young female ERα knockout and ERß knockout mice. Results shown are mean±SEM. *; P<0.05 vs. respective vehicle group (Veh). #; P<0.05 vs. respective CRP group.
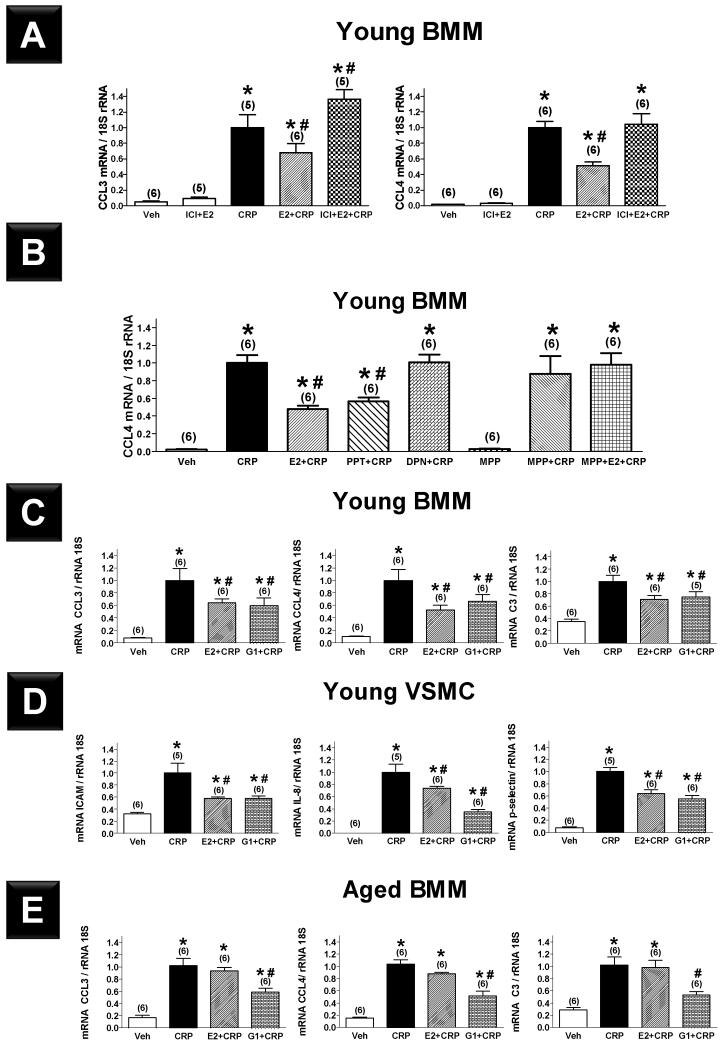
The findings in VSMCs were consistent with the previous studies from our laboratory that demonstrated using E2 agonists and antagonists that E2 inhibits proinflammatory mediator expression in VSMCs through an ERβ-mediated mechanism.4 To confirm the findings in BMMs, BMMs from young mice were pretreated with the selective ER down-regulator ICI 182,780 or vehicle for 2 h followed by E2 for 24 h. Cells were then treated with CRP or vehicle for 4 h, and CCL3 and CCL4 mRNA expression was measured. Pre-treatment with ICI completely blocked the inhibitory effect of E2 on CRP-induced expression of CCL3 and CCL4 (Figure 4A). After establishing the ER-dependence of the E2 effect, we used selective ER agonists and antagonists to identify the specific ER subtype responsible. PPT, a selective ERα agonist, reproduced the effects previously seen with E2, while MPP, a selective ERα antagonist, completely blocked the E2 effect (Figure 4B). In contrast, DPN, a selective ERß agonist, had no effect on CRP-induced expression of CCL4. These data confirm our findings in ER knockout mice that E2 acts through ERα and not ERß on BMMs to abrogate CRP-induced mRNA expression of CCL4.
Figure 4.
