Abstract
Ras superfamily proteins participate in TGFβ-mediated developmental pathways that promote either tumor suppression or progression. However, the specific Ras proteins which integrate in vivo with TGFβ signaling pathways are unknown. As a general approach to this question, we activated all Ras proteins in vivo by genetic deletion of the RasGAP protein Nf1 and examined mice doubly deficient in a Ras protein to determine its requirement in formation of TGFβ-dependent neurofibromas that arise in Nf1-deficient mice. Animals lacking Nf1 and the Ras-related protein R-Ras2/TC21 displayed a delay in formation of neurofibromas but an acceleration in formation of brain tumors and sarcomas. Loss of R-Ras2 was associated with elevated expression of TGFβ in Nf1-deficient Schwann cell precursors (SCP), blockade of a Nf1/TGFβRII/AKT-dependent autocrine survival loop in tumor precursor cells, and decreased precursor cell numbers. Further, the increase in size of sarcomas from xenografts doubly deficient in these genes was also found to be TGFβ-dependent, in this case resulting from cell non-autonomous effects on endothelial cells and myofibroblasts. Extending these findings in clinical specimens, we documented an increase in TGFβ ligands and an absence of TGFβ receptor II in malignant peripheral nerve sheath tumors which correspond to tumors in the Nf1-deficient mouse model. Together, our findings reveal RRas2 as a critical regulator TGFβ signaling in vivo.
Keywords: neurofibromatosis, Ras, sarcoma, TC21, TGF
Introduction
Ras proteins are molecular switches that cycle between an inactive GDP-bound form and an active GTP-bound form and signal through effector pathways including Ral, phosphoinositide-3-kinase (PI3K)-AKT and Raf-MEK-ERK to regulate proliferation, cell death and cell differentiation (1, 2). Individual members of the Ras superfamily can have unique roles in diverse cell compartments (3, 4). The commonly studied Ras oncogenic proteins encoded by the H-Ras, K-Ras and N-Ras genes (Ras) are activated by mutation in up to 50% of human cancers (5). The related R-Ras family, encoded by the R-Ras, TC21/R-Ras2 (subsequently TC21) and R-Ras3/M-Ras genes, also has oncogenic potential (6). Here we focus on TC21, a transforming oncogene mutated in human tumor cell lines (7) and shown to induce lymphoma in vivo (8), in the context of the NF1 Ras GTPase activating protein (GAP).
Ras signaling is inactivated by GAPs, including the NF1 tumor suppressor protein neurofibromin. Neurofibromin is a GAP for Ras proteins (9), so that sustained activation of each expressed Ras protein is predicted in cells that rely on neurofibromin function. Mutations in the NF1 gene result in neurofibromatosis type 1 (NF1) (10). NF1 patients develop disfiguring benign peripheral nerve tumors, neurofibromas; plexiform neurofibroma can transform into sarcomas known as malignant peripheral nerve sheath tumors (MPNST), a leading cause of death in adults with NF1 (11). We reasoned that specific roles of individual Ras proteins would be revealed in the setting of NF1 loss in peripheral nerve cells. Consistent with this idea, increased migration of Nf1−/− Schwann cells was rescued by a dominant negative allele of TC21 (12).
Ras signaling is critical to activate a variety of downstream kinase cascades. While mitogen-activated protein kinases (MAPKs) Erk1/2, c-Jun N-terminal kinase, and p38SAPK MAPK act as downstream effectors of TC21 in certain cell lines (6), PI3K-AKT is currently believed to be a major TC21 effector (13, 14). A recent in vivo study implicates TC21 in PI3K signaling downstream of the antigen receptor in T-cells (14). In addition to PI3K signaling, TC21 was recently linked to TGFβ in vitro; overexpression of an activated TC21 allele caused cells to lose responsiveness to the growth inhibitory effects of transforming growth factor β (TGFβ) (13).
