Abstract
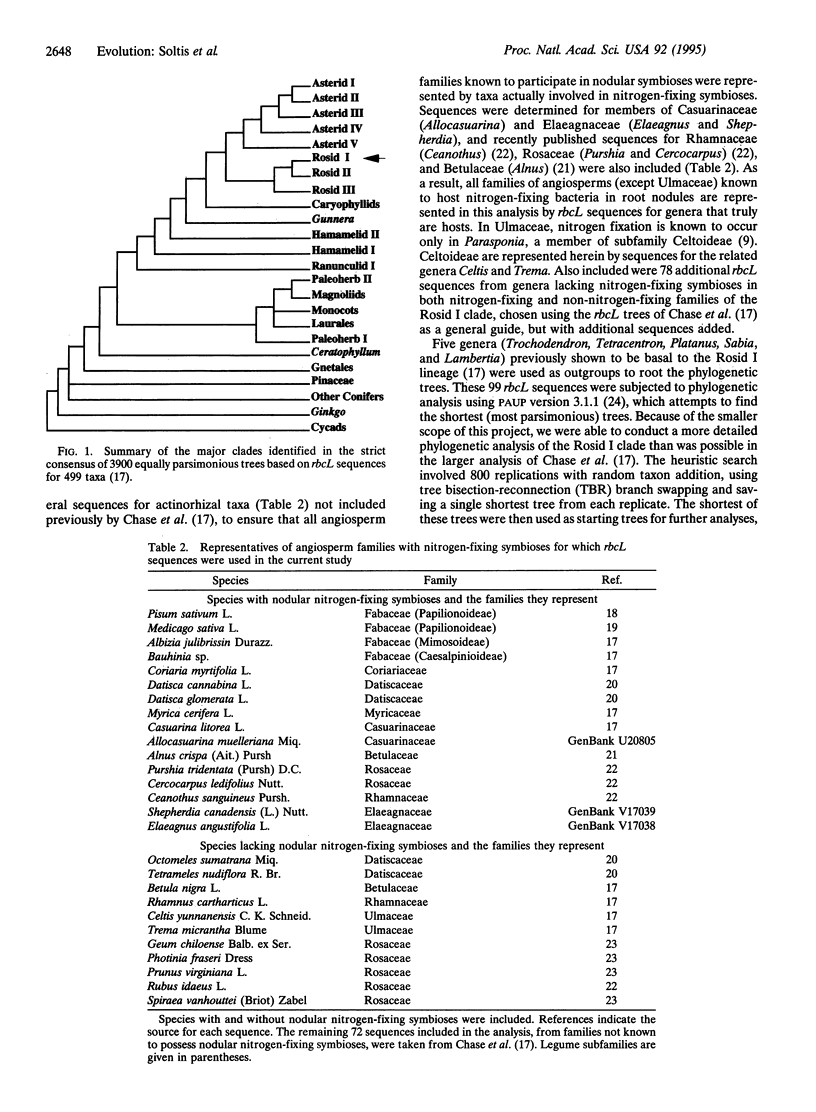
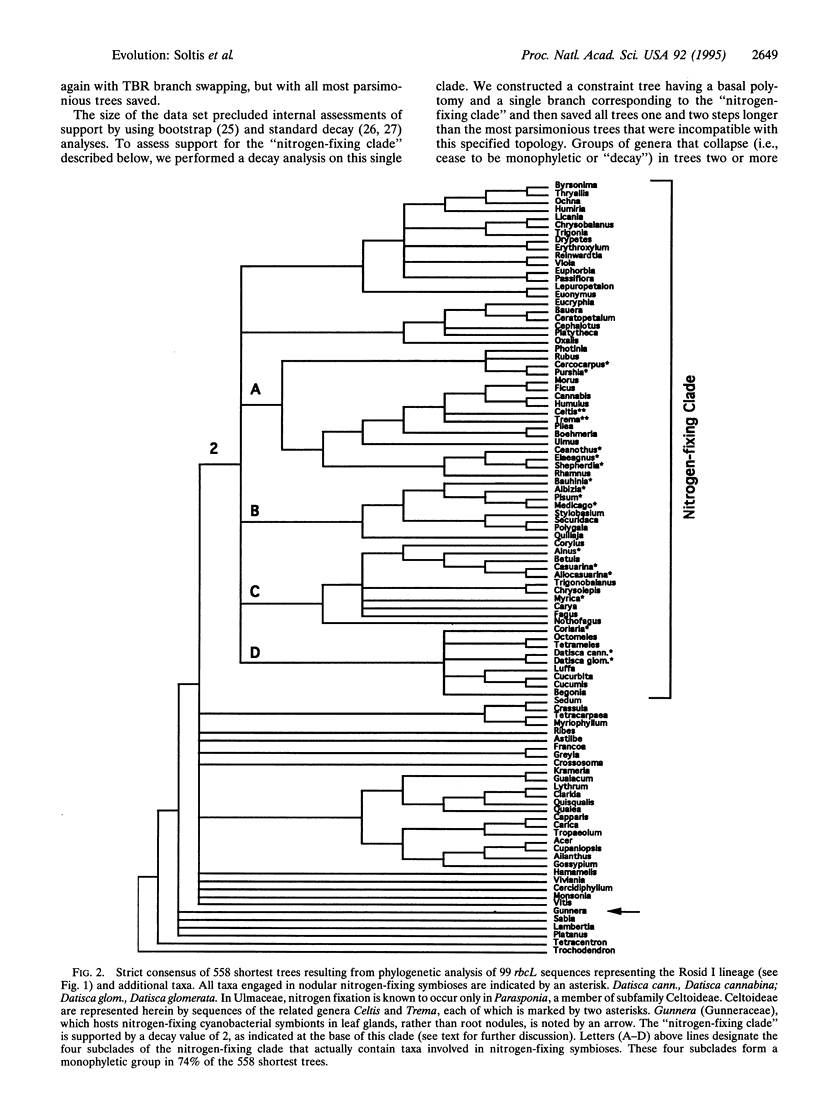
Of the approximately 380 families of angiosperms, representatives of only 10 are known to form symbiotic associations with nitrogen-fixing bacteria in root nodules. The morphologically based classification schemes proposed by taxonomists suggest that many of these 10 families of plants are only distantly related, engendering the hypothesis that the capacity to fix nitrogen evolved independently several, if not many, times. This has in turn influenced attitudes toward the likelihood of transferring genes responsible for symbiotic nitrogen fixation to crop species lacking this ability. Phylogenetic analysis of DNA sequences for the chloroplast gene rbcL indicates, however, that representatives of all 10 families with nitrogen-fixing symbioses occur together, with several families lacking this association, in a single clade. This study therefore indicates that only one lineage of closely related taxa achieved the underlying genetic architecture necessary for symbiotic nitrogen fixation in root nodules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich J., Cherney B., Merlin E., Palmer J. Sequence of the rbcL gene for the large subunit of ribulose bisphosphate carboxylase-oxygenase from alfalfa. Nucleic Acids Res. 1986 Dec 9;14(23):9535–9535. doi: 10.1093/nar/14.23.