Effects of C-reactive protein (CRP, 50 μg·mL−1) alone and pretreatment with 17β-estradiol (E2, 10−7 M), nonselective ER antagonist ICI (10−6 M) in addition to E2 (A), selective ERα agonist PPT (10−7 M), selective ERß agonist DPN (10−7 M), and selective ERα antagonist MPP (10−5 M) in addition to E2 (B) prior to CRP administration on mRNA expression of proinflammatory mediators in bone marrow macrophages (BMMs) derived from young female C57BL/6 mice. Effects of CRP alone and pretreatment with E2 or selective G-protein coupled estrogen receptor agonist (G1, 10−7 M) prior to CRP administration on mRNA expression of proinflammatory mediators in BMMs (C) and vascular smooth muscle cells (VSMCs, D) derived from young and BMMs (E) derived from aged female C57BL/6 mice. Results shown are mean±SEM. *; P<0.05 vs. respective vehicle group (Veh). #; P<0.05 vs. respective CRP group.
We then examined the effect of the selective GPR30 agonist G1 on CRP-mediated inflammation in BMMs and VSMCs. BMMs and VSMCs derived from young female mice were pretreated with G1 for 24 h followed by CRP for 4 h. Pretreatment with G1 significantly attenuated the CRP-induced increased expression of inflammatory mediators in BMMs (Figure 4C, CCL3, CCL4 and C3) and VSMCs (Figure 4D, ICAM, IL-8 and p-selectin) derived from young mice. However, in BMMs derived from aged mice, G1 but not E2 significantly inhibited CRP-derived inflammation (Figure 4E). Both G15 and G36 (GPR30 antagonists) had pro-inflammatory effects in our system. Therefore, we could not use these antagonists to address the hypothesis that blocking GPR30 will block the effects of E2 and G1 (Figure SI).
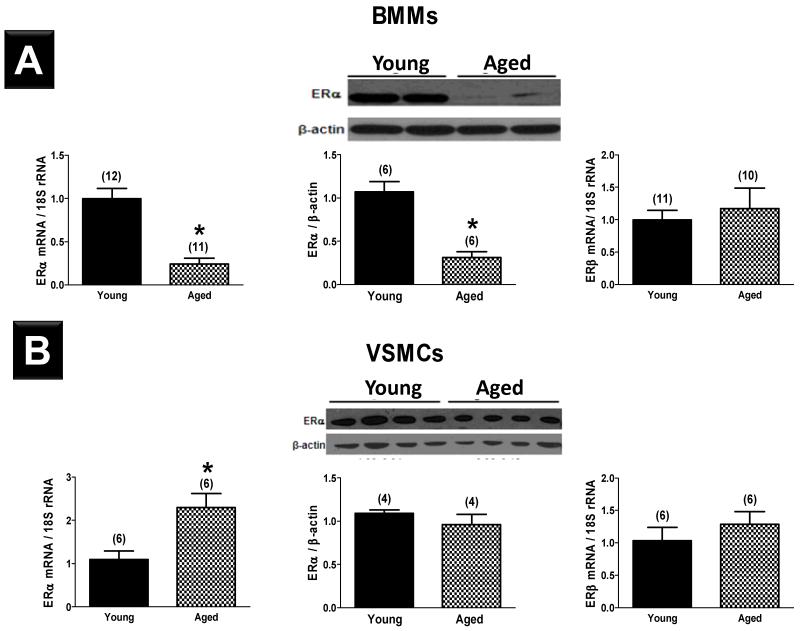
ERα mRNA and protein were detectable in BMMs and VSMCs derived from C57BL/6 mice. In comparison to BMMs derived from young mice, BMMs from aged mice expressed 76±7% less ERα mRNA and 71±7% less ERα protein (Figure 5A). In contrast, expression of ERα mRNA by VSMCs derived from aged mice was significantly higher than that in VSMCs from young animals, while ERα protein levels did not differ between groups (Figure 5B). BMMs and VSMCs derived from aged female mice expressed ERβ mRNA at a level similar to their young counterparts. We were unable to reliably measure ERβ protein levels due to limitations in currently available mouse ERβ antibodies.18
Figure 5.
mRNA expression (left) and protein expression (middle) of ERα in bone marrow macrophages (BMMs, A) and vascular smooth muscle cells (VSMCs, B) derived from young and aged female C57BL/6 mice. mRNA expression of ERβ in cells derived from young and aged female C57BL/6 mice is shown (right). Results shown are mean±SEM. *; P<0.05 vs. young mice.
