Abstract
Background
Inhaled atropine is being developed as a systemic and pulmonary treatment for the extended recovery period after chemical weapons exposure. We performed a pharmacokinetics study comparing inhaled atropine delivery using the MicroDose Therapeutx Dry Powder Inhaler (DPIA) with intramuscular (IM) atropine delivery via auto-injector (AUTO).
Methods
The MicroDose DPIA utilizes a novel piezoelectric system to aerosolize drug and excipient from a foil dosing blister. Subjects inhaled a 1.95-mg atropine sulfate dose from the dry powder inhaler on one study day [5 doses×0.4 mg per dose (nominal) delivered over 12 min] and received a 2-mg IM injection via the AtroPen® auto-injector on another. Pharmacokinetics, pharmacodynamic response, and safety were studied for 12 hr.
Results
A total of 17 subjects were enrolled. All subjects completed IM dosing. One subject did not perform inhaled delivery due to a skin reaction from the IM dose. Pharmacokinetic results were as follows: area under the curve concentration, DPIA=20.1±5.8, AUTO=23.7±4.9 ng hr/mL (means±SD); maximum concentration reached, DPIA=7.7±3.5, AUTO=11.0±3.8 ng/mL; time to reach maximum concentration, DPIA=0.25±0.47, AUTO=0.19±0.23 hr. Pharmacodynamic results were as follows: maximum increase in heart rate, DPIA=18±12, AUTO=23±13 beats/min; average change in 1-sec forced expiratory volume at 30 min, DPIA=0.16±0.22 L, AUTO=0.11±0.29 L. The relative bioavailability for DPIA was 87% (based on output dose). Two subjects demonstrated allergic responses: one to the first dose (AUTO), which was mild and transient, and one to the second dose (DPIA), which was moderate in severity, required treatment with oral and intravenous (IV) diphenhydramine and IV steroids, and lasted more than 7 days.
Conclusions
Dry powder inhalation is a highly bioavailable route for attaining rapid and consistent systemic concentrations of atropine.
Key words: systemic aerosol delivery, anticholinergic, chemical weapon, atropine allergy, nerve gas antidote
Introduction
Inhalation has long been recognized as a viable means for delivering medications to the systemic circulation. Although often described, this route has rarely been applied for systemic delivery of medications. Efforts to develop inhaled insulin have demonstrated the viability of systemic delivery through inhalation and have furthered the technologies required to deliver medications efficiently through this route. Here we consider a unique application of an inhaled drug intended both to treat the lung and to provide systemic dosing through inhalation. Specifically, we consider the inhaled delivery of atropine for treatment after exposure to organophosphate chemical weapons and pesticides. Atropine acts as an antidote in this condition, competitively blocking muscarinic receptors that would otherwise be affected by excessive levels of acetylcholine present at the synaptic junctions. Organophosphate poisons inhibit the normal enzymatic breakdown of acetylcholine that would occur after neural signaling, resulting in a range of devastating symptoms affecting multiple systems in the body.(1) Treatment with parenteral atropine and an oxime therapy (typically pralidoxime chloride in the United States), which restores normal acetylcholinesterase activity, is typical practice, and exposure is not unusual in regions where organophosphate pesticides are in use.(2) Atropine auto-injectors are available in 0.25–2.0-mg doses (atropine sulfate, AtroPen®, King Pharmaceuticals, Bristol, TN). Therapeutic requirements of 40–60 mg per day of atropine have been reported for subjects with mild to moderate organophosphate poisoning, with some subjects requiring hundreds of milligrams.(1,3)
The need for an inhaled atropine product to complement currently available parenteral forms stems from several sources. An inhaled product can be designed to deliver smaller systemic atropine doses for the extended recovery period after exposure in a form that allows for self-administration without the discomfort that might be associated with multiple auto-injector applications. The inhaled product also offers direct pulmonary treatment along with systemic dosing. Bronchospasm and bronchorrhea are reported effects of organophosphate poisoning.(1) Atropine is a bronchodilator that also suppresses secretions in the airway. Nebulized atropine has been used to suppress secretions ahead of bronchoscopy(4) and has been used successfully to treat the pulmonary manifestations of organophosphate poisoning.(3) An inhaled product allows for the packaging of many atropine doses in a small container, which may be a priority in military medical use. Based on these factors, a chlorofluorocarbon (CFC)-based atropine metered dose inhaler (MDI) was developed by the US military and stocked through 2005.(5,6) This device delivered 0.43 mg of atropine sulfate per inhalation and contained more than 240 doses. Eight inhalations from this atropine MDI provided systemic drug concentrations similar to a 2-mg intramuscular (IM) injection of atropine when compared through area under the curve (AUC) measurements.(7) Based on this comparison, a single inhaler contained the therapeutic equivalent of approximately 30 auto-injectors and consumed significantly less storage space. The discontinuation of CFC propellants for use in MDIs necessitated the design of a new delivery method for inhaled atropine.(8)
Here we compare the pharmacokinetics of atropine delivery using a prototype dry powder inhaler to IM atropine delivery by auto-injector (AUTO). These studies were performed to help establish the dosing regimen for the final form of an atropine dry powder inhaler (DPIA). They also serve as an example of systemic drug delivery via inhalation that may be useful for developing other similar applications.
Methods
The MicroDose Therapeutx Dry Powder Inhaler
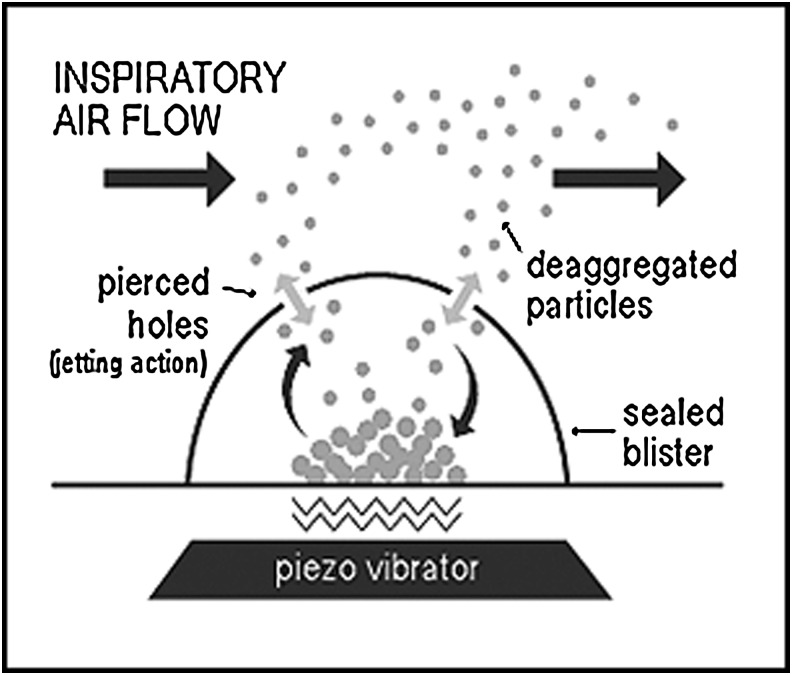
The MicroDose Therapeutx Dry Powder Inhaler (DPIA) uses a novel piezoelectric system to aerosolize drug and excipient from a foil dosing blister (see Fig. 1). The system further causes the formation of synthetic jets that disperse and transport the aerosol through pierced holes in the blister into the inhalation channel of the device. The system is triggered by detection of an inhalation flow signal and controlled using electronics that can be used to control dosing from the device. Dispersion of the aerosol is independent of inhalation velocity and inhaler orientation.