Interestingly, TGFβ proteins can act as either tumor suppressors or promoters (15, 16). TGFβ ligands 1, 2 and 3 regulate cell proliferation, death and differentiation through interaction with receptors TGFβRI (ALK5) and TGFβRII, and the TGFβRIII co-receptor (17). Downstream of TGFβ receptors (TGFβR), activation of SMAD proteins and PI3K-AKT signaling are thought to control TGFβ tumor suppression, while activation of RhoA, TAK1, and PI3K-AKT are implicated in TGFβ oncogenesis (16). Decreased or altered TGFβ responsiveness and increased expression or activation of TGFβ ligands is common as tumors progress. For example, TGFβ can promote tumorigenesis through sequestration of mutant p53 (18), and have non-autonomous effects on tumor stroma (19).
We identify TC21 as a regulator of TGFβ function in vivo. Previous studies examined crosstalk between the Ras and TGFβ signaling pathways, mainly in vitro (20–22). Ras/MAPK activates the TGFβ promoter (23, 24), and Ras/Erk signaling blocks SMAD translocation to the nucleus through phosphorylation of SMAD2/3 (20). In vivo, RAS/MAPK can phosphorylate p53 which then interacts with SMAD proteins (25).
Neurofibromas and MPNSTs derive from neural crest lineage cells, more mature Schwann cell precursors (SCP), and/or differentiated mature Schwann cells (26). To study development, we crossed TC21 deficient mice (TC21−/−) (14) to Nf1+/− mice (27). To study tumorigenesis, we crossed TC21−/− mice to Nf1fl/fl;DhhCre mice that form neurofibromas, and Nf1+/−;Trp53+/− (NPCis) mice that serve as a model of soft tissue sarcoma (28) and brain tumors with histology of glioblastoma (29), and used a xenograft model of human MPNST.
We found that Nf1 mutation renders SCPs insensitive to TGFβ–mediated cell death and define a TGFβ autocrine survival loop, correlating with benign neurofibroma formation. Nf1 mutants lacking TC21 restore TGFβ sensitivity and benign tumorigenesis is delayed. Conversely, loss of TC21 increases TGFβ-induced malignancy in the NPCis model. In NF1 MPNST xenografts, loss of TC21 accelerates tumor growth in a non-cell autonomous manner. In summary, TC21 is a major regulator of TGFβ production that functions in development and tumorigenesis in vivo.
Materials and Methods
Mice
We housed mice in a temperature- and humidity-controlled vivarium on a 12-hour dark-light cycle with free access to food and water. TC21+/− and R-Ras+/− mice were obtained on C57Bl/6/129 mixed background after four generations of backcross onto C57/Bl6 (14), then bred to homozygosity. We mated TC21−/− and R-Ras−/− mice to Nf1+/− C57/Bl6 mice (27) to obtain TC21−/−;Nf1+/− and R-Ras−/−;Nf1+/− mice, which were intercrossed to obtain mutant embryos. TC21−/− mice were mated to Nf1fl/fl;DhhCre mice (26) to obtain TC21+/−;Nf1fl/+;DhhCre mice. F1 mice were intercrossed to obtain TC21−/− ;Nf1fl/fl;DhhCre mice and TC21−/−;Nf1fl/+;DhhCre littermates. We bred TC21−/− mice to NPCis C57BL/6 mice (28) to obtain TC21+/−;NPCis mice. These mice were mated with TC21−/− to obtain TC21−/−;NPCis mice. TC21+/−;NPCis littermates were bred to C57BL/6 wild type mice for parallel controls. For tumor experiments we analyzed male mice.