Discussion
This study demonstrates for the first time that E2 regulates human CRP-induced inflammation in murine BMMs and VSMCs in an age-dependent manner; E2 inhibits inflammation in cells derived from young but not aged mice. Unlike in tumor necrosis factor (TNF)-α treated rat VSMCs, in which we have shown that the anti-inflammatory effect of E2 is mediated via ERβ using both selective agonists/antagonists4 and ER knockout mice, we demonstrate that the effect of E2 on human CRP-induced inflammation in mouse BMMs is mediated via ERα and that expression of ERα on mouse BMMs is diminished with aging. We also show that G1, a selective GPR30 agonist, reproduces the anti-inflammatory effects of E2 in VSMCs and BMMs. Further, G1 suppresses the inflammatory response in BMMs derived from aged mice which are unresponsive to E2. These findings, if confirmed in humans, may help reconcile the seemingly paradoxical effects of E2 seen in clinical trials and could potentially define a subpopulation of women who may derive benefit from hormone therapy.
Previous studies from our laboratory and others have shown that E2 has an anti-inflammatory effect in animal models of acute vascular injury.15 In rodents, inflammatory cells, including macrophages, infiltrate the adventitia of the injured artery within hours of the insult.5,13 These inflammatory cells are attracted to and infiltrate the site of injury due to increased expression of inflammatory mediators by VSMCs in the medial domain of the artery.3 Administration of E2 to OVX rodents attenuates expression of inflammatory mediators and the resultant infiltration of inflammatory cells into injured carotid arteries, limiting the neointimal response to vascular injury.3,5,14 In vitro studies showed that E2 inhibits inflammation in rat VSMCs by two distinct mechanisms: 1) enhancing expression of IκBα, a direct inhibitor of NFκB activation, thus accelerating a negative feedback loop in NFκB signaling, and 2) directly inhibiting binding of NFκB to promoters of inflammatory genes, thereby inhibiting their expression.19 The effect of E2 on inflammation may be context dependent. A recent study by Calippe et al. demonstrated that E2 treatment, via ERα activation in macrophages in vivo, enhances their ability to produce inflammatory mediators and cytokines upon subsequent TLR activation.20 In the current study, E2 treatment of isolated BMMs and VSMCs derived from young female mice inhibited the CRP-induced inflammatory response of the cells. These data provide mechanistic insights into how E2 protects the vasculature of young female CRPtg against inflammation and adverse remodeling in response to acute vascular injury. Neointima formation in the carotid artery ligation model closely resembles that seen after reconstructive procedures such as angioplasty, vascular stenting, vascular grafts, and vein grafts. The model also offers an excellent opportunity to study vascular inflammation in response to acute injury, an essential component of all vascular diseases. Furthermore, since BMMs and VSMCs are intimately involved in many disease processes (eg: atherosclerosis) the findings could have wider implications than those studied here.