FIG. 1.
The atropine drug formulation is contained in a sealed blister, which protects it from the environment until delivery. Upon actuation, the blister is pierced and placed in contact with a piezo vibrator. When the patient inhales, an airflow sensor automatically turns on the piezo, which deaggregates the particles of powder and aerosolizes them into the user's inhalation airstream. The electronic flow sensor assures proper inspiratory airflow before activating the piezo, eliminating the need for coordinated activities. The piezo vibrator generates the energy needed to efficiently deaggregate and deliver the dose, eliminating the need for high inspiratory flow rates to assure good dose delivery.
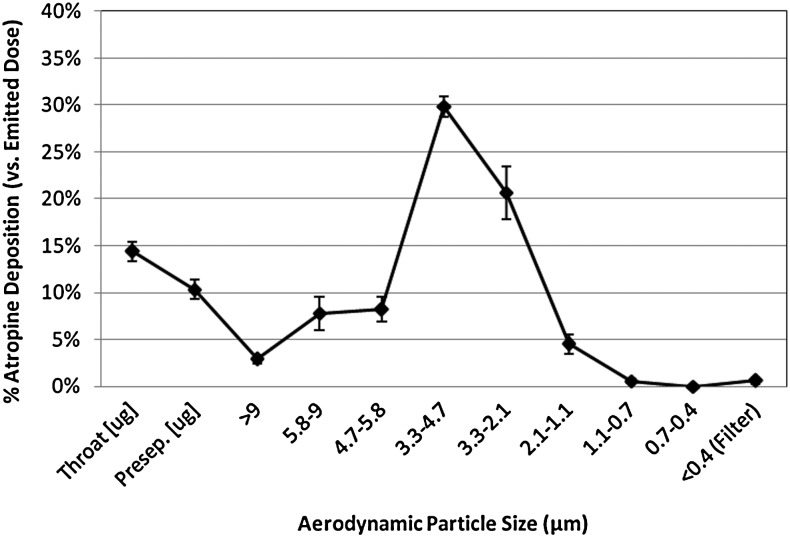
The prototype inhaler used in these studies delivered a nominal dose of 0.4 mg of atropine sulfate per inhalation. A lactose excipient was used along with atropine in the dosing blisters. Preclinical studies using cascade impaction demonstrated that 57% of this nominal dose was in particle sizes smaller than 5.8 μm and 25% was in sizes smaller than 3.3 μm (mass aerodynamic diameters) (see Fig. 2). Mass median aerosol diameter was 3.7 μm (geometric standard deviation=1.5). Deposition fraction in the lung is strongly dependent on aerosol size as well as other factors related to the patient (age, disease state) and delivery (inhalation rate, breath hold).(9,10) Aerosol delivery via MDI often includes significant oral deposition, which limits the efficiency of delivery. We therefore anticipated similar efficacy with lower nominal doses with the DPIA in comparison with the previously available atropine MDI. An attempt was made to further increase the dose per inhalation from the DPIA to accommodate the packaging of more total drug per device. Comparisons of preclinical DPIA studies with available data from the atropine MDI led us to hypothesize that five to six DPIA inhalations would provide systemic exposure equivalent to eight MDI inhalations or one IM delivery by auto-injector (2 mg).
FIG. 2.
Aerosol performance of atropine sulfate delivered from the MicroDose Inhaler (n=19, Andersen Cascade Impactor, 28.3 L/min).
Study design
Nonsmoking male and female subjects between the ages of 18 and 55, weighing more than 100 lb, were included. Subjects performed a series of screening studies, including blood tests, urinalysis, pulmonary function testing, an optical exam, a history and physical, urine pregnancy testing, and an electrocardiogram (EKG), to ensure they had no medical conditions that might be adversely affected by atropine administration. The study design included two testing sessions that were separated by 5–8 days.
A two-period randomized crossover design was used. On one study day, subjects inhaled five doses of atropine from the DPIA. The doses were delivered every 3 min (t=0, 3, 6, 9, and 12 min) with the clock for pharmacokinetic measurements being started immediately after the first dose. The DPIA was set to deliver drug when inhalation flow rates between 25 and 35 L/min were detected. Subjects used visual signals of inhalation flow rate from a modified spirometer to maintain their inhalation rate within this range for 4 sec during each inhaled cycle. Subjects performed a full exhalation prior to each dose and a 10-sec breath hold after each dose. On the other study day, subjects received a 2-mg IM injection of atropine sulfate (1.67 mg of atropine base) using an AtroPen auto-injector delivered into the anterior thigh by study personnel. The clock for pharmacokinetic measurements was started 10 sec after injection. The order of administration was randomized.
Blood samples (6 mL) for pharmacokinetic measurements were drawn pre-study (single measurement) and then at 4, 8, 15, and 30 min and 1, 2, 4, 6, 8, and 12 hr. Blood samples for clinical labs were drawn pre-study and at t=10 hr after delivery. Urine pregnancy screening was performed before dosing on all testing days. Vital signs were measured pre-study and then immediately after all blood draws except the 4- and 8-min draws. Optical measurements were also performed at these times. Atropine dilates the pupil and paralyzes accommodation.(11) Pupil diameter was measured using a Neuroptics digital pupilometer (Neuroptics Inc., Irvine, CA). Near point of accommodation (NPA), which represents the closest point to the face at which an object can be seen in focus, was measured using an RAF gauge (Clement Clark Int., Harlow, UK). Subjects performed pulmonary function testing pre-study and then at 30 min and 12 hr after delivery. Collected values included 1-sec forced expiratory volume (FEV1) and forced vial capacity (FVC). EKGs (12 lead) were performed pre-study and at 12 hr. Continuous cardiac monitoring was used for the first 5 hr of testing. Subjects were asked to rate a series of known anticholinergic effects on a 0–3 scale (none, mild, moderate, severe) pre-study and at 15 and 30 min and 1, 2, 4, 6, 8, 10, and 12 hr.