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J., Strauss S. H., Li P. Complete congruence between morphological and rbcL-based molecular phylogenies in birches and related species (Betulaceae). Mol Biol Evol. 1992 Nov;9(6):1076–1088. doi: 10.1093/oxfordjournals.molbev.a040779. [DOI] [PubMed] [Google Scholar]
- Dénarié J., Debellé F., Rosenberg C. Signaling and host range variation in nodulation. Annu Rev Microbiol. 1992;46:497–531. doi: 10.1146/annurev.mi.46.100192.002433. [DOI] [PubMed] [Google Scholar]
- Fisher R. F., Long S. R. Rhizobium--plant signal exchange. Nature. 1992 Jun 25;357(6380):655–660. doi: 10.1038/357655a0. [DOI] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Scheres B., Van De Wiel C., Zalensky A., Horvath B., Spaink H., Van Eck H., Zwartkruis F., Wolters A. M., Gloudemans T., Van Kammen A. The ENOD12 gene product is involved in the infection process during the pea-Rhizobium interaction. Cell. 1990 Jan 26;60(2):281–294. doi: 10.1016/0092-8674(90)90743-x. [DOI] [PubMed] [Google Scholar]
- Vandenbosch K. A., Bradley D. J., Knox J. P., Perotto S., Butcher G. W., Brewin N. J. Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J. 1989 Feb;8(2):335–341. doi: 10.1002/j.1460-2075.1989.tb03382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Whitfeld P. R., Bottomley W. Sequence of the gene for the large subunit of ribulose 1,5-bisphosphate carboxylase from pea chloroplasts. Nucleic Acids Res. 1986 May 12;14(9):3975–3975. doi: 10.1093/nar/14.9.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]