Observational studies in humans show benefit associated with E2 in women who choose to use menopausal hormones. In the Nurses’ Health Study, which followed 70,533 postmenopausal women for up to 20 years and documented 1,258 major coronary events (nonfatal myocardial infarction or fatal coronary disease), current use of hormone therapy resulted in a multi-variate adjusted relative risk of 0.61 (95%CI 0.52-0.71) compared to never users.6 However, randomized controlled trials, such as WHI and Heart and Estrogen/progestin Replacement Study have shown harm, or at least no benefit, of menopausal hormone treatment on CVD events.7,21,22 The reason for this apparent paradox is not fully understood, but several explanations have been proposed, including the formulation used (conjugated equine estrogen vs. E2), dose, route of administration (oral vs. transdermal), duration of use, the potential deleterious effect of medroxyprogesterone acetate on the vasculature, the presence of comorbidities and pre-existing vascular disease, and the timing of E2 administration relative to onset of menopause and age.10,23,24 The intriguing ‘timing hypothesis’ proposes that the effect of E2 administration on the vasculature is dependent on the age and hormonal milieu of the recipient.8,9 Indeed, age has been shown to be an important determinant of the vascular effects of E2 in women, with beneficial effects observed in the early post-menopausal years but not in older (≥60 years) post-menopausal women.25 In observational studies, and in real-life, women generally start menopausal hormones in the peri-menopausal or early post-menopausal period, while randomized controlled trials enrolled women with an average age of 63-67 years.6,7,21,22 Subgroup analysis of one of these studies, the WHI, showed that when hormone therapy was initiated at a young age (50-59 years) it was associated with reduced risk of CVD.26,27 These findings were recently confirmed in the Danish Osteoporosis Prevention Study which randomized 1,006 younger women (mean age 50 years) to receive hormone therapy vs. no therapy. After 10 years of treatment, hormone therapy was associated with 52% reduction in the primary endpoint of death, myocardial infarction or heart failure.28 Interestingly, even in this relatively young population, an age-effect was evident (RR 0.35 for <50 years vs. 0.63 for ≥50 years).
Our laboratory has previously shown that aged OVX rats subjected to balloon injury of the carotid artery lose the vasoprotective and anti-inflammatory responses to exogenous E2 seen in younger animals.29 We confirmed here this age-dependent attenuation of the E2 effect in aged CRPtg subjected to ligation injury of the carotid artery. Further, we explored the effect of age on the actions of E2 on the two cell types central to the mouse carotid ligation model of vascular injury. Macrophages30 and VSMCs31 have been shown to express ERs and E2 has been shown to inhibit the expression of pro-inflammatory mediators in macrophages32 and VSMCs4 derived from young animals. Unlike in cells derived from young animals, E2 treatment of BMMs and VSMCs derived from aged mice either did not attenuate (BMMs) or exacerbated (VSMCs) CRP-induced inflammation. In vivo, we have previously demonstrated that E2 inhibits CRP-mediated vascular remodeling in young CRPtg,14 and we demonstrate here that this effect is lost and may be reversed in aged animals. These data support the concept that E2 modulates the vascular inflammatory response in an age-dependent manner and may help explain the lack of benefit of E2 treatment in clinical trials in elderly postmenopausal women. It is plausible that the findings reported here are related to prolonged deprivation from physiological levels of E2 in aged mice rather than aging itself. We did not directly measure E2 levels in our mice due to limitations of the available assays, which are unable to differentiate serum E2 levels between OVX and intact animals.33 However, uterine weight was significantly lower in aged compared to young mice (62±11 vs. 119±15 mg, p<0.05), suggesting E2 deprivation. Prior studies using different models have demonstrated that the anti-inflammatory effects of E2 are lost after an extended period of hypoestrogenicity.34,35 The effects of age vs. time from menopause (or OVX) as a determinant of E2-induced modulation of vascular inflammation and remodeling in response to acute or chronic injury is an important topic for future basic and translational studies.
The anti-inflammatory/vasoprotective effects of E2 in vascular injury are known to be ER-mediated,15 but the specific ER subtype(s) responsible for these effects have been the subject of controversy. Studies in knockout mice demonstrated that ERα, but not ERβ, mediates the facilatatory effects of E2 on re-endothelialization after electric vascular injury.36 Krom et al. used selective ERα and ERβ agonists and antagonists in a mouse model of vascular injury to show that local activation of ERβ with DPN inhibited neointima formation in a dose-dependent manner, while ERα activation using PPT inhibited the formation of neointima at low but not high doses.37 The effects of E2 and DPN on neointima formation were not blocked with the ERα selective antagonist MPP, supporting their ERβ dependence. Thus, both ER subtypes appear to contribute to vasoprotection in murine models. We show here that E2 inhibits proinflammatory mediator expression in both BMMs and VSMC and that its effects in BMMs are mediated via ERα and in VSMC via ERβ. These data taken together indicate that the effects of systemic E2 treatment on the vasculature are mediated via different ER subtypes expressed by different cell types.