Pharmacokinetic and pharmacodynamic methods
Blood samples were collected in heparinized tubes that were placed on ice prior to being spun at 2,500 rpm for 15 min at 4°C, and then divided into aliquots in polypropylene vials and frozen at −70°C. The concentration of atropine in plasma samples was analyzed by a validated HPLC-MS-MS methodology.
Sample preparation
Stock solutions of atropine and the internal standard were prepared in glassware that was pretreated with Sigmacote to prevent adsorption loss. Calibration curves, controls, and the clinical samples were thawed at room temperature. Exactly 0.5 mL of plasma was transferred to 12×75-mm glass tubes, and 0.5 mL of 0.05 M phosphate buffer (pH 8) was added. Twenty-five microliters of the internal standard (100 ng/mL D3-atropine in 50% methanol) was added, and the sample was subjected to solid-phase extraction. The OASIS HLB Cartridges used for solid-phase extraction were preconditioned with 1 mL×2 of methanol (Optima LCMS). One milliliter of water (HPLC) was added twice and allowed to drip to waste. The entire treated sample was then loaded onto the solid-phase extraction column. The content of the column was washed with 1 mL of water. One milliliter of 10% methanol in water was added and followed up with vacuum drying of the column. Atropine and the internal standard were eluted from the solid-phase extraction column with 1 mL of methanol. The methanol was dried under normal air. The residue was reconstituted in 100 μL of mobile phase buffer A/mobile phase B (2/1). Ten microliters of this was injected onto the analytical column.
HPLC-MS instrumentation
The analytical column consisted of a 2.1×50 mm, 3.5 μm, Xbridge C18 column. The mobile phase consisted of buffer A (2 mM ammonium acetate, 0.1% formic acid, and 5% methanol in water) and buffer B (2 mM ammonium acetate, 0.1% formic acid in methanol). The initial composition of the mobile phase was 70% buffer A and 30% buffer B, which was maintained for 1 min and changed to 10% buffer A and 90% buffer B starting from 1.6 min to 2.0 min and was returned to the original conditions in 5.9 min. The flow rate of the mobile phase was maintained at 0.2 mL.
The HPLC-MS-MS instrument used was Waters Micromass Quattro Micro Triple Quadrupole Mass Spectrometer (Waters Corporation, MA, USA) running with Mass Lynx Software. The following conditions were used in the mass spectrometry: The capillary voltage was 2.0 kV; the source temperature was 100°C; the desolvation temperature was 450°C; the desolvation gas flow was 550 L/hr; the cone gas flow was 50 L/hr; the argon pressure was 20±10 psig; and the nitrogen pressure was 100±20 psig. The following ions were monitored: MRM transitions and condition for atropine: 290.20>124.04 (ES+), cone: 35 V, collision: 23 eV; and for D3-atropine: 293.23>127.01 (ES+), cone: 35 V, collision: 23 eV.
Quality control data
The assay was linear in the concentration range of 0.5 ng/mL to 20 ng/mL. The lower limit of quantitation of the assay was 0.5 ng/mL. Quality control samples (1.5, 7.5, and 15 ng/mL) were run with every batch of samples assayed, and the assay results were accepted only if two of the three controls were within acceptable limits.
The intraday bias in the concentration measurements was below 9%. The intraday variation of the assay, expressed as CV, was below 6%, and the intraday CVs were below 3% at 15 ng/mL, 7.5 ng/mL, and 1.5 ng/mL.
Six blank plasma samples from healthy volunteers and every first blank sample collected from the subjects who participated in the study were tested to make sure that there was no assay interference. The relative recovery of atropine was 80%. The long-term stability of the plasma samples was investigated over a 6-month time period. All the samples were within a range of 93–112% of the initial values over this time period.
The concentration versus time profile was then analyzed using WINNONLIN (version 5.0.1). A noncompartmental analysis of the data was carried out. A minimum of at least three concentration data points was used for calculation of the terminal disposition rate constant. For each profile, various pharmacokinetic parameters were determined, including disposition rate constant (kelim), plasma half-life (T1/2), time to reach maximal concentration (Tmax), maximum concentration reached (Cmax), area under the plasma concentration versus time curve from time 0 to time infinity (AUC), and apparent clearance (CL/F). Given that the drug will be cleared exactly the same way once in the systemic circulation, differences in CL/F estimates essentially reflect a difference in bioavailability between the two formulations tested.
Previous studies on atropine had used radioimmunoassay (RIA) or GC-MS assay methods. Whereas RIA is a nonspecific assay, GC-MS assays are expected to be specific to the drug measured. The GC-MS and HPLC-MS-MS assays are expected to provide similar concentration measurements from the plasma. Although pharmacokinetic data using two different assay methodologies cannot be directly compared, they provide some initial preliminary comparison of the data.
To assess pharmacodynamic response, we considered the maximum increase from baseline of a series of measurements known to demonstrate anticholinergic response. These included heart rate, blood pressure, temperature, the ocular measurements of pupil diameter and NPA, and pulmonary function, specifically FEV1 and FVC. All study time points after delivery were included when maximum increase was assessed. If no increase from baseline occurred during the testing day, a zero value was included in the average.
Statistics
Comparisons of pharmacokinetic values were performed using the Wilcoxon sign rank test (paired, nonparametric) based on the difference between values for the devices (DPIA – AUTO) and including all subjects who tested with both devices (n=16). Linear mixed models were also used with natural log transformed values of AUC and Cmax in order to determine the effect of device, test day, and treatment sequence, along with subject factors including age and gender. Least squares means were computed for each categorical covariate. Differences of least squares means were exponentiated in order to express effect sizes as fold changes, adjusted for the other covariates in the model. For age, we report the fold change associated with a 5-year age difference. Maximum increases in pharmacodynamic variables were compared by Wilcoxon based on the difference between values for the devices. Changes in heart rate from baseline were compared between devices at individual study time points also by Wilcoxon. Pulmonary function values (specifically FEV1 and FVC) were compared at t=30 min versus baseline for each device. Pulmonary function testing changes over that time period were also compared by device, both by Wilcoxon. Comparisons of pharmacokinetic effects by gender were performed using a Mann-Whitney test (unpaired, nonparametric).
Results
A total of 29 subjects were enrolled and screened. Eight of these subjects failed screening tests and were excluded from the study. Four subjects withdrew from the study prior to performing testing. Seventeen subjects participated in the study. Table 1 includes demographic information for these subjects, along with height and weight and pulmonary function data. Sixteen of these subjects performed testing with both devices. One subject was excluded from DPIA testing after experiencing a slight allergic reaction to atropine delivered by AUTO. Instances of atropine allergy are discussed in detail below.
Table 1.
