Abstract
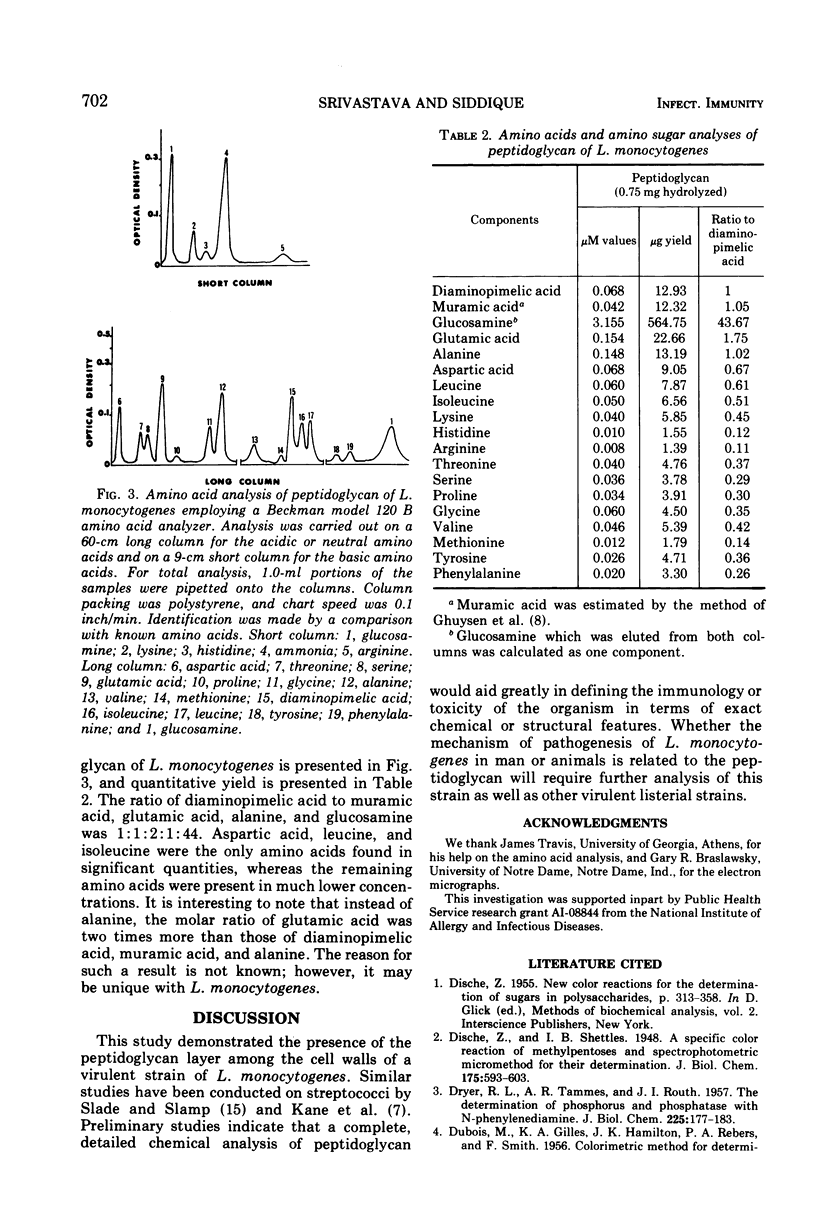
The qualitative and quantitative chemical composition of the peptidoglycan layer of Listeria monocytogenes cell walls was determined. The peptidoglycan layer was isolated from intact cells after treatment with sodium lauryl sulfate and digestion with Pronase. The isolated peptidoglycan contained carbohydrates which included hexose, rhamnose, and amino sugars. The amino sugar was identified as glucosamine which was present in very high micromole concentration, as compared with the amino acids, muramic acid, or diaminopimelic acid. In addition, protein and phosphorus were also present. However, the presence of teichoic acid was not detected in the peptidoglycan. Protein moiety contained a total of 18 amino acids with lysine as the N-terminal amino acid.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- Jenkins E. M., Watson B. B. Serum protein changes: an activity of the extracellular hemolytic-lipolytic protein from Listeria monocytogenes. Am J Vet Res. 1969 Apr;30(4):669–677. [PubMed] [Google Scholar]
- Kane J., Lackland H., Karakawa W. W., Krause R. M. Chemical studies on the structure of mucopeptide isolated from Streptococcus bovis. J Bacteriol. 1969 Jul;99(1):175–179. doi: 10.1128/jb.99.1.175-179.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Wicken A. J. Serological properties of the wall and membrane teichoic acids from Lactobacillus helveticus NCIB 8025. J Gen Microbiol. 1970 Oct;63(2):237–248. doi: 10.1099/00221287-63-2-237. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Ensign J. C. Isolation and chemical structure of the peptidoglycan of Spirillum serpens cell walls. J Bacteriol. 1968 Jan;95(1):201–210. doi: 10.1128/jb.95.1.201-210.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rimington C. Seromucoid and the bound carbohydrate of the serum proteins. Biochem J. 1940 Jun;34(6):931–940. doi: 10.1042/bj0340931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F. The arrangement of amino acids in proteins. Adv Protein Chem. 1952;7:1–67. doi: 10.1016/s0065-3233(08)60017-0. [DOI] [PubMed] [Google Scholar]
- Slade H. D., Slamp W. C. Peptidoglycan composition and taxonomy of group D, E, and H streptococci, and Streptococcus mutans. J Bacteriol. 1972 Feb;109(2):691–695. doi: 10.1128/jb.109.2.691-695.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]