In addition to the classical ERs, GPR30 has been identified as an important ER that mediates many estrogenic effects on the vasculature.16 G1, a selective GPR30 agonist, reproduced the anti-inflammatory effects of E2 in VSMC and BMMs derived from young mice. Interestingly, G1 also exhibited anti-inflammatory effects in BMMs derived from aged mice in which E2 had no effect (Figure 4E). Further, E2 did not have anti-inflammatory effects in ERα knockout BMMs or ERβ knockout VSMCs (Figure 3). These observations are consistent with results of recent studies demonstrating that G1 may have GPR30 independent activities,38 or alternatively, with findings of functional cross-talk between the traditional ERs and GPR30, as suggested by other reports.16,39 These exciting findings may reveal new therapeutic targets for vasoprotection that are effective in aging women.
The cellular mechanisms responsible for the decrease in E2-induced vasoprotection seen with aging are poorly understood. A limited number of studies have examined the effect of aging on ER expression in the vasculature. In an early study that was not able to differentiate ER-subtypes, coronary arteries from postmenopausal women tended to have fewer ERs than those of premenopausal women, independent of the presence of atherosclerosis.40 In a more recent study, ERα levels were decreased in endothelial cells from postmenopausal vs. premenopausal women, and ERα expression was strongly related to endothelial function.41 Consistent with our observation that the inhibitory effect of E2 on CRP-induced inflammation in BMMs is ERα dependent, we demonstrated that macrophages derived from aged mice expressed significantly less ERα than those derived from young mice. This novel finding may help explain why E2 administration is vasoprotective in young animal models of CVD and in young peri-menopausal women but increases risk of CVD in older postmenopausal women. The change in expression of ERα with aging could be regulated by epigenetic modification of the gene promoter. For example, variation in methylation-associated inactivation of ER gene promoters in various tissue-types, including in the cardiovascular system, has been described in aging rodents and humans.42,43 The hypermethylation of ERα with aging is more impressive when taken in context of the global hypomethylation of the genome that accompanies the aging process.44 It is important to mention that the observed changes in ERα expression with aging are cell-type specific since they are not seen in VSMCs and that age-dependent change in ERα expression does not account for the paradoxical effect of E2 on CRP-induced inflammatory mediator expression in VSMCs. We were not able to explore whether ERβ levels are decreased in VSMCs with aging due to limitations in available antibodies to this receptor in the mouse.18
Several limitations apply to our work. First, we recognize the limitations of using pharmacologic ER agonists and antagonists to delineate biological processes. In order to optimize selectivity, we have carried out dose-response analyses4 and have used the lowest effective doses of these agents. We verified our results using cells derived from ER knockout animals. We note that the ERα knockout mouse that we have used has been shown to have residual E2 binding to uterine tissue, likely due to a truncated ERα isoform.45-47 Therefore, we can’t exclude the possibility that residual ERα activity in our mouse model may have been responsible for the non-significant trend for E2 effect in Figure 3A. Further, we have not dissected the mechanism by which E2 inhibits inflammation in our cells. Since the effects of CRP in our model are attributed to its activation of immunoglobulin G Fc receptors (FcγRs),12 the observation that E2, via its interaction with ERs, influences expression of FcγRs on the surface of macrophages48 may partially explain the effect of E2 on CRP-mediated vascular inflammation seen in our study. However, since the anti-inflammatory effects of E2 seen in BMMs and VSMCs are not restricted to CRP-induced inflammation, but are also evident in TNF-α-induced inflammation (Figures SII-III), the mechanism is more likely to involve a common downstream pathway (e.g. at level of NFκB as described earlier).