Demographics and Characteristics of Tested Subjects
| (A) | ||
|---|---|---|
| n | % | |
| Sex | ||
| Female | 6 | 35% |
| Male | 11 | 65% |
| Ethnicity | ||
| Caucasian | 9 | 53% |
| African American | 6 | 35% |
| Asian/Pacific Islander | 1 | 6% |
| Other | 1 | 6% |
| (B) | ||
|---|---|---|
| Mean | SD | |
| Characteristic | ||
| Age (yr) | 29.9 | 9.6 |
| Height (in) | 68.7 | 3.5 |
| Weight (lb) | 175.3 | 37.7 |
| Body mass index (BMI) | 25.1 | 5.5 |
| Pulmonary function | ||
| FEV1%p | 94.4 | 14.2 |
| FVC%p | 98.5 | 12.9 |
The demographics (A) and height, weight, and pulmonary function values (B) of the tested subjects (n=17) are presented. %p, percent of predicted value.
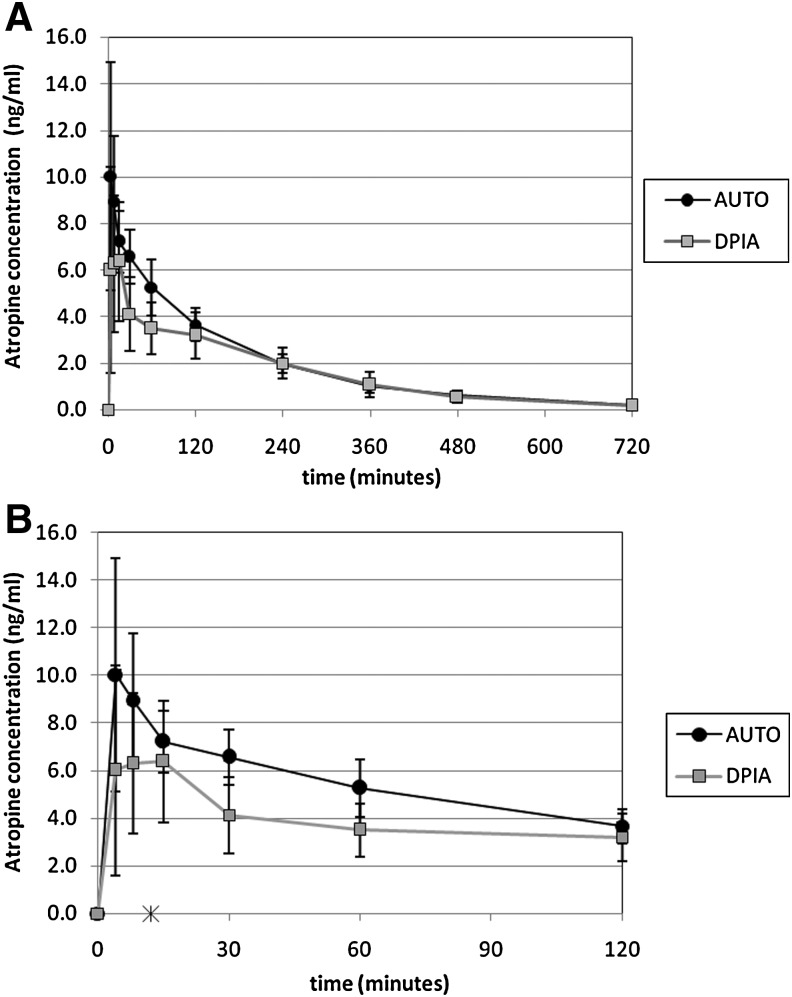
Table 2 includes the comparative pharmacokinetic data for the devices. In Table 2A, comparisons are made through paired nonparametric testing. In Table 2B, a linear mixed model was used to compare log transformed values of Cmax and AUC. Atropine peak concentrations (Cmax) and area under the curve concentration (AUC) were significantly higher with AUTO versus DPIA. Apparent clearance (CL/F) was significantly higher with DPIA. Time to peak (Tmax) was similar for both devices despite the extended interval used to deliver the five inhaled doses (12 min). There were no effects of sequence or test day demonstrated. Neither age nor gender contributed significantly to the results of the linear mixed model. Figure 3 shows average plasma atropine concentration versus time for DPIA and AUTO.
Table 2.
The Pharmacokinetics of Atropine Sulfate Delivery
| (A) | |||||
|---|---|---|---|---|---|
| DPIA (n=16) | AUTO (n=17) | ||||
| Parameter (units) | Mean | SD | Mean | SD | p value (n=16) |
| AUC 0–inf (ng hr/mL) | 20.1 | 5.8 | 23.7 | 4.9 | 0.002 |
| Cmax (ng/mL) | 7.7 | 3.5 | 11 | 3.8 | 0.001 |
| Tmax (hr) | 0.25 | 0.47 | 0.19 | 0.23 | 0.44 |
| T1/2 (hr) | 2.4 | 1.0 | 2.4 | 0.6 | 0.90 |
| Kelim (L/hr) | 0.33 | 0.12 | 0.31 | 0.08 | 0.62 |
| CL/F (L/hr) | 110 | 43 | 87 | 17 | 0.006 |
| MRT inf (hr) | 3.8 | 1.3 | 3.2 | 0.6 | 0.09 |
| (B) | ||||
|---|---|---|---|---|
| AUC | Cmax | |||
| Variable (effect) | Fold change | p value | Fold change | p value |
| Treatment (AUTO:DPIA) | 1.27 | 0.04 | 1.59 | 0.047 |
| Test day (2:1) | 1.07 | 0.73 | 1.12 | 0.70 |
| Treatment sequence (AUTO-DPIA:DPIA-AUTO) | 1.06 | 0.39 | 1.13 | 0.37 |
| Gender (F:M) | 1.24 | 0.11 | 0.71 | 0.18 |
| Age (+5 years) | 1.05 | 0.08 | 1.05 | 0.43 |
(A) Comparisons performed using the Wilcoxon signed rank test comparing differences between values (DPIA–AUTO). (B) Comparisons performed using linear mixed models for log transformed AUC and Cmax, considering treatment, test day, sequence, and subject variables. Fold change values represent exponentiated differences in least squares means for each variable. For age, the fold change is based on a 5-year age difference. MicroDose Therapeutx dry powder inhaler (DPIA): nominal dose 2 mg, output dose 1.95 mg; the AtroPen Auto-Injector (AUTO): 2 mg IM.
AUC, area under the curve of drug concentration vs. time; Cmax, maximum plasma drug concentration; Tmax, time of maximum concentration; T1/2, plasma half-life; Kelim, elimination rate constant; F, bioavailability; CL, clearance; MRT, mean residence time.
FIG. 3.
