Abstract
Melanoma exhibits variable resistance to the alkylating agent temozolomide (TMZ). We evaluated the potential of adenovirus expressing forkhead human transcription factor like 1 triple mutant (Ad-FKHRL1/TM) to sensitize melanoma cells to TMZ. Four melanoma cell lines were treated with Ad-FKHRL1/TM and TMZ, alone or in combination. Apoptosis was assessed by activation and inhibition of caspase pathway, nuclei fragmentation, and annexin V staining. The potential therapeutic efficacy of Ad-FKHRL1/TM with TMZ was also assessed in a mouse melanoma xenograft model. Combination therapy of Ad-FKHRL1/TM and TMZ resulted in greater cell killing (<20% cell viability) compared with single therapy and controls (p<0.05). Combination indices of Ad-FKHRL1/TM and TMZ therapy indicated significant (p<0.05) synergistic killing effect. Greater apoptosis induction was found in cells treated with Ad-FKHRL1/TM and TMZ than with Ad-FKHRL1/TM or TMZ-treated cells alone. Treatment with TMZ enhanced adenovirus transgene expression in a cell type-dependent manner. In an in vivo model, combination therapy of Ad-FKHRL1/TM with TMZ results in greater tumor growth reduction in comparison with single treatments. We suggest that Ad-FKHRL1/TM is a promising vector to sensitize melanoma cells to TMZ, and that a combination of both approaches would be effective in the clinical setting.
Introduction
Cutaneous melanoma is a tremendous health problem in the United States and globally, and the incidence is increasing in the United States (Simard et al., 2012). While localized disease is readily treated with potential curative resection, the effectiveness of systemic chemotherapies is limited. Patients with stage IV metastatic melanoma have 5-year survival rates of 10% (Balch et al., 2009). Temozolomide (TMZ) is an oral chemotherapy agent used for glioma, and as a second-line therapy with limited efficacy in melanoma (Friedman et al., 2000). TMZ is converted at physiologic pH to the metabolically active agent 5-(3-methyl)-1-triazeu-1-yl)imid-azole-4-carboxamide (MTIC); this is the same metabolically active end product of dacarbazine (Newlands et al., 1992). Because response rates to TMZ are limited in metastatic melanoma (Patel et al., 2011; Kottschade et al., 2013), alternative strategies are needed. One promising approach to sensitizing melanoma cells to TMZ is the transduction of cancer cells with adenovirus encoding a tumor suppressor gene.
The members of the forkhead box O (FoxO) family of transcription factors play important roles in many physiological and pathological processes. These factors have been postulated to be tumor suppressors and have been studied as potential gene therapy agents. Previous investigators have shown that the rational combinations of gene therapy treatments and TMZ are effective against malignant gliomas, which are the other solid tumor against which TMZ is used (Rainov et al., 2001; Huang et al., 2013; Stedt et al., 2013). We hypothesized that similar effects may be seen in melanoma, which is also treated by TMZ clinically. In this study, we evaluated the potential of adenovirus expressing forkhead human transcription factor-like 1 triple mutant (Ad-FKHRL1/TM) to sensitize melanoma cells to TMZ. Adenoviral therapy in combination with chemotherapy is a promising approach to cancer treatment. With lower doses of both agents, one may limit the inherent side effects and dose-limiting toxicities without compromising tumoricidal activity. We hypothesized that combination therapy for the treatment of melanoma using TMZ with Ad-FKHRL1/TM would be effective in the clinical setting.
Materials and Methods
Cell lines and culture conditions
Human melanoma cell lines SK-MEL-28, A2058, and A375 were purchased from the American Type Culture Collection (Rockville, MD). Human melanoma cell line DM6 was kindly provided by DS Tyler (Duke University, Durham, NC). SK-MEL-28 cells were cultured in α-MEM. A2058 and A375 cells were cultured in DMEM. DM6 cells were cultured in IMDM. All media were supplemented with 10% of fetal bovine serum and penicillin/streptomycin (100 U/ml). All cell culture reagents were purchased from Life Technologies (Grand Island, NY). Cells were cultured in a 5% CO2 incubator at 37°C.
Adenoviral vectors and drug therapies
Two replication-deficient adenoviral vectors were used. Ad-LacZ that expresses β-galactosidase was used as a control as previously described (Gomez-Gutierrez et al., 2010). Ad-FKHRL1/TM was used for treatment as described previously (Gomez-Gutierrez et al., 2006). TMZ was purchased from Sigma-Aldrich (St. Louis, MO), and stock solutions of 100 mmol were prepared in dimethyl sulfoxide and stored at −20°C.
Cell viability, annexin V staining, and nuclear localization analysis
For infections and chemotherapy treatments, 1×105 melanoma cells were plated in 12-well plates and treated 24 hr later with indicated therapy. Viral infection was performed at indicated multiplicity of infection (MOI). For control infection, we used cell line-specific media alone without virus or drug. Cell viability was assessed 72 hr after experimental therapy by measuring the conversion of the tetrazolium salt (MTT) to formazan, according to the manufacturer's instructions (Boehringer Mannheim, Indianapolis, IN) and as was described previously (Mosmann, 1983). For annexin V staining, cells were stained with annexin V-PE and 7-AAD, according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). Cells were analyzed by FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ) and with FlowJo software (Tree Star Inc., Ashland, OR). Nuclei morphology inspection was performed with Hoechst 33342 staining (AnaSpec Inc., Fremont, CA) at a final concentration of 2 μM 48 hr after treatment as described above. Nucleus fragmentation was visualized at 40× magnification with the EVOS FL Imaging System (Advanced Microscopy Group, Mill Creek, WA) under 357/44 (nm) and 447/60 (nm) excitation and emission visualization.