In conclusion, the current study reveals that the anti-inflammatory effects of E2 seen in BMMs and VSMCs derived from young female mice and in injured carotid arteries of young mice are lost with aging. G1, a GPR30 agonist, reproduced the effects of E2 in BMMs and VSMCs. E2 lost it anti-inflammatory effect in BMMs derived from ERα−/− and in VSMCs from ERβ−/−. ERα expression in BMMs is greatly diminished with aging. These results may help explain the lack of benefit of E2 treatment seen in clinical trials of menopausal hormone therapy and pave the way for novel therapeutic interventions that are tailored to preserve E2 responsiveness in aging vascular cells.
Supplementary Material
Significance.
The Women’s Health Initiative (WHI) reported increased cardiovascular events in women treated with menopausal hormones compared to placebo. These results were in contrast to the well-established anti-inflammatory and vasoprotective effects of 17β-Estradiol (E2) seen in in vitro preparations, young adult laboratory animals, and observational studies in pre- and peri-menopausal women. Importantly, WHI enrolled older women than the observational studies. We showed in a mouse model of vascular injury and in macrophages and vascular smooth muscle cells in culture that E2 inhibits inflammation in cells derived from young but not aged mice and in injured carotid arteries of young but not aged animals. This demonstration that the vasoprotective effects of E2 disappear with advancing age may explain the vasotoxic effects of E2 seen in clinical trials of postmenopausal women, e.g, WHI, and pave the way for novel therapeutic interventions designed to preserve E2 responsiveness in aging vascular cells.
Acknowledgements
a) Acknowledgments: none.
b) Sources of Funding:
This work was supported, in part, by a Veterans Affairs Biomedical Laboratory Research & Development Service Merit Award (to Dr. Hage); American Heart Association NCRP Scientist Development Grant 0930098N (to Dr. Hage) and a Greater Southeast Affiliate grant 09BGIA2250367 (to Dr. Xing); National Heart, Lung, and Blood Institute grants R01 HL087980 (to Dr. Oparil) and HL080017, HL044195 (to Dr. Chen).
Abbreviations
- BMM
bone marrow-derived macrophages
- CRP
C-reactive protein
- CRPtg
C-reactive protein transgenic mice
- CVD
cardiovascular disease
- E2
17β-Estradiol
- ER
estrogen receptor
- GPR30
G-protein coupled receptor 30
- OVX
ovariectomized
- TNF
tumor necrosis factor
- VSMC
vascular smooth muscle cells
- WHI
Women’s Health Initiative
Footnotes
c) Disclosures: none.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Bakir S, Mori T, Durand J, Chen YF, Thompson JA, Oparil S. Estrogen-induced vasoprotection is estrogen receptor dependent: evidence from the balloon-injured rat carotid artery model. Circulation. 2000;101:2342–2344. doi: 10.1161/01.cir.101.20.2342. [DOI] [PubMed] [Google Scholar]
- 3.Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;110:1664–1669. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- 4.Xing D, Feng W, Miller AP, Weathington NM, Chen YF, Novak L, Blalock JE, Oparil S. Estrogen modulates TNF-alpha-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-beta activation. Am J Physiol Heart Circ Physiol. 2007;292:H2607–2612. doi: 10.1152/ajpheart.01107.2006. [DOI] [PubMed] [Google Scholar]
- 5.Xing D, Miller A, Novak L, Rocha R, Chen YF, Oparil S. Estradiol and progestins differentially modulate leukocyte infiltration after vascular injury. Circulation. 2004;109:234–241. doi: 10.1161/01.CIR.0000105700.95607.49. [DOI] [PubMed] [Google Scholar]
- 6.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Phillips LS, Langer RD. Postmenopausal hormone therapy: critical reappraisal and a unified hypothesis. Fertil Steril. 2005;83:558–566. doi: 10.1016/j.fertnstert.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy: physiological complexity belies therapeutic simplicity. Science. 2004;304:1269–1273. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- 10.Bowling M, Oparil S, Hage FG, Hilgers RH, Xing D. Sex hormones and vascular function. In: Dubey RK, editor. Sex hormones. InTech; Rijeka, Croatia: 2012. ISBN: 978-953-307-856-4. [Google Scholar]