Average plasma atropine concentration after delivery using the MicroDose Therapeutx dry powder inhaler (DPIA: nominal dose 2 mg, output dose 1.95 mg; n=16) and the AtroPen Auto-Injector (AUTO: 2 mg IM; n=17). (A) Values over 12 hr. (B) Values over the first 2 hr. *The DPIA dose was delivered in five inhalations over 12 min.
The relative bioavailability (Frel) of the two devices can be calculated as: Frel=(dose AUTO/dose DPIA)×(AUC DPIA/AUC AUTO). The nominal dose delivered from DPIA was 2.0 mg (5 inhalations×0.4 mg/inhalation). The total output dose, excluding losses in the device, was 1.95 mg as determined through dose uniformity testing of the drug/device combination.(12) Calculations using DPIA nominal dose yield Frel=85%. Calculations using DPIA output dose yield Frel=87%. The 90% confidence intervals for Cmax and AUC ratios (DPIA/AUTO) were 50.1–79.7% and 73.7–92.4%, respectively, and therefore the devices are not bioequivalent based on FDA definitions, which require both of these intervals to be within 80–125%.
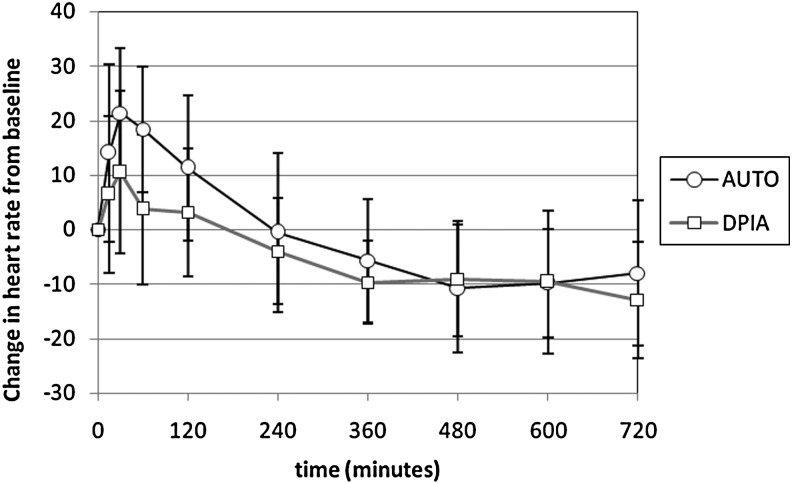
Table 3 describes the pharmacodynamic response to DPIA and AUTO based on a series of measurements likely to be increased by atropine exposure. In all cases, the maximum increases from baseline values are reported. There was a trend toward increased heart rate response with AUTO versus DPIA (p=0.07). All other indicators of response were similar. Figure 4 compares changes in heart rate from baseline with DPIA and AUTO over 12 hr. Values at t=15, 30, 60, and 120 min were significantly different for DPIA and AUTO. Baseline heart rate was similar on both testing days.
Table 3.
Pharmacodynamic Response to Atropine Sulfate Delivery
| DPIA (n=16) | AUTO (n=17) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p value (n=16) | |
| Heart rate (bpm) | 18 | 12 | 23 | 13 | 0.07 |
| Systolic BP (mm Hg) | 9.3 | 7.8 | 10.5 | 12.4 | 0.92 |
| Diastolic BP (mm Hg) | 6.1 | 5.7 | 9.1 | 7.0 | 0.25 |
| Temperature (°C) | 1.0 | 0.5 | 0.7 | 0.5 | 0.11 |
| Pupil diameter R (mm) | 0.8 | 0.6 | 1.0 | 0.4 | 0.63 |
| Pupil diameter L (mm) | 0.8 | 0.7 | 0.8 | 0.4 | 0.99 |
| NPA (cm) | 5.9 | 5.7 | 8.9 | 8.4 | 0.18 |
| FEV1 (L) | 0.2 | 0.2 | 0.2 | 0.2 | 0.98 |
| FVC (L) | 0.1 | 0.1 | 0.1 | 0.2 | 0.83 |
MicroDose Therapeutx dry powder inhaler (DPIA): nominal dose 2 mg, output dose 1.95 mg; and AtroPen Auto-Injector (AUTO): 2 mg IM. Maximum increases from baseline are reported and compared. Comparisons were performed using the Wilcoxon signed rank test comparing differences between values (DPIA–AUTO). NPA, near point of accommodation.
FIG. 4.
Change in heart rate after atropine sulfate delivery using the MicroDose Therapeutx dry powder inhaler (DPIA: nominal dose 2 mg, output dose 1.95 mg; n=16) and the AtroPen Auto-Injector (AUTO: 2 mg IM; n=17). Values are means±SD. The DPIA dose was delivered in five inhalations over 12 min.
FEV1 increased by 0.11±0.29 L with AUTO versus 0.16±0.22 L with DPIA on average when measured 30 min after delivery and compared with baseline. The change from baseline was significant with DPIA (n=16; p=0.02, Wilcoxon) and approached significance with AUTO (n=17; p=0.06). Changes were similar by device (n=16; p=0.91). Changes in FVC were minimal and not statistically different from baseline (AUTO=−0.02±0.36 L, p=0.93; DPIA=−0.06±0.20 L, p=0.59). FVC changes by device were similar (p=0.93).
Table 4 reports gender-averaged results for AUC, Cmax, Tmax, and maximum increase in heart rate. Previous studies with the atropine MDI had included only male subjects, and the label for AUTO discusses some pharmacokinetic differences by gender, specifically increased AUC and Cmax in female subjects.(13) The linear mixed model did not indicate any effect of gender on AUC or Cmax. Tmax was significantly increased in female subjects using DPIA and approached significance after use of AUTO.
Table 4.
The Effect of Gender on the Pharmacokinetics of Atropine Sulfate Delivery
| (A) | |||||
|---|---|---|---|---|---|
| DPIA | |||||
| Male (n=11) | Female (n=5) | ||||
| Mean | SD | Mean | SD | p value | |
| AUC 0–inf (ng hr/ml) | 18.7 | 4.7 | 23.2 | 7.4 | 0.53 |
| Cmax (ng/ml) | 8.1 | 3.7 | 6.8 | 3.0 | 0.57 |
| Tmax (hr) | 0.10 | 0.03 | 0.56 | 0.81 | 0.02 |
| Max. increase in heart rate | 16 | 9 | 21 | 17 | 0.49 |
| (B) | |||||
|---|---|---|---|---|---|
| AUTO | |||||
| Male (n=11) | Female (n=6) | ||||
| Mean | SD | Mean | SD | p value | |
| AUC 0–inf (ng hr/ml) | 21.7 | 3.1 | 27.4 | 5.8 | 0.07 |
| Cmax (ng/ml) | 12.2 | 4.0 | 8.8 | 2.5 | 0.12 |
| Tmax (hr) | 0.13 | 0.13 | 0.30 | 0.35 | 0.07 |
| Max. increase in heart rate | 19 | 9 | 30 | 16 | 0.14 |
(A) MicroDose Therapeutx dry powder inhaler (DPIA): nominal dose 2 mg, output dose 1.95 mg. (B) AtroPen Auto-Injector (AUTO): 2 mg IM. Comparisons were performed using the Mann-Whitney test (nonparametric, unpaired).