Western blot analysis
Western blotting was performed by standard procedure as described previously (Gomez-Gutierrez et al., 2012). The primary antibodies used were rabbit antihuman FKHRL1, rabbit antihuman cleaved caspase-3, rabbit antihuman cleaved caspase-9 (Cell Signaling, Danvers, MA), rabbit anti-LC3 polyclonal antibody (Novus Biologicals, Littleton, CO), and rabbit antihuman-α-actin (Sigma-Aldrich). Next, the membranes were incubated with antirabbit Ig peroxidase-linked species-specific whole Abs (GE Healthcare Bio-Sciences, Pittsburgh, PA). Electrochemiluminescent (ECL) reagents were used to detect the signals, according to the manufacturer's instructions (GE Healthcare Bio-Sciences). The scanned band intensity was quantitated by Gel-pro Analyzer 4.0 software (Media Cybernetics, Bethesda, MD) according to the manufacturer's tutorial. Densitometric value for each band was expressed as integrated optical density and normalized with actin. The results were reported as the ratios of normalized band intensities of FKHRL1/TM to actin.
Caspase pathway inhibition
Melanoma cells were cultured in the absence or presence of general caspase inhibitor z-VAD-fmk at a concentration of 50 μM. The cells were then treated with cisplatin at a concentration of 25 μM as positive control. Cells also were either infected with Ad-FKHRL1/TM or treated with TMZ alone or combination of both at their respective LD50 (Supplementary Figs. S1 and S2). Three days after infection, an MTT assay was performed to determine cell viability, as described previously (Mosmann, 1983).
Mouse melanoma xenograft model
Subcutaneous tumors were formed in the flanks of 6-week-old athymic BALB/c nu/nu male mice (Charles River Laboratories, Wilmington, MA) by injecting 5×106 DM6 human melanoma cells in 100 μl of phosphate buffered saline (PBS). Seven days following injection, palpable tumors were formed. Treatment groups were as follows: Ad-LacZ, Ad-FKHRL1/TM, TMZ, and Ad-FKHLR1/TM+TMZ. Mice were randomly selected and injected in the flank with Ad-FKHRL1/TM (1×109 plaque forming units [pfu]) or Ad-LacZ (1×109 pfu) (n=5 for each group) every 3 days for a total of 4 treatments. TMZ was administered via intraperitoneal injections of 50 mg/kg (diluted in PBS) daily for 5 consecutive days. Tumor size was measured with calipers in 2 dimensions (length and width) every 3 days for 31 days, and tumor volume was estimated by the following equation: V=(L×W2)/2, where V is volume, L is length, and W is width. Animal experiments were performed in accordance with institutional guidelines and were approved by the University of Louisville Institutional Animal Care and Use Committee.
Immunohistochemistry
Tumors were excised 24 hr after the fourth injection following euthanization, fixed in 10% formalin, embedded in paraffin blocks, and processed for histological analysis. Expression levels of cleaved caspase-3 and FKHRL1/TM were evaluated. Rabbit antihuman FoxO3a (1:200) or rabbit antihuman cleaved caspase-3 (Asp175)(5A1E) (1:200) (Cell Signaling) was used to detect FKHRL1/TM or cleaved caspase-3 expression, respectively. The slides were then washed with PBS, incubated with the standard ultra-sensitive ABC peroxidase staining kit (Pierce, Rockford, IL), and detected with diaminobenzidine tetrahydrochloride solution containing 0.006% H2O2. Hematoxylin was used as a counterstain. Tissue sections stained without primary antibodies were used as negative controls. Photographs were taken with×20 magnification and analyzed with NIS-Elements BR 3.0 software (Nikon instruments Inc.).
Statistical analysis
One- and two-way ANOVA was used to determine differences in cell viability across different virus treatments and doses as appropriate. Statistically significant differences between control (Ad-LacZ) and active (Ad-FKHRL1/TM) virus therapy were determined by the significance of the interaction effect of dose and virus. Differences in cell viability across combination therapies were analyzed by one-way ANOVA. Post-hoc testing was performed with Tukey's adjustment to control for a significance level of 0.05. Synergistic effect was determined by analysis using Calcusyn software, version 2.1 (Biosoft, Cambridge, United Kingdom). Combination indices were calculated by the Chou–Talalay method. Significant synergistic effect at the p<0.05 level was considered if the 95% confidence interval for the combination index was less than 1.0 (Chou and Talalay, 1984). Tumor sizes were compared with nonparametric Kruskal–Wallis tests. Statistical analyses were performed with SAS 9.3 (SAS, Cary, NC).
Results
Determination of Ad-FKHRL1/TM and TMZ median lethal doses in melanoma cells
TMZ has limited response rates in metastatic melanoma; therefore, approaches that sensitize melanoma cells to TMZ are needed. The median lethal dose (LD50) of Ad-FKHRL1/TM or TMZ was determined. Four melanoma cell lines were infected with Ad-FKHRL1/TM or Ad-LacZ at increasing MOI. Three days postinfection, cell killing effects of Ad-FKHRL1/TM increased in a dose-dependent manner in melanoma cells. Ad-FKHRL1/TM had a statistically significant cytotoxic effect compared with Ad-LacZ control (two-way ANOVA for interaction effect, p<0.0001). Sensitivity to Ad-FKHRL1/TM infection was variable across cell lines. Estimated LD50 ranged from 15 to 50 MOI (LD50 ranges—A375: 20 MOI; A2058: 50 MOI; DM6: 50 MOI; SK-MEL-28: 20 MOI) (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/humc). Similarly, TMZ significantly reduced cell viability in multiple human melanoma cell lines. Micromolar doses of TMZ significantly reduced cell viability in all four human melanoma cell lines (p<0.0001). Again, varying degrees of sensitivity were seen. Estimated LD50 values were in the range of 400 to>800 μM (LD50 ranges—A375: 400 μM; A2058: 400 μM; DM6: 400 μM; SK-MEL-28: >800 μM) (Supplementary Fig. S2).