Cell culture
We dissociated DRGs from E12.5 embryos and plated cells in serum-free medium (29). We used cells direct from embryos for precursor numbers. At passage 2–3, 500 cells/well were plated then inhibitors, antibody or lentivirus added after 24-hours. Spheres were counted 3 days later. Each experiment shown represents ≥three independent experiments. Anti-TGFβ antibody, rhTGFβ1 and rabbit IgG were from R&D Systems, MN. TGFβR1, MEK1 (Cayman Chemicals, MI), AKT (Selleck Chemicals, TX), p38SAPK and ROCK (Calbiochem, NJ) inhibitors were dissolved in DMSO.
MPNST cell lines included 26T, T265, 8814 [WT for p53 (30)] and S462TY [derived from the S462 cell line by two rounds of in vivo growth as xenografts (31)]. Cells were maintained in DMEM/10% FBS/1% penicillin/streptomycin.
Immunohistochemistry and Histology
Formalin-fixed 6µM paraffin sections or 12µM 4% paraformaldehyde-fixed frozen sections were stained with phospho-SMAD2/3, phospho-AKT (Cell Signal), SMA (Dako, CA), anti-neurofilament and meca-32 (DSHB, IO). Secondary incubations used hostappropriate secondary antibodies. Neurofibroma sections were submitted to the CCHMC Pathology Laboratory for H&E, toluidine blue and rabbit polyclonal anti-cow S100β (Dako).
Western analysis
We lysed cells, tissue and tumor sections as described (32). We separated proteins on 4–20% TrisHCl acrylamide gels (Biorad, CA), transferred to PVDF membranes (Millipore, MA) and probed membranes with: anti-TC21 (Abnova, Taiwan), phospho-AKT, phospho-SMAD2/3, phospho-42/44-MAPK and β-Actin (Cell Signaling). Detection used horseradish peroxidase-conjugated secondary antibodies (BioRad) and ECL Plus developing system (Amersham Biosciences, NJ).
qRT-PCR
We isolated total RNA with an RNeasy kit (Qiagen) and carried out cDNA synthesis (Invitrogen Superscript III). We used triplicate reactions to perform qRT–PCR (ABI 7500-Sequence Detection System, CA) (32). Values for genes of interest were normalized to GAPDH (mouse samples) or β-actin (human samples) and fold change calculated by the ΔΔct method.
Lentiviral infection
We infected MPNST cells at 50% confluence with TRIPZ shTC21 or non-target control (Open Biosystems, AL). We incubated lentiviral particles with MPNST cells in the presence of polybrene (8µg/ml; Sigma-Aldrich) daily for 3 days, followed by selection in puromycin (2.5µg/ml; Sigma-Aldrich) then maintained cells in media containing puromycin; 2µg/ml doxycycline (MP Biomedicals, OH) induced shRNA expression.
Mouse xenograft
We injected 2.3×106 S462TY cells in 150µl with 30% matrigel (BD Biosciences, MD) into flanks of 5/6-week-old female athymic nude (nu/nu) mice (Harlan, IN). We maintained mice on 1875ppm doxycycline feed (Test Diet, IN). Tumor volumes and weight were measured twice weekly. We sacrificed mice before tumor size reached 10% body weight. For anti-TGFβ treatment, we injected mice intraperitoneally with 3mg/kg of IgG or anti-TGFβ antibody every 2 weeks. We dissected tumors; we fixed tumors in 4% paraformaldehyde or flash-froze and stored tumors at −80°C.
Gene expression analysis
All samples except for normal nerves were previously described (33). Affymetrix probes were remapped to RefSeq genes (version 11.0.1). Comparisons and data visualization were performed using GeneSpring GX v7.3.1 (Agilent Technologies).
Results
Loss of TC21 extends neurofibroma-bearing mice survival but decreases NPCis mice survival
To define roles for TC21, we used TC21−/− mice (Supplemental Figure 1A). TC21 mRNA was <10-fold and protein expression lost (Supplemental Figure 1B, 1C), confirming that the TC21 mutation is a null allele (14). Nf1−/− embryos die by embryonic day 12.5 (E12.5) (27), but 90% of TC21−/−;Nf1−/− embryos survived to E14.5 and 10% survived to E16.5 (Supplemental Table 1). Partial rescue of embryo viability was not observed in R-Ras−/−;Nf1−/− embryos (Supplemental Table 1). Thus, TC21 plays a role in Nf1 embryonic development.