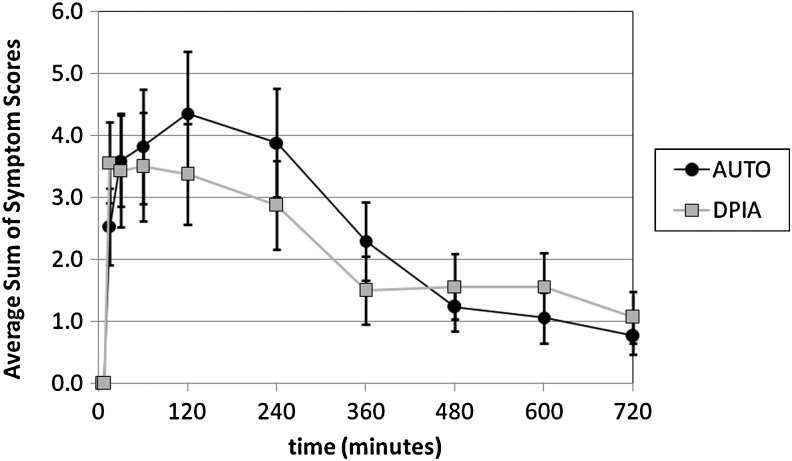
Table 5 shows the percentage of subjects reporting specific anticholinergic symptoms associated with use of the two devices, based on specific inquiries made throughout the testing days. Severe symptoms were reported in the following instances: aftertaste (2 subjects DPIA, 1 AUTO), difficulty urinating (2 AUTO), dizziness (1 AUTO), dry mouth (1 AUTO), excessive thirst (1 AUTO), and fatigue (1 AUTO). Figure 5 shows the average sum of anticholinergic symptom scores for the subjects by time point. Subjects were asked to rate the effects on a 0–3 scale (none, mild, moderate, severe). This measure would increase with both the number of symptoms reported and the severity of those symptoms. Clinical lab values measured at t=10 hr after atropine dosing were compared with pre-study lab values. With DPIA, seven of 42 measured values demonstrated significant changes on average, including platelet volume (+), CO2 (+), blood urea nitrogen (BUN) (–), albumin (–), alanine aminotransferase (ALT) (–), aspartate aminotransferase (AST) (–), and phosphorus (+). With AUTO, seven values demonstrated significant changes, specifically eosinophil count (+), Na (–), K (+), BUN (–), ALT (–), AST (–), and total bilirubin (+). These changes do not indicate any consistent pattern and are likely coincidental. The maximum increases in heart rate for both DPIA and AUTO were both recorded from subject 4 at t=15 min (ΔHR=46 and 57, respectively). No subject demonstrated any clinically significant changes in EKG over the study period.
Table 5.
The Percentage of Subjects Reporting Anticholinergic Symptoms After Atropine Sulfate Delivery
| (A) | |
|---|---|
| Anticholinergic effect | % reporting |
| Aftertaste | 94 |
| Dry mouth | 88 |
| Fatigue | 44 |
| Dizziness | 38 |
| Excessive thirst | 38 |
| Headache | 38 |
| Sore throat | 38 |
| Coughing | 25 |
| Blurred vision | 19 |
| Decreased sweating | 19 |
| Difficulty urinating | 19 |
| Difficulty swallowing | 13 |
| Eye pain | 13 |
| Nausea | 13 |
| Palpitations | 13 |
| Difficulty walking | 6 |
| Excessive warmth | 6 |
| Facial flushing | 0 |
| Shortness of breath | 0 |
| Pain at injection site | NR |
| (B) | |
|---|---|
| Dry mouth | 100 |
| Excessive thirst | 47 |
| Headache | 41 |
| Aftertaste | 35 |
| Dizziness | 35 |
| Fatigue | 35 |
| Blurred vision | 29 |
| Difficulty swallowing | 29 |
| Difficulty urinating | 29 |
| Pain at injection site | 24 |
| Sore throat | 24 |
| Coughing | 18 |
| Decreased sweating | 18 |
| Nausea | 18 |
| Excessive warmth | 12 |
| Eye pain | 12 |
| Palpitations | 12 |
| Difficulty walking | 6 |
| Facial flushing | 0 |
| Shortness of breath | 0 |
(A) MicroDose Therapeutx dry powder inhaler (DPIA): nominal dose 2 mg, output dose 1.95 mg. (B) AtroPen Auto-Injector (AUTO): 2 mg IM.
FIG. 5.
Average sum of the anticholinergic symptom scores by time point for DPIA and AUTO. Subjects were asked to rate a series of known anticholinergic effects on a 0–3 scale (none, mild, moderate, severe) at different time points during the study. These scores were summed for each subject time point and then averaged across all subjects.
There were two adverse events reported during the study. In one instance, a female subject experienced an allergic reaction (urticaria) that began approximately 4 hr after the delivery of inhaled atropine via DPIA. This reaction was experienced on the second testing day. The subject had received IM atropine by AUTO 1 week prior without incident. The reaction was treated with oral and intravenous (IV) diphenhydramine and IV steroids, and resolved by the end of the testing day. However, the subject experienced multiple recurrences of urticaria in the days after discharge that required multiple oral doses of diphenhydramine. Symptoms persisted for more than 8 days before resolving. A second subject experienced minor urticaria around the injection site after IM injection of atropine via AUTO on testing day 1. Symptoms in this case resolved without treatment, and the subject was discharged at the end of the testing day without recurrence. This subject was withdrawn from further testing and did not receive an inhaled dose.
Discussion
The primary purpose of this study was to compare the pharmacokinetics of atropine delivery by inhalation and IM injection. Inhalational delivery was performed using a prototype dry powder inhaler (DPIA), whereas IM injection was performed using the AtroPen auto-injector (AUTO). This comparison will allow for the establishment of a dosing regimen for the new inhaler. Pharmacokinetic measurements provide the best means of comparative evaluation of these systemic delivery systems, because no clinical methods are available to directly evaluate their efficacy in the treatment of organophosphate poisoning—the primary indication for both devices. Pharmacodynamic measurements of systemic response were also performed. DPIA is intended as a secondary therapy for organophosphate poisoning for use during the extended recovery period after initial application of parenteral atropine and pralidoxime chloride. Individual inhaled doses are intended to deliver drug in smaller increments, allowing systemic concentrations to be developed that are sufficient to relieve symptoms without causing excessive side effects. More generally, DPIA provides an example of systemic drug delivery via inhalation and provides a means of evaluating this route for the delivery of small-molecule pharmaceuticals.