Combination therapy of Ad-FKHRL1/TM with TMZ has synergistic melanoma killing effect
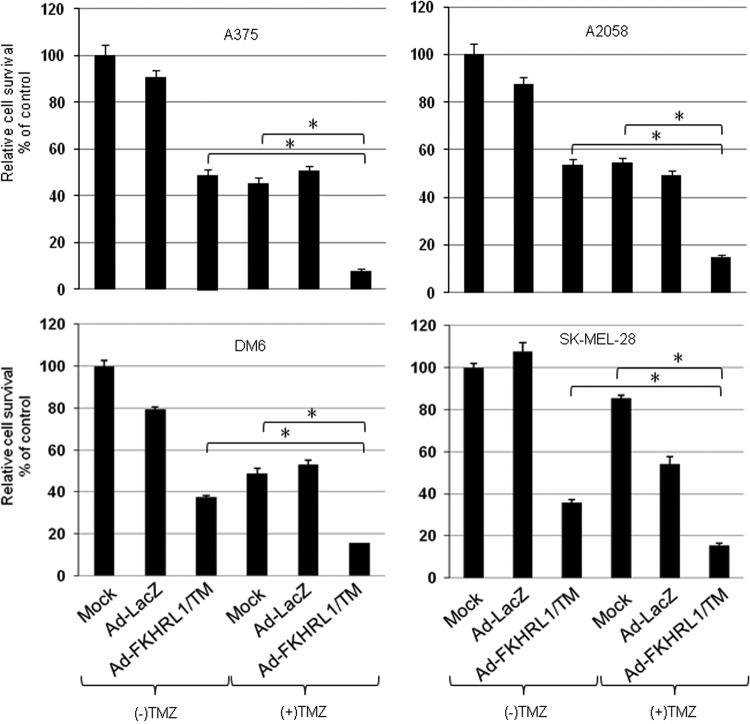
The effect of combined therapy with the two agents (Ad-FKHRL1/TM or TMZ) on cell viability (LD50) was analyzed for synergy. In all four cell lines, the estimated combination index was less than 1.0, indicating statistically significant synergistic effect (p<0.05). Estimated combination indices ranged from 0.271 to 0.660, levels that suggest moderate to high degrees of synergism. Furthermore, post-hoc analysis, which controlled for multiple hypothesis testing, confirmed that cell viability with combination therapy (Ad-FKHRL1/TM+TMZ) was significantly reduced compared with single therapies (Ad-FKHRL1/TM alone, TMZ alone, and Ad-LacZ+TMZ) and controls (mock infection and Ad-LacZ) (Fig. 1). These results indicate that the combination therapy (Ad-FKHRL1/TM+TMZ) resulted in synergistic cytotoxicity in multiple human melanoma cell lines.
FIG. 1.
Combination therapy of Ad-FKHRL1/TM with temozolomide (TMZ) has synergistic killing effect in multiple melanoma cell lines in vitro. Multiple human melanoma cell lines were treated with Ad-FKHRL1/TM and TMZ at the following doses (Ad-FKHRL1/TM, TMZ): A375 (20 MOI, 400 μM), A2058 (50 MOI, 400 μM), DM6 (50 MOI, 400 μM), and SK-MEL-28 (20 MOI, 800 μM). Cell viability was determined by MTT assay 72 hr following treatment and reported as percentage of control (mock). Results represent the mean of three repeated measurements±standard deviation (SD; error bars) (*p<0.001 for all cell lines). MOI, multiplicity of infection.
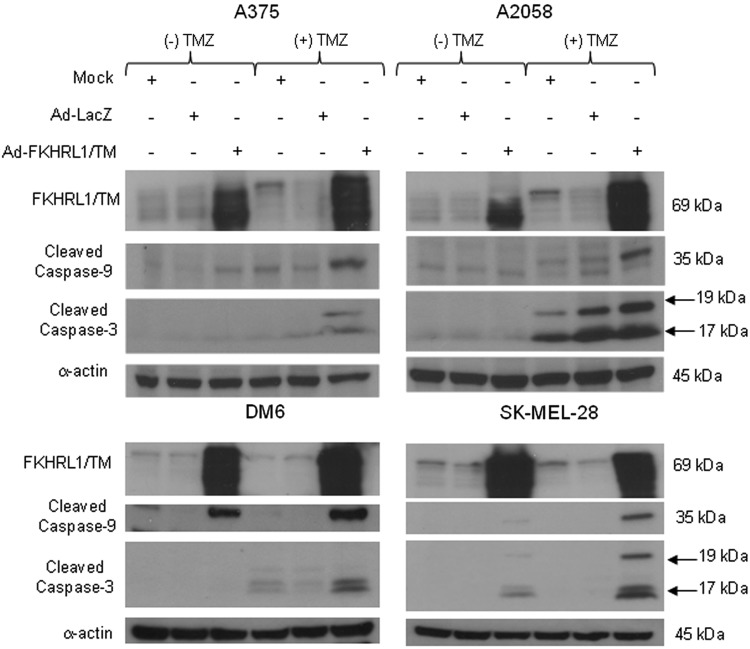
Combination therapy increases caspase activation
To determine whether synergistic killing effect was, at least in part, caused by increased activation of the caspase pathway, cleaved caspases-3 and -9 expressions were analyzed. An immunoblot revealed that combination therapy using Ad-FKHRL1/TM+TMZ induced greater levels of cleaved caspase-9 and -3 than cells infected or treated with Ad-FKHRL1/TM or TMZ alone (Fig. 2). The addition of TMZ also appeared to increase FKHRL1/TM expression compared with Ad-FKHRL1/TM alone in some melanoma cell lines. These findings suggest that synergistic melanoma cell killing effect was, at least in part, because of increased caspase pathway activation.
FIG. 2.
Treatment with Ad-FKHRL1/TM and TMZ significantly increases caspase pathway activation. Multiple human melanoma cell lines were not treated (mock) or treated with Ad-LacZ alone, Ad-FKHRL1/TM alone, or TMZ alone, or in combination at the following doses: (Ad-FKHRL1/TM, TMZ): A375 (20 MOI, 400 μM), A2058 (50 MOI, 400 μM), DM6 (50 MOI, 400 μM), and SK-MEL-28 (20 MOI, 800 μM). Whole cell protein lysates were collected 72 hr following indicated treatment. Expression levels of cleaved caspase-9 and -3 were detected by Western blotting, and a representative experiment is shown from three performed.