- 11.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 12.Xing D, Hage FG, Chen YF, McCrory MA, Feng W, Skibinski GA, Majid-Hassan E, Oparil S, Szalai AJ. Exaggerated neointima formation in human C-reactive protein transgenic mice is IgG Fc receptor type I (Fc gamma RI)-dependent. Am J Pathol. 2008;172:22–30. doi: 10.2353/ajpath.2008.070154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hage FG, Oparil S, Xing D, Chen YF, McCrory MA, Szalai AJ. C-reactive protein-mediated vascular injury requires complement. Arterioscler Thromb Vasc Biol. 2010;30:1189–1195. doi: 10.1161/ATVBAHA.110.205377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Oparil S, Chen YF, McCrory MA, Skibinski GA, Feng W, Szalai AJ. Estrogen treatment abrogates neointima formation in human C-reactive protein transgenic mice. Arterioscler Thromb Vasc Biol. 2005;25:2094–2099. doi: 10.1161/01.ATV.0000179602.85797.3f. [DOI] [PubMed] [Google Scholar]
- 15.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrabarti S, Davidge ST. G-protein coupled receptor 30 (GPR30): a novel regulator of endothelial inflammation. PLoS One. 2012;7:e52357. doi: 10.1371/journal.pone.0052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernelot Moens SJ, Schnitzler GR, Nickerson M, Guo H, Ueda K, Lu Q, Aronovitz MJ, Nickerson H, Baur WE, Hansen U, Iyer LK, Karas RH. Rapid estrogen receptor signaling is essential for the protective effects of estrogen against vascular injury. Circulation. 2012;126:1993–2004. doi: 10.1161/CIRCULATIONAHA.112.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder MA, Smejkalova T, Forlano PM, Woolley CS. Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. J Neurosci Methods. 2010;188:226–234. doi: 10.1016/j.jneumeth.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing D, Oparil S, Yu H, Gong K, Feng W, Black J, Chen YF, Nozell S. Estrogen modulates NFkappaB signaling by enhancing IkappaBalpha levels and blocking p65 binding at the promoters of inflammatory genes via estrogen receptor-beta. PLoS ONE. 2012;7:e36890. doi: 10.1371/journal.pone.0036890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lelu K, Krust A, Pipy B, Bayard F, Arnal JF, Guery JC, Gourdy P. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185:1169–1176. doi: 10.4049/jimmunol.0902383. [DOI] [PubMed] [Google Scholar]
- 21.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 22.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Hage FG, Oparil S. Ovarian hormones and vascular disease. Curr Opin Cardiol. 2013;28:411–416. doi: 10.1097/HCO.0b013e32836205e7. [DOI] [PubMed] [Google Scholar]
- 24.Barton M, Meyer MR, Haas E. Hormone replacement therapy and atherosclerosis in postmenopausal women: does aging limit therapeutic benefits? Arterioscler Thromb Vasc Biol. 2007;27:1669–1672. doi: 10.1161/ATVBAHA.106.130260. [DOI] [PubMed] [Google Scholar]
- 25.Sherwood A, Bower JK, McFetridge-Durdle J, Blumenthal JA, Newby LK, Hinderliter AL. Age moderates the short-term effects of transdermal 17beta-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler Thromb Vasc Biol. 2007;27:1782–1787. doi: 10.1161/ATVBAHA.107.145383. [DOI] [PubMed] [Google Scholar]
- 26.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 27.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J, Investigators WHI Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, Kober L, Jensen JE. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. doi: 10.1136/bmj.e6409. [DOI] [PubMed] [Google Scholar]
- 29.Miller AP, Xing D, Feng W, Fintel M, Chen YF, Oparil S. Aged rats lose vasoprotective and anti-inflammatory actions of estrogen in injured arteries. Menopause. 2007;14:251–260. doi: 10.1097/01.gme.0000235366.39726.f6. [DOI] [PubMed] [Google Scholar]