To determine TC21 relevance to tumorigenesis, we generated Nf1fl/fl;DhhCre;TC21−/− and NPCis;TC21−/− mice. Loss of TC21 significantly extended survival in Nf1fl/fl;DhhCre mice (Figure 1A). Nf1fl/fl;DhhCre;TC21−/− (n=15) mice survived up to 20 months while littermate controls died by 15 months. Mice required sacrifice due to morbidity secondary to paralysis that correlated with neurofibroma formation and spinal cord compression. A second cohort of mice (n=15) showed identical results (not shown).
Figure 1. Loss of TC21 extends survival of neurofibroma-bearing mice but decreases survival of NPCis mice.
Kaplan-Meyer survival curves for (A) Nf1fl/fl;DhhCre and Nf1fl/fl;DhhCre;TC21−/− mice, (log-rank test, p < 0.0001) and (B) NPCis and NPCis;TC21−/− mice, (log-rank test, p = 0.0001).
To test for effects of TC21 loss in malignancy, we generated NPCis;TC21−/− mice. NPCis;TC21−/− (n=13) mice died by 7 months while littermate controls (n=7) survived up to 13 months (Figure 1B). Therefore, loss of TC21 in benign tumors extends survival, while paradoxically in a model of aggressive tumors, loss of TC21 decreases survival. Additionally, NPCis;TC21−/− mice died early due to rapid formation of aggressive brain tumors (Supplemental Figure 2).
A role for TC21 in tumor initiation
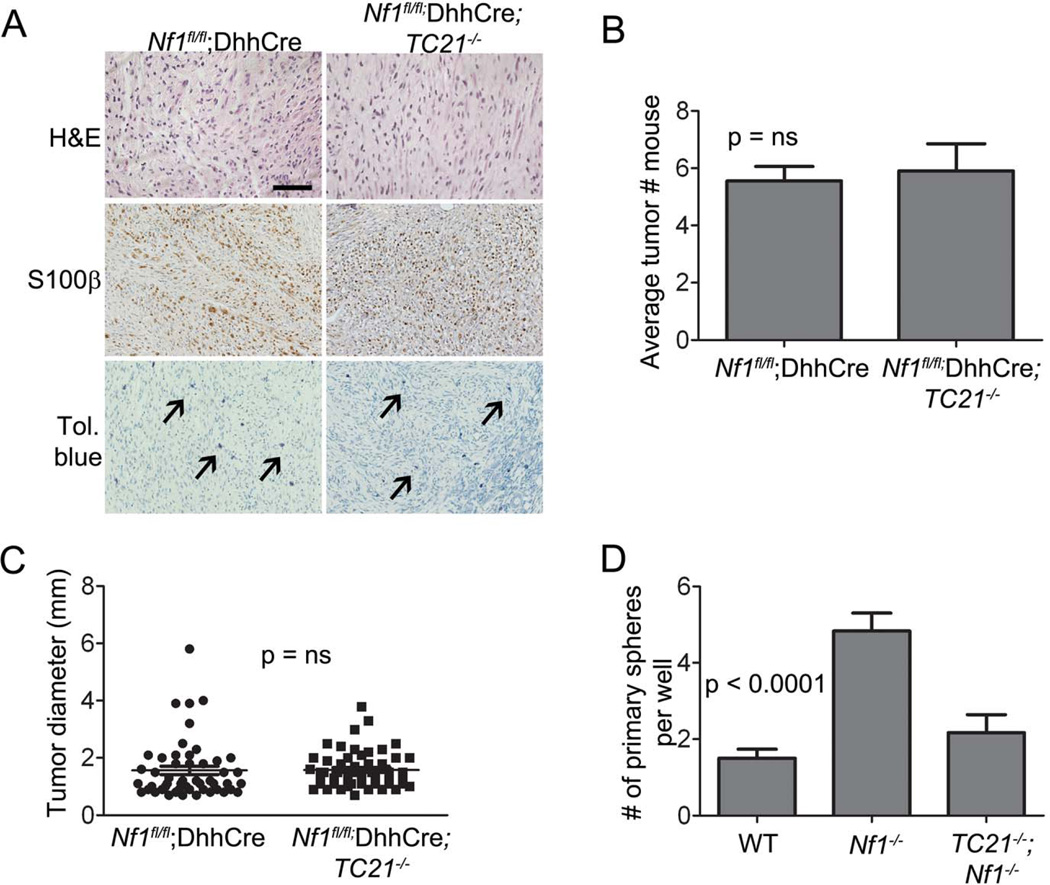
Grade 1 GEM neurofibromas from Nf1fl/fl;DhhCre mice with or without TC21 did not differ in histology on H&E staining, or anti-S100β staining to mark Schwann cells (34) (Figure 2A). Toluidine blue+ metachromatic mast cells increased slightly in the absence of TC21 (not shown). At the time of sacrifice there was no difference in number or size (diameter) of neurofibromas in the two strains of mice (Figures 2B, 2C).