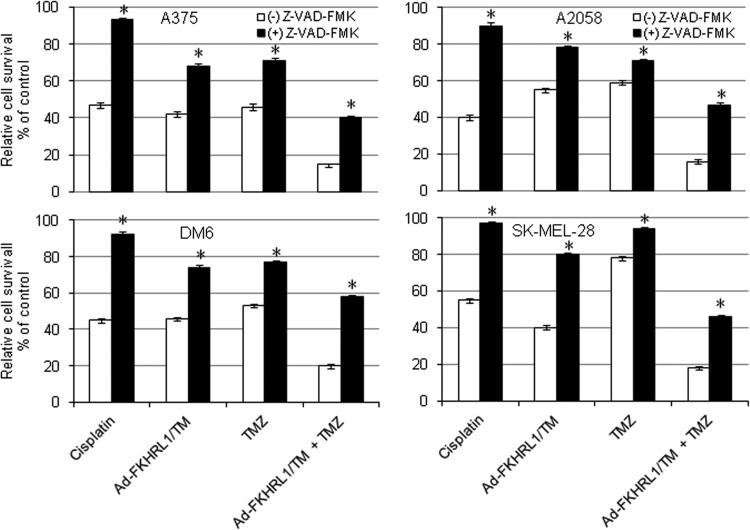
Caspase inhibition partially protects melanoma cells from Ad-FKHRL1/TM+TMZ-mediated cell death
We next investigated the effect of caspase inhibition on Ad-FKHRL1/TM and TMZ-mediated cancer cell killing. Four melanoma cells were cultured in the absence or presence of general caspase inhibitor Z-VAD-FMK (50 μM of concentration), and then treated with cisplatin (25 μM), TMZ alone, Ad-FKHRL1/TM alone, or the two agents combined (Ad-FKHRL1/TM+TMZ). Cisplatin was used as positive control because it triggers typical caspase-associated cell death (Dong et al., 2002). After treatment with cisplatin, an MTT assay revealed that Z-VAD-FMK increased the relative survival rate from 47% to 93% for A375; 40% to 90% for A2058; 45% to 92% for DM6; and 56% to 97% for SK-MEL-28. In the absence of Z-VAD-FMK, treatment with Ad-FKHRL1/TM alone or TMZ alone resulted in a relative cell survival of 42% and 46% in A375; 55% and 59% in A2058; 46% and 53% in DM6; and 40% and 78% in SK-MEL-28, respectively. In the presence of Z-VAD-FMK, cell survival increased to 68% and 71% in A375; 78% and 73% in A2058; 74% and 77% in DM6; and 80% and 94% in SK-MEL-28, respectively (p<0.05; Fig. 3). Cells treated with combination therapy along with the addition of Z-VAD-FMK increased cell viability to 40% in A375; 47% in A2058; 58% in DM6; and 46% in SK-MEL-28. Without the addition of Z-VAD-FMK, cell viability was reduced to 15% in A375; 16% in A2058; 20% in DM6; and 18% in SK-MEL-28 (p<0.05; Fig. 3). These results further suggest that cancer cell death induced by Ad-FKHRL1/TM and TMZ alone or in combination is, at least in part, because of caspase activation.
FIG. 3.
Caspase pathway inhibition partially blocks Ad-FKHRL1/TM- and TMZ-mediated cell death. Melanoma cells were cultured in the absence or presence of general caspase inhibitor z-VAD-fmk at a concentration of 50 μM. The cells then were treated with cisplatin (25 μM), treated with TMZ, infected with Ad-FKHRL1/TM alone, or treated in combination (TMZ+Ad-FKHRL1/TM) at their respective LD50. Three days after treatments, cell viability was determined by MTT assay and reported as percentage of control (mock). Results represent the mean of three repeated measurements±standard deviation (SD; error bars) (*p<0.05 for all cell lines).
TMZ enhances adenovirus-mediated FKHRL1/TM expression in A2058 melanoma cell line
Interestingly, it seems that FKHRL1/TM expression was increased in the presence of TMZ in A2058 and A375; the effect on FKHRL1/TM expression in DM6 and SK-MEL-28 cell lines is less clear in this immunoblotting (Fig. 2). An immunoblot assay revealed that FKHRL1/TM expression increased in a TMZ dose-dependent manner in A2058 melanoma cells, whereas TMZ did not further increase FKHRL1/TM expression in DM6 (Fig. 4). Although we do not know the mechanism by which TMZ increases adenovirus-mediated FKHRL1/TM expression in certain cell lines, this finding suggests that the synergistic killing effect in A2058 was, at least in part, by increased FKHRL1/TM expression.
FIG. 4.
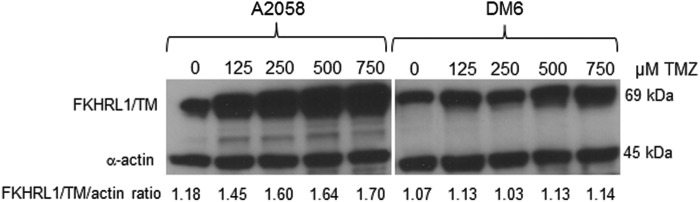
TMZ enhances adenovirus-mediated FKHRL1/TM expression in a dose-dependent manner in A2058 melanoma cells. A2058 and DM6 human melanoma cell lines were infected with Ad-FKHRL1/TM at their respective LD50 doses followed by increasing doses of TMZ (0, 125, 250, 500, and 750 μM). Forty-eight hours postinfection, expression of FKHRL1/TM was analyzed by Western blotting. Actin was used as a loading control. The values indicate the ratios of normalized band intensities of FKHRL1/TM to actin. A representative experiment is shown from three performed.