Atropine was previously available for inhalation in an FDA-approved CFC-based MDI. Eight inhalations from this MDI provided the pharmacokinetic equivalent of a single dose from a 2-mg auto-injector, based on AUC comparisons. The nominal dose per inhalation for the MDI was 0.43 mg. Similar nominal dosing is provided by DPIA (nominal: 0.40 mg/inhalation); however, significantly higher pulmonary dosing efficiency was anticipated based on the technologies applied in the device. MDI technologies typically emit drug in high-velocity aerosols that can deposit in large amounts in the mouth and throat. With the atropine MDI, such oral deposition might have limited the dose available for absorption in the lungs, but contributed to the pharmacokinetic result through buccal or gastrointestinal (GI) absorption. Dry powder devices generally emit aerosols without any propulsion. Also, the DPIA incorporates specific technologies to detect inhalation and time drug delivery to improve device efficiency. Based on preclinical studies, we hypothesized that five to six inhalations from DPIA would provide the AUC equivalent to a single 2-mg IM dose delivered by auto-injector. We used five inhalations in the study to ensure subject safety and to minimize the chance of overdosing. Our studies indicated that the five DPIA inhalations provided 85% of the AUC associated with the auto-injector. Simple scaling would imply that six inhalations would provide equivalent AUC values. Although we believe that oral and/or GI absorption was limited with DPIA, we cannot exclude the possibility of GI contribution to the observed results (charcoal block was not used in these studies). Time to peak concentration (Tmax) occurred on average at 15 min after the first inhalation with DPIA and at 11.5 min after injection with AUTO, with no statistical difference between the devices. DPIA doses were delivered over a 12-min period during testing, however (one dose every 3 min). A more rapid delivery sequence might have resulted in a more rapid time to peak for DPIA. Although the nominal doses for DPIA and AUTO were similar (5×0.4 mg versus 2 mg), the actual dose delivered from DPIA was slightly less—1.95 mg. The higher apparent clearance for the DPIA is a reflection of the lower bioavailability of atropine from DPIA and does not indicate differences in systemic disposition of atropine. Relative bioavailability can be calculated as 85% or 87% based on nominal or output dose, respectively. Although the devices do not meet criteria for bioequivalence based on comparisons with five inhalations, it seems likely that they would if a sixth inhalation was added within the same time sequence of delivery (one inhalation every 3 min). Both the number of doses and the time sequence of delivery will affect the pharmacokinetic outcomes. The time period between inhalations in this study was designed to accommodate reloading of the prototype inhaler. Information from this study will be used to design the dosing regimen or regimens to be tested when the final form of the device is available. The relative bioavailability calculated assumes linear pharmacokinetics of atropine at dose used and concentrations observed. Given the similar range of concentrations observed (low ng/ml) after AUTO and MDI in this study, assumption of linear pharmacokinetics appears reasonable.
Previous studies performed in the 1980s to gain FDA approval for MDI atropine did not include any female subjects, based on labeling for military use and the profile of military personnel in that era. Studies reported on the AtroPen label indicate that both AUC and Cmax were increased in female subjects.(13) Although the utility and statistical significance of our results is ultimately limited by the small number of female subjects included (n=6), our studies do indicate an extended period to Tmax in female subjects. These studies do not allow us to draw specific conclusions about the effects of gender, but they do suggest a need to stratify the enrollment of future studies by gender to ensure that the results are applicable to current military populations.
Our studies demonstrated a few instances of allergy. In one instance, the allergic reaction occurred on the first testing day at the site of injection and was mild and transient. In the second instance, the reaction occurred on the second testing day (inhalation), had a delayed onset (approximately 4 hr), and resulted in urticaria over the surface of the entire upper body. This was treated successfully with diphenhydramine and corticosteroids, but recurred multiple times over a period of more than a week. Oral diphenhydramine was successful in suppressing these recurrences. Cases of atropine allergy have been previously reported rarely after ocular use,(14) but we are not aware of previous reports of such persistence. Cases of extreme sensitivity to atropine have been reported.(15) Our study presents a less investigated scenario, because it includes two atropine administrations separated by a period of days to weeks. An immunogenic response to the first dose may have set the stage for the systemic reaction that occurred after the second. It is difficult to speculate on whether allergy might be a significant factor in a mass-casualty setting involving organophosphate poisoning where repeated atropine doses are being given to many patients over the course of days. Although organophosphate poisoning is common in parts of the world where these chemicals are still in use as pesticides, the literature describing atropine use in this role is still limited. We are not aware of any reports of allergy after the use of oral atropine preparations. Although these preparations would likely be administered multiple times, the atropine dose included in them is very small. Mast cell degranulation has been described as the mechanism for atropine-induced urticaria.(16) There were no excipients common to DPIA (lactose) and AUTO (glycerin, phenol, citrate buffer, and water for injection)(13); however, we cannot rule out the possibility that unrelated reactions to different components from the individual preparations occurred in this study.
Our studies demonstrated the typical symptoms associated with atropine delivery after both inhaled and IM delivery. For DPIA, the most common symptoms reported (≥30% of subjects) included aftertaste, dry mouth, fatigue, dizziness, excessive thirst, headache, and sore throat. For AUTO, the symptoms included dry mouth, excessive thirst, headache, aftertaste, dizziness, and fatigue. No subjects experienced the excessive manifestation of anticholinergic symptoms associated with atropine toxicity. Symptoms, when they did occur, resolved quickly in all cases, and no subjects were exhibiting symptoms that prevented their discharge at the end of the study day. No subject demonstrated any clinically significant EKG changes in response to the drug.