Figure 2. A role for TC21 in tumor initiation.
(A) Neurofibroma sections stained with H&E, anti-S100β antibody (brown) and toluidine blue for mast cell infiltration (black arrows). Scale bar: 50µm. (B) Quantification of average tumor numbers/mouse. (C) Quantification of tumor size (diameter, mm). (D) Number of primary spheres from E12.5 DRG plated at clonal density.
To explain how loss of TC21 extends survival in Nf1fl/fl;DhhCre mice we postulated that TC21 diminishes numbers of neurofibroma-initiating or sustaining multipotent self-renewing cells (29). To test this, we used an in vitro model. SCPs from Nf1−/− DRG cells give rise to more spheres than do wild-type (WT) or Nf1+/− DRG cells, and Nf1−/− DRG sphere cells form neurofibroma-like lesions upon xenotransplantation (29). Nf1−/− DRGs formed significantly more primary spheres at clonal density than cells from WT DRG. Importantly, TC21−/−;Nf1−/− DRG cells formed WT levels of spheres (Figure 2D) consistent with a role of TC21 regulating numbers of tumor-initiating cells early in Nf1 tumorigenesis.
A TGFβ autocrine loop in Nf1−/− SCPs
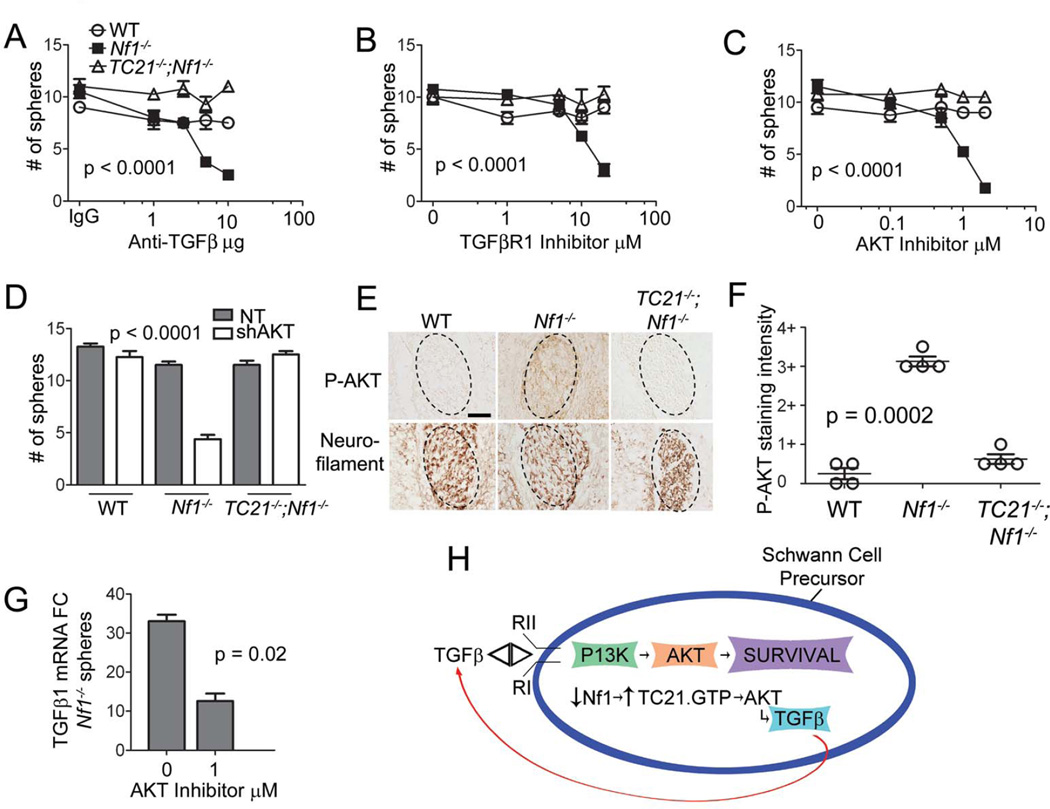
Since TGFβ proteins can act as tumor suppressors or tumor promoters and TC21 acts similarly (Figure 1), and because TC21 and TGFβ have been linked in vitro (13), we examined TGFβ signaling in the context of Nf1 and TC21 null alleles. Cells from secondary spheres were plated at clonal density and tested for response to TGFβ1. WT SCP numbers were reduced by exposure to TGFβ1 in a dose-dependent manner. WT SCP cells died but Nf1−/− SCPs did not die when exposed to TGFβ1 (Supplemental Figure 3A). Loss of TC21 restored sensitivity to TGFβ1 (Figure 3A).