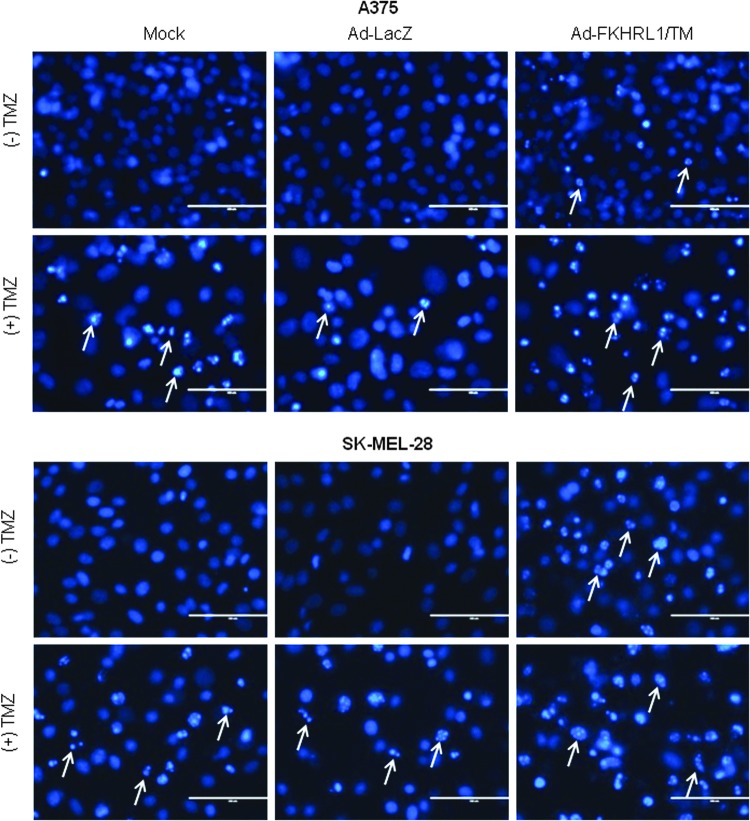
Ad-FKHRL1/TM in combination with TMZ results in increased apoptosis
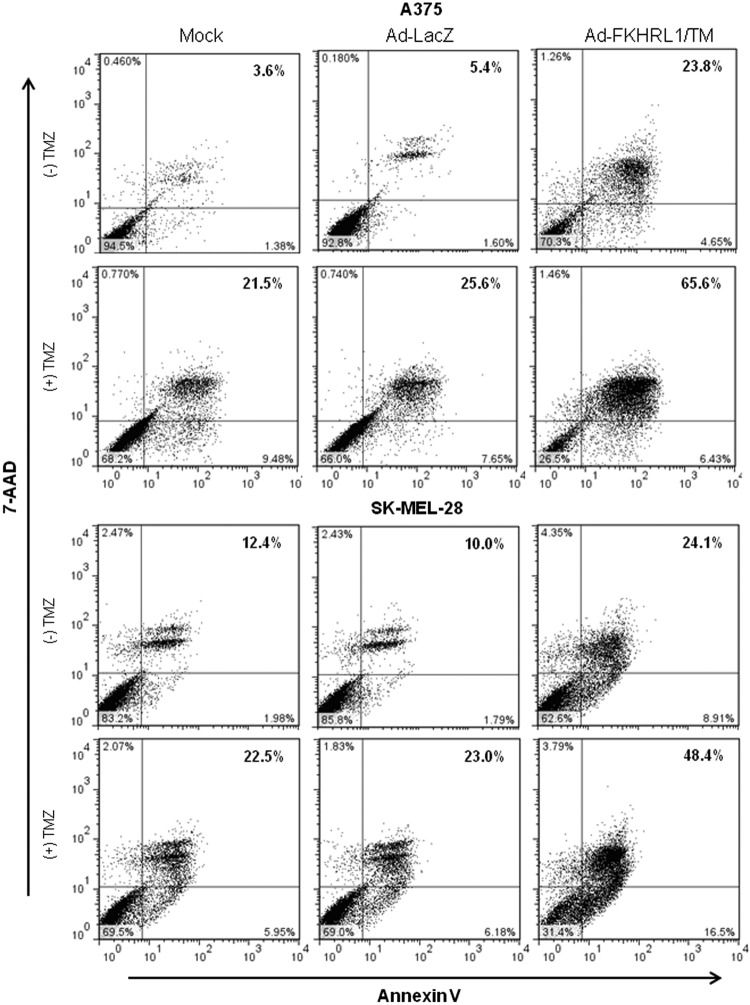
No changes in nuclear morphology were observed in mock or Ad-LacZ, while nuclei fragmentation increased slightly in cells treated with Ad-FKHRL1 or TMZ alone. In contrast, marked nuclear condensation and fragmentation was observed in cells treated with combination therapy in A375 and SK-MEL-28 cell lines (Fig. 5). Similar findings were observed in A2058 and DM6 cell lines (Supplementary Fig. S3). The analysis of annexin V staining by flow cytometry revealed that Ad-FKHRL1/TM+TMZ-treated cells induced greater percentages of apoptosis (65.6% and 48.4%) than single treatments (23.8% and 21.5%, and 24.1% and 22.5%) in A375 and SK-MEL-28 cell lines, respectively (Fig. 6). Similar findings were observed in A2058 and DM6 cell lines (Supplementary Fig. S4). These results confirm that Ad-FKHRL1/TM sensitizes melanoma cells to TMZ-induced apoptosis, and the combination of both results in significantly greater apoptosis than single treatments.
FIG. 5.
Combination of Ad-FKHRL1/TM with TMZ significantly increases nuclei fragmentation. Human melanoma cell lines were treated with Ad-FKHRL1/TM alone, TMZ alone, or in combination (Ad-FKHRL1/TM+TMZ) at the following doses: A375 (20 MOI, 400 μM) and SK-MEL-28 (20 MOI, 800 μM). Forty-eight hours after treatment, Hoechst staining was performed by adding Hoechst 33342 dye to a final concentration of 2 μM. Condensation and fragmentation of nuclei (examples indicated by white arrows) were visualized at 40× magnification with the EVOS FL Imaging System under 357/44 (nm) and 447/60 (nm) excitation and emission visualization (scale bar=400 μm). One representative experiment is shown from three performed. Color images available online at www.liebertpub.com/humc
FIG. 6.