- 30.McLaren J, Prentice A, Charnock-Jones DS, Millican SA, Muller KH, Sharkey AM, Smith SK. Vascular endothelial growth factor is produced by peritoneal fluid macrophages in endometriosis and is regulated by ovarian steroids. J Clin Invest. 1996;98:482–489. doi: 10.1172/JCI118815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation. 1994;89:1943–1950. doi: 10.1161/01.cir.89.5.1943. [DOI] [PubMed] [Google Scholar]
- 32.Frazier-Jessen MR, Kovacs EJ. Estrogen modulation of JE/monocyte chemoattractant protein-1 mRNA expression in murine macrophages. J Immunol. 1995;154:1838–1845. [PubMed] [Google Scholar]
- 33.Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152:4443–4447. doi: 10.1210/en.2011-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pechenino AS, Lin L, Mbai FN, Lee AR, He XM, Stallone JN, Knowlton AA. Impact of aging vs. estrogen loss on cardiac gene expression: estrogen replacement and inflammation. Physiol Genomics. 2011;43:1065–1073. doi: 10.1152/physiolgenomics.00228.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103:423–428. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- 37.Krom YD, Pires NM, Jukema JW, de Vries MR, Frants RR, Havekes LM, van Dijk KW, Quax PH. Inhibition of neointima formation by local delivery of estrogen receptor alpha and beta specific agonists. Cardiovasc Res. 2007;73:217–226. doi: 10.1016/j.cardiores.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 38.Wang C, Lv X, Jiang C, Davis JS. The putative G-protein coupled estrogen receptor agonist G-1 suppresses proliferation of ovarian and breast cancer cells in a GPER-independent manner. Am J Transl Res. 2012;4:390–402. [PMC free article] [PubMed] [Google Scholar]
- 39.Filice E, Recchia AG, Pellegrino D, Angelone T, Maggiolini M, Cerra MC. A new membrane G protein-coupled receptor (GPR30) is involved in the cardiac effects of 17beta-estradiol in the male rat. J Physiol Pharmacol. 2009;60:3–10. [PubMed] [Google Scholar]
- 40.Losordo DW, Kearney M, Kim EA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;89:1501–1510. doi: 10.1161/01.cir.89.4.1501. [DOI] [PubMed] [Google Scholar]
- 41.Gavin KM, Seals DR, Silver AE, Moreau KL. Vascular endothelial estrogen receptor alpha is modulated by estrogen status and related to endothelial function and endothelial nitric oxide synthase in healthy women. J Clin Endocrinol Metab. 2009;94:3513–3520. doi: 10.1210/jc.2009-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa JP. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43:985–991. doi: 10.1016/s0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- 43.Novensa L, Novella S, Medina P, Segarra G, Castillo N, Heras M, Hermenegildo C, Dantas AP. Aging negatively affects estrogens-mediated effects on nitric oxide bioavailability by shifting ERalpha/ERbeta balance in female mice. PLoS ONE. 2011;6:e25335. doi: 10.1371/journal.pone.0025335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalo S. Epigenetic alterations in aging. J Appl Physiol. 2010;109:586–597. doi: 10.1152/japplphysiol.00238.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- 46.Kos M, Denger S, Reid G, Korach KS, Gannon F. Down but not out? A novel protein isoform of the estrogen receptor alpha is expressed in the estrogen receptor alpha knockout mouse. J Mol Endocrinol. 2002;29:281–286. doi: 10.1677/jme.0.0290281. [DOI] [PubMed] [Google Scholar]
- 47.Pendaries C, Darblade B, Rochaix P, Krust A, Chambon P, Korach KS, Bayard F, Arnal JF. The AF-1 activation-function of ERalpha may be dispensable to mediate the effect of estradiol on endothelial NO production in mice. Proc Natl Acad Sci U S A. 2002;99:2205–2210. doi: 10.1073/pnas.042688499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer PR, Winger V, Kramer SF. 17beta-Estradiol utilizes the estrogen receptor to regulate CD16 expression in monocytes. Mol Cell Endocrinol. 2007;279:16–25. doi: 10.1016/j.mce.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.