Figure 3. A TGFβ autocrine loop in Nf1−/− SCPs.
(A) SCP spheres treated with TGFβ1 then counted (p = 0.005 ANOVA). (B) qRT-PCR mRNA expression of TGFβ1 in spheres compared to WT spheres (t-test: Nf1−/− vs TC21−/−;Nf1−/−). (C) ELISA for TGFβ1 protein. (D, E) Phase contrast micrographs of Nf1−/− spheres after treatment. (F) Quantification of Nf1−/− spheres after treatment.
Nf1−/− SCPs had 20-fold higher levels of TGFβ1 mRNA and protein compared to WT precursors (Figure 3B, 3C) and loss of TC21−/− rescued TGFβ1 levels in Nf1−/− spheres. TGFβ produced by Nf1−/− spheres was functional, as Nf1−/− SCPs plated at clonal density with function-blocking anti-TGFβ antibody died but spheres in control IgG formed healthy spheres (Figures 3D, 3E). In contrast, treatment of WT or TC21−/−;Nf1−/− SCPs with anti-TGFβ antibody did not alter sphere numbers (Figure 4A). Production of TGFβ and effects of function blocking antibody suggest that Nf1−/− SCPs secrete TGFβ, enhancing their survival. The data support the hypothesis that Nf1−/− SCPs are dependent on TGFβ for survival.
Figure 4. Survival of Nf1 mutant SCPs is dependent on TGFβ and AKT.
SCP spheres treated with (A) anti-TGFβ antibody (B) TGFβR1 inhibitor, SB 431542 and (C) AKT inhibitor, MK-2206 or (D) shAKT compared to NT. Spheres counted after treatment. (E) E12.5 DRGs stained with anti-phospho-AKT. Adjacent sections stained with anti-neurofilament (to highlight neurons in DRG). Scale bar: 50µm. (F) Quantification of staining intensity for phospho-AKT in DRG sections. (G) qRT-PCR, TGFβ1 mRNA expression in Nf1−/− SCP spheres after treatment. (H) Model of TC21 dependent TGFβ autocrine loop in Nf1−/− SCPs.