Combination of Ad-FKHRL1/TM with TMZ significantly increases the percentage of apoptosis. Human melanoma cell lines were treated with Ad-FKHRL1/TM alone, TMZ alone, or in combination (Ad-FKHRL1/TM+TMZ) at the following doses: A375 (20 MOI, 400 μM) and SK-MEL-28 (20 MOI, 800 μM). Forty-eight hours after treatment, cells were stained with annexin V-PE and 7-Aminoactinomycin D (7-AAD). Positive cells for annexin V-PE and 7-AAD staining were analyzed by FACScan flow cytometer with FlowJo software. Similar results were obtained in three independent experiments. A representative experiment is shown.
Combination therapy of Ad-FKHRL1/TM with TMZ efficiently suppresses tumor growth in vivo
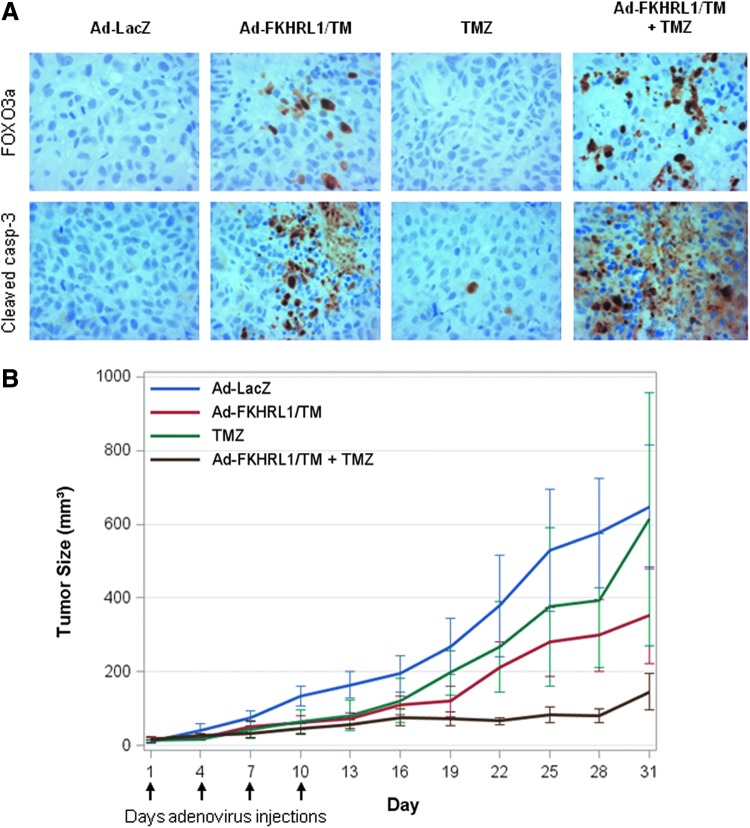
Combination therapy was further assessed for its antitumor activity in a melanoma mouse subcutaneous xenograft model. DM6 melanoma cells were injected subcutaneously into the flanks of nude mice. Seven days later, mice containing palpable tumors were randomized and directly injected with Ad-LacZ, Ad-FKHRL1/TM, TMZ, and Ad-FKHLR1/TM+TMZ. Intratumoral injections with adenovirus were performed every 3 days for a total of 4 treatments, whereas TMZ was administered via intraperitoneal. The tumors were harvested 24 hr after the final treatment following euthanization and subjected to histopathological analysis to assess whether transgene expression and apoptosis are possible mechanisms that induce tumor suppression. Immunohistochemical analysis revealed that expression of FKHRL1/TM was identified only in the Ad-FKHRL1/TM groups and was associated with the detection of cleaved caspase-3-positive cells, suggesting apoptosis induction. A greater number of cleaved caspase-3-positive cells were observed in the tumors of mice treated with the combination of the two agents (Ad-FKHRL1/TM+TMZ) in comparison with single treatments (Ad-FKHRL1/TM or TMZ) and control (Ad-LacZ) (Fig. 7A).
FIG. 7.
Combination therapy with Ad-FKRHL1/TM and temozolomide is effective in an in vivo mouse xenograft melanoma model. Flank melanoma xenografts were developed by injection of DM6 human melanoma cells subcutaneously. Therapy was initiated 7 days following tumor injection when palpable tumors were formed. Treatments (n=5) were as follows: Ad-LacZ (control), Ad-FKHRL1/TM, temozolomide, and Ad-FKHRL1/TM+temozolomide. Virus therapy was delivered by intratumoral injection every 3 days for 4 total doses (vertical arrows). Temozolomide was administered by intraperitoneal injection daily for 5 days. Tumor volume (V) was plotted against time and was determined by the equation V=(L×W2)/2, where L is the length, and W is the width of the tumor. (A) Immunohistochemistry of excised tumor samples with FOXO3a and caspase-3 indicates increased FOXO3a expression and apoptosis activation with combined treatment of Ad-FKHRL1/TM and temozolomide in a mouse xenograft model of human melanoma. (B) Mean tumor volumes over the 31-day experiment estimated by caliper measurements every 3 days. Tumor sizes were compared with nonparametric Kruskal–Wallis tests. Color images available online at www.liebertpub.com/humc
There was greater tumor suppression in the combination therapy with Ad-FKHRL1/TM and TMZ. Tumor size reduction was approximately 40% in Ad-FKHRL1/TM-treated mice, whereas in Ad-FKHRL1/TM+TMZ it was ∼90% in comparison with control virus Ad-LacZ-treated mice (Fig. 7B). Final tumor volume was similar in the control group and the TMZ-treated groups. The differences in median tumor volume at the conclusion of the experiment were not statistically significant across all four treatment groups (p value of 0.12). These results suggest that Ad-FKHRL1/TM may increase the sensitivity of melanoma cells to TMZ, and that tumor growth was suppressed, at least in part, by caspase pathway activation.
Discussion
In this work, we show that TMZ sensitivity in human melanoma cells can be increased by augmenting apoptosis with Ad-FKHRL1/TM, which is a novel approach to improving sensitivity to TMZ that has not been described previously.