Limitations of the current study include a small number of total subjects and, specifically, a small number of female subjects. Also, dry powder atropine delivery was performed using a prototype delivery device and not the final form of DPIA. This prototype incorporated visual guidance to maintain an inhalation flow rate within the ideal range for the device, which will not be available in the final commercial design. The intent of this study was to inform the design of the final device, and such steps are necessary and appropriate for pilot studies. As previously described, a substantial period of time was required to deliver the total inhaled dose (12 min), and future studies using a more rapid delivery sequence may demonstrate more rapid development of systemic concentrations. Additional pharmacokinetic studies will play an important role in the future development of DPIA. Based on the results of the current study, we anticipate that six inhalations from DPIA will provide pharmacokinetic equivalency to AUTO. Other dry powder atropine formulations are being developed by other investigators.(17)
This study demonstrates the utility of inhaled medications as systemic therapies, especially when the current generation of precision dosing technologies is applied. The use of the lung as a route to the systemic circulation has been long discussed but rarely utilized. Only a few inhaled systemic therapies have been developed, most notably inhaled insulin.(18) Many of the technologies developed to deliver inhaled insulin have now been applied to other systemic therapies. Products intended for the treatment of conditions such as migraine headaches(19,20) and chronic pain are under development. Here the MicroDose DPIA provided consistent systemic dosing (AUC coefficient of variation for DPIA=0.29 versus 0.21 for AUTO), and these concentrations were developed rapidly after inhalation (Tmax DPIA=15 min). Inhaled delivery provides a needleless alternative for systemic therapy and may be particularly useful when rapid onset is needed (e.g., pain control) or when the GI route is undesirable. Here we have demonstrated that dry powder atropine inhalation provides an effective and efficient means of self-administered systemic delivery. This delivery route also provides the advantage of simultaneous pulmonary treatment and logistical advantages in terms of packaging large amounts of drug in small device volumes.
Acknowledgments
This study was sponsored by Chemical Biological Medical Systems Joint Project Management Office under U.S. Army Space and Missile Defense Command Contract No. W9113M-05-C-0200.
Author Disclosure Statement
Dr. Cook and Mr. Oakum are employees of MicroDose Therapeutx, which is developing the Atropine Dry Powder Inhaler. No other authors have any conflicts of interest.
References
- 1.Schrickel JW. Lewalter T. Luderitz B. Nickenig G. Klehr HU. Rabe C. Recovery from ultra-high dose organophosphate poisoning after "In-the-field" antidote treatment: potential lessons for civil defense. J Emerg Med. 2009;37:279–282. doi: 10.1016/j.jemermed.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Pawar KS. Bhoite RR. Pillay CP. Chavan SC. Malshikare DS. Garad SG. Continuous pralidoxime infusion versus repeated bolus injection to treat organophosphorus pesticide poisoning: a randomised controlled trial. Lancet. 2006;368(9553):2136–2141. doi: 10.1016/S0140-6736(06)69862-0. [DOI] [PubMed] [Google Scholar]
- 3.Orma PS. Middleton RK. Aerosolized atropine as an antidote to nerve gas. Ann Pharmacother. 1992;26:937–938. [PubMed] [Google Scholar]
- 4.Zavala DC. Godsey K. Bedell GN. The response to atropine sulfate given by aerosol and intramuscular routes to patients undergoing fiberoptic bronchoscopy. Chest. 1981;79:512–515. doi: 10.1378/chest.79.5.512. [DOI] [PubMed] [Google Scholar]
- 5.Harrison LI. Smallridge RC. Lasseter KC. Goldlust MB. Shamblen EC. Gam VW. Chang SF. Kvam DC. Comparative absorption of inhaled and intramuscularly administered atropine. Am Rev Respir Dis. 1986;134:254–257. doi: 10.1164/arrd.1986.134.2.254. [DOI] [PubMed] [Google Scholar]
- 6.Kehe CR. Lasseter KC. Miller NC. Wick KA. Shamblen EC. Ekholm BP. Sandahl JH. Chang SF. Goldlust MB. Kvam DC. Comparative absorption of atropine from a metered-dose inhaler and an intramuscular injection. Ther Drug Monit. 1992;14:132–134. doi: 10.1097/00007691-199204000-00009. [DOI] [PubMed] [Google Scholar]
- 7.3M Riker: NDA 20-056, application to market a new drug for human use, atropine sulfate metered-dose aerosol. 1990.
- 8.United States Food and Drug Administration. Use of ozone-depleting substances; removal of essential use designations (direct final rule) Fed Regist. 2006;71:235. [PubMed] [Google Scholar]
- 9.Heyder J. Gebhardt J. Rudolf G. Schiller CF. Stahlhofen W. Deposition of particles in the human respiratory tract in the size range of 0.005–15 micron. J Aerosol Sci. 1986;17:811–825. [Google Scholar]
- 10.Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1:315–320. doi: 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]
- 11.Brunton L. Goodman L. Blumenthal D. Buxton I. Goodman and Gilman's Manual of Pharmacology and Therapeutics. McGraw-Hill Professional; New York, NY: 2007. [Google Scholar]
- 12.United States Pharmacopeial Convention. The United States Pharmacopeia, Chapter 601: Aerosols, Metered-Dose Inhalers, and Dry Powder Inhalers. 2004.
- 13.AtroPen® drug label. Meridian Medical Technologies, Inc., subsidiary of King Pharmaceuticals; Bristol, TN: [Google Scholar]
- 14.Decraene T. Goossens A. Contact allergy to atropine and other mydriatic agents in eye drops. Contact dermatitis. 2001;45:309–310. doi: 10.1034/j.1600-0536.2001.450519.x. [DOI] [PubMed] [Google Scholar]
- 15.Hague JD. Derr JJ. Military implications of atropine hypersensitivity. Mil Med. 2004;169:389–391. doi: 10.7205/milmed.169.5.389. [DOI] [PubMed] [Google Scholar]
- 16.Shipley D. Ormerod AD. Drug-induced urticaria. Recognition and treatment. Am J Clin Dermatol. 2001;2:151–158. doi: 10.2165/00128071-200102030-00004. [DOI] [PubMed] [Google Scholar]
- 17.Ali R. Jain GK. Iqbal Z. Talegaonkar S. Pandit P. Sule S. Malhotra G. Khar RK. Bhatnagar A. Ahmad FJ. Development and clinical trial of nano-atropine sulfate dry powder inhaler as a novel organophosphorous poisoning antidote. Nanomedicine. 2009;5:55–63. doi: 10.1016/j.nano.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Rosenstock J. Lorber DL. Gnudi L. Howard CP. Bilheimer DW. Chang PC. Petrucci RE. Boss AH. Richardson PC. Prandial inhaled insulin plus basal insulin glargine versus twice daily biaspart insulin for type 2 diabetes: a multicentre randomised trial. Lancet. 2010;375(9733):2244–2253. doi: 10.1016/S0140-6736(10)60632-0. [DOI] [PubMed] [Google Scholar]
- 19.Dinh K. Myers DJ. Glazer M. Shmidt T. Devereaux C. Simis K. Noymer PD. He M. Choosakul C. Chen Q. Cassella JV. In vitro aerosol characterization of Staccato® Loxapine. Int J Pharm. 2011;403:101–108. doi: 10.1016/j.ijpharm.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 20.Aurora SK. Silberstein SD. Kori SH. Tepper SJ. Borland SW. Wang M. Dodick DW. MAP0004, orally inhaled DHE: a randomized, controlled study in the acute treatment of migraine. Headache. 2011;51:507–517. doi: 10.1111/j.1526-4610.2011.01869.x. [DOI] [PubMed] [Google Scholar]