In contrast, TGFβ1 mRNA was only slightly elevated in mature Schwann cells in sciatic nerves from Nf1fl/fl;DhhCre mice compared to WT sciatic nerve (2-fold change); Supplemental Figure 3B). There was no significant difference in TGFβ1 levels between neurofibromas from Nf1fl/fl;DhhCre (n=8) and Nf1fl/fl;DhhCre;TC21−/− (n=10) mice (Supplemental Figure 3C). That elevation and rescue of TGFβ1 mRNA expression levels in the TC21−/−;Nf1−/− setting is most pronounced early in Schwann cell development suggests that TC21 activation plays roles in SCP viability and growth, not neurofibroma growth. The mechanism(s) by which TC21 loss enhances TGFβ expression may include alterations in signaling pathways that change in cell maturation and/or alterations due to as-yet-unidentified mutations in neurofibroma Schwann cells during tumor formation.
Survival of Nf1−/− SCPs requires TGFβ and AKT
To determine how TGFβ affects survival of Nf1−/− SCPs, we used specific inhibitors. Treatment of Nf1−/− SCPs with a TGFβR1 inhibitor blocked sphere formation but did not affect WT or TC21−/−;Nf1−/− spheres (Figure 4B). PI3K/AKT has been implicated downstream of TGFβ signaling, and treatment with an AKT inhibitor significantly reduced Nf1−/− sphere formation (Figure 4C). In contrast, there was no of MEK1, ROCK, nor p38SAPK inhibitors on Nf1−/− sphere formation (Supplemental Figure 4A). Efficacy of inhibitors was validated by western blotting (not shown). Confirming a role for AKT signaling in a TGFβ autocrine loop, formation of Nf1−/− but not WT or TC21−/− ;Nf1−/− spheres was significantly reduced by shAKT (Figure 4D). Thus, formation and survival of Nf1−/− spheres in vitro are dependent on TC21, TGFβ, TGFβRI and AKT.
We tested whetehr loss of TC21 affects signaling in vivo by immunochemistry. We stained E12.5 DRG tissue sections containing SCPs, the targets for Nf1 loss in the Nf1fl/fl;DhhCre model, with anti-phospho-AKT. Nf1−/− DRG SCPs showed elevated levels of phospho-AKT compared to WT and TC21−/−;Nf1−/− embryos (Figures 4E, 4F). Nf1−/− spheres from E12.5 DRG grown in vitro also contained increased phospho-AKT compared to WT and TC21−/−;Nf1−/− spheres (Supplemental Figure 4B). P-AKT was similar in neurofibromas from Nf1fl/fl;DhhCre and Nf1fl/fl;DhhCre;TC21−/− mice, consistent with TC21 affecting events shortly after Nf1 loss (not shown). Phospho-SMAD2/3 staining, marking canonical TGFβ signaling was similar across genotypes (Supplemental Figures 4C, 4D). Thus, losing TC21 in an Nf1−/− background correlated with reduced expression of phospho-AKT in SCPs in vivo.
To test if TGFβ1 mRNA production in SCPs requires AKT, we examined levels of TGFβ1 mRNA in Nf1−/− SCPs treated with the AKT inhibitor; the inhibitor decreased TGFβ1 mRNA (Figure 4G). These data support a existence of a TC21 dependent TGFβ autocrine survival loop, requiring AKT, in Nf1−/− SCP cells (Figure 4H). We propose that absence of the autocrine loop causes the observed delay in benign tumor formation and decreased survival of neurofibroma-bearing mice.
Loss of TC21 in MPNST cells increases tumor growth