TMZ forms several DNA methylation products, most predominantly N7-methylguanine, the accumulation of which induces cell cycle arrest and initiates apoptosis (Newlands et al., 1997; Plummer et al., 2005). Several recent trials have tried novel combination therapies with TMZ and newer agents in an effort to improve the response rates to TMZ alone (Tawbi et al., 2012; Dronca et al., 2013; Kottschade et al., 2013; Ott et al., 2013). None of these trials have shown breakthrough improvements in durable response rates to TMZ therapy. We found greater apoptosis induction in cells treated with Ad-FKHRL1/TM+TMZ than Ad-FKHRL1/TM- or TMZ-treated cells alone. The inhibition of caspase with Z-VAD-FMK partially protected melanoma cells from Ad-FKHRL1/TM+TMZ-mediated cell death.
It is possible that other cell death mechanisms independent of caspase are being activated by FKHRL1/TM alone, TMZ alone, or when the two agents are combined. Recently, it has been reported that melanoma cells treated with TMZ undergo autophagy, a caspase-independent programmed cell death process (Cheng et al., 2012). It has been also reported that FOXO3 induces a transcription-dependent autophagy, and FOXO1 is required for this process (Zhou et al., 2012). We tested whether FKHRL1/TM induces expression of autophagy markers such as LC3-I and -II in melanoma cells. LC3-II accumulation, which is a hallmark of increased autophagy, was examined (Kabeya et al., 2000; Tanida et al., 2005). An immunoblot revealed that Ad-FKHRL1/TM induced significant accumulation of LC3-II in A2058 and DM6 melanoma cells as compared with controls (Supplementary Fig. S5). This result suggests that, in addition to apoptosis, Ad-FKHRL1/TM-induced melanoma cell death may be also due in part to autophagy activation.
Interestingly, it was observed that TMZ increased FKHRL1/TM expression in a dose-dependent manner in A2058, and to a lesser degree in DM6 (Fig. 4). It was reported that TMZ produced a 6.4-fold greater induction of TNF-alpha after infection with Ad.Egr-TNF compared with Ad.Egr-TNF alone (Yamini et al., 2004). This vector is a replication-defective adenoviral vector encoding the cDNA for TNF-alpha under the control of chemo-inducible elements of the egr1 gene promoter. However, in our study FKHRL1/TM is under regulation of the cytomegalovirus (CMV) promoter. The CMV promoter is sensitive to silencing because of methylation (Brooks et al., 2004), and some cancer cells are much more prone to methylating the promoters to silence gene expression (Ushijima and Okochi-Takada, 2005). The mechanisms by which TMZ increases adenovirus-mediated FKHRL1/TM expression are unknown. More work is needed to identify the reasons why TMZ-induced FKHRL1/TM expression is greater in some melanoma cell lines than in others. While this induced FKHRL1/TM expression cannot be generalized to all melanoma cell types, it is an important finding to note that select melanoma cell lines may be more susceptible to combination therapy of TMZ and Ad-FKHRL1/TM based on the potential for TMZ to induce FKHRL1/TM expression. If the genotypes associated with these susceptible cell lines can be identified, then patients possessing the susceptible genotype can be identified who would be expected to benefit from combination therapy with TMZ+Ad-FKHRL1/TM in an effort to reduce the adenovirus dose needed for efficacy. These experiments are ongoing and are the subject of future research efforts.
There does appear to be some degree of enhanced antitumor activity in select instances in which TMZ is combined with the control virus Ad-LacZ. There is some increased cell death in the SK-MEL-28 cell line (Fig. 1), and some degree of enhanced caspase-3 activation in the A2058 cell line when TMZ is combined with Ad-LacZ (Fig. 2). There is likely some small degree of adenovirus-mediated cell death in these instances. For this reason, as in any adenovirus vector-based studies, it is important to include adenovirus controls to evaluate for adenovirus-based tumoricidal activity. These findings are what led us to use the Ad-LacZ-infected tumors as our control group in the mouse xenograft model. There may be a small degree of enhanced anticancer activity when TMZ is combined with adenovirus alone, but this effect is inconsistent.
Recently, we reported that intratumoral injections with Ad-FKHRL1/TM in a mouse xenograft melanoma model reduced tumor growth and induced apoptosis (Gomez-Gutierrez et al., 2012). The tumors created in the previously published mouse xenograft model were made with A2058 cell line, rather than the DM6 cell line used in this report, and thus comparison of tumor sizes between these two sets of experiments is inappropriate. Manipulation of FKHRL1 expression is a promising anticancer therapy, the translational relevance of which has been demonstrated previously. In this current study, in which we have used a subcutaneous mouse xenograft model of human melanoma, we have demonstrated differences in tumor volume after 31 days across controls, single-agent therapy, and combination therapy with Ad-FKHRL1/TM and TMZ. The in vivo results showed a promising trend toward enhanced tumoricidal activity with the combination therapy of Ad-FKHRL1/TM and TMZ. The differences did not quite reach statistical significance, likely because of the high variability of tumor sizes and small sample sizes. These findings, though not conclusive, are promising and have laid the foundation for future work in the rational combination of TMZ and Ad-FKHRL1/TM in animal models of melanoma.
Future work will focus on alternatively drug delivery methods for both TMZ and Ad-FKHRL1/TM in an effort to refine the best method to deliver this combination therapy. For our in vivo model, we chose the DM6 human melanoma cell line, which has previously been shown to be relatively resistant to TMZ treatment (Augustine et al., 2009). Future work with different melanoma cell line may identify differential sensitivity to this combination approach, thus providing evidence for potential biomarkers that may identify patients in whom this approach would be particularly effective. These studies are ongoing. The results do suggest that such combination therapy of Ad-FKHRL1/TM and TMZ is feasible in preclinical animal models and may have limited toxicity and promising reductions in tumor volume growth.
In conclusion, combination therapy with Ad-FKHRL1/TM and TMZ is effective against human melanoma both in vitro and in vivo. Novel therapeutics that target the PI3/Akt pathway, such as Ad-FKHRL1/TM, are promising innovations that may increase the efficacy and applicability of TMZ treatment in melanoma.
Supplementary Material
Acknowledgments
This work was supported by the Lung Cancer Research Foundation (J.G.G.-G.), award number R01CA90784 (K.M.M), and by grant R25-CA-134283 from the National Cancer Institute (J.N.). We thank Margaret Abby for editing.
Author Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- Augustine C.K., Yoo J.S., Potti A., et al. (2009). Genomic and molecular profiling predicts response to temozolomide in melanoma. Clin. Cancer Res. 15, 502–510 [DOI] [PubMed] [Google Scholar]
- Balch C.M., Gershenwald J.E., Soong S.J., et al. (2009). Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 27, 6199–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A.R., Harkins R.N., Wang P., et al. (2004). Transcriptional silencing is associated with extensive methylation of the CMV promoter following adenoviral gene delivery to muscle. J. Gene Med. 6, 395–404 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Sk U.H., Zhang Y., et al. (2012). Rational incorporation of selenium into temozolomide elicits superior antitumor activity associated with both apoptotic and autophagic cell death. PLoS One 7, e35104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T.C., and Talalay P. (1984). Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22, 27–55 [DOI] [PubMed] [Google Scholar]
- Dong Y.B., Yang H.L., Elliott M.J., and Mcmasters K.M. (2002). Adenovirus-mediated E2F-1 gene transfer sensitizes melanoma cells to apoptosis induced by topoisomerase II inhibitors. Cancer Res. 62, 1776–1783 [PubMed] [Google Scholar]
- Dronca R.S., Allred J.B., Perez D.G., et al. (2014). Phase II study of temozolomide (TMZ) and everolimus (RAD001) therapy for metastatic melanoma: a North Central Cancer Treatment Group Study, N0675. Am. J. Clin. Oncol. 37, 369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman H.S., Kerby T., and Calvert H. (2000). Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 6, 2585–2597 [PubMed] [Google Scholar]
- Gomez-Gutierrez J.G., Souza V., Hao H.Y., et al. (2006). Adenovirus-mediated gene transfer of FKHRL1 triple mutant efficiently induces apoptosis in melanoma cells. Cancer Biol. Ther. 5, 875–883 [DOI] [PubMed] [Google Scholar]
- Gomez-Gutierrez J.G., Garcia-Garcia A., Hao H., et al. (2010). Adenovirus-mediated expression of truncated E2F-1 suppresses tumor growth in vitro and in vivo. Cancer 116, 4420–4432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gutierrez J.G., Egger M.E., Hao H., et al. (2012). Adenovirus-mediated expression of mutated forkhead human transcription like-1 suppresses tumor growth in a mouse melanoma xenograft model. Cancer Biol. Ther. 13, 1195–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T.T., Hlavaty J., Ostertag D., et al. (2013). Toca 511 gene transfer and 5-fluorocytosine in combination with temozolomide demonstrates synergistic therapeutic efficacy in a temozolomide-sensitive glioblastoma model. Cancer Gene Ther. 10, 544–551 [DOI] [PubMed] [Google Scholar]
- Kabeya Y., Mizushima N., Ueno T., et al. (2000). LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottschade L.A., Suman V.J., Perez D.G., et al. (2013). A randomized phase 2 study of temozolomide and bevacizumab or nab-paclitaxel, carboplatin, and bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N0775. Cancer 119, 586–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 16, 55–63 [DOI] [PubMed] [Google Scholar]
- Newlands E.S., Blackledge G.R., Slack J.A., et al. (1992). Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br. J. Cancer 65, 287–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlands E.S., Stevens M.F., Wedge S.R., et al. (1997). Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat. Rev. 23, 35–61 [DOI] [PubMed] [Google Scholar]
- Ott P.A., Chang J., Madden K., et al. (2013). Oblimersen in combination with temozolomide and albumin-bound paclitaxel in patients with advanced melanoma: a phase I trial. Cancer Chemother. Pharmacol. 71, 183–191 [DOI] [PubMed] [Google Scholar]
- Patel P.M., Suciu S., Mortier L., et al. (2011). Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV melanoma: final results of a randomised phase III study (EORTC 18032). Eur. J. Cancer 47, 1476–1483 [DOI] [PubMed] [Google Scholar]
- Plummer E.R., Middleton M.R., Jones C., et al. (2005). Temozolomide pharmacodynamics in patients with metastatic melanoma: dna damage and activity of repair enzymes O6-alkylguanine alkyltransferase and poly(ADP-ribose) polymerase-1. Clin. Cancer Res. 11, 3402–3409 [DOI] [PubMed] [Google Scholar]
- Rainov N.G., Fels C., Droege J.W., et al. (2001) Temozolomide enhances herpes simplex virus thymidine kinase/ganciclovir therapy of malignant glioma. Cancer Gene Ther. 8, 662–668 [DOI] [PubMed] [Google Scholar]
- Simard E.P., Ward E.M., Siegel R., and Jemal A. (2012). Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J. Clin. 62, 118–128 [DOI] [PubMed] [Google Scholar]
- Stedt H., Samaranayake H., Pikkarainen J., et al. (2013). Improved therapuetic effect on malignant glioma with adenovirual suicide gene therapy combined with temozolomide. Gene Ther. 20, 1165–1171 [DOI] [PubMed] [Google Scholar]
- Tanida I., Minematsu-Ikeguchi N., Ueno T., and Kominami E. (2005). Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 1, 84–91 [DOI] [PubMed] [Google Scholar]
- Tawbi H.A., Beumer J.H., Tarhini A.A., et al. (2013). Safety and efficacy of decitabine in combination with temozolomide in metastatic melanoma: a phase I/II study and pharmacokinetic analysis. Ann. Oncol. 24, 1112–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima T., and Okochi-Takada E. (2005). Aberrant methylations in cancer cells: where do they come from? Cancer Sci. 96, 206–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamini B., Yu X., Gillespie G.Y., et al. (2004). Transcriptional targeting of adenovirally delivered tumor necrosis factor alpha by temozolomide in experimental glioblastoma. Cancer Res. 64, 6381–6384 [DOI] [PubMed] [Google Scholar]
- Zhou J., Liao W., Yang J., et al. (2012). FOXO3 induces FOXO1-dependent autophagy by activating the AKT1 signaling pathway. Autophagy 8, 1712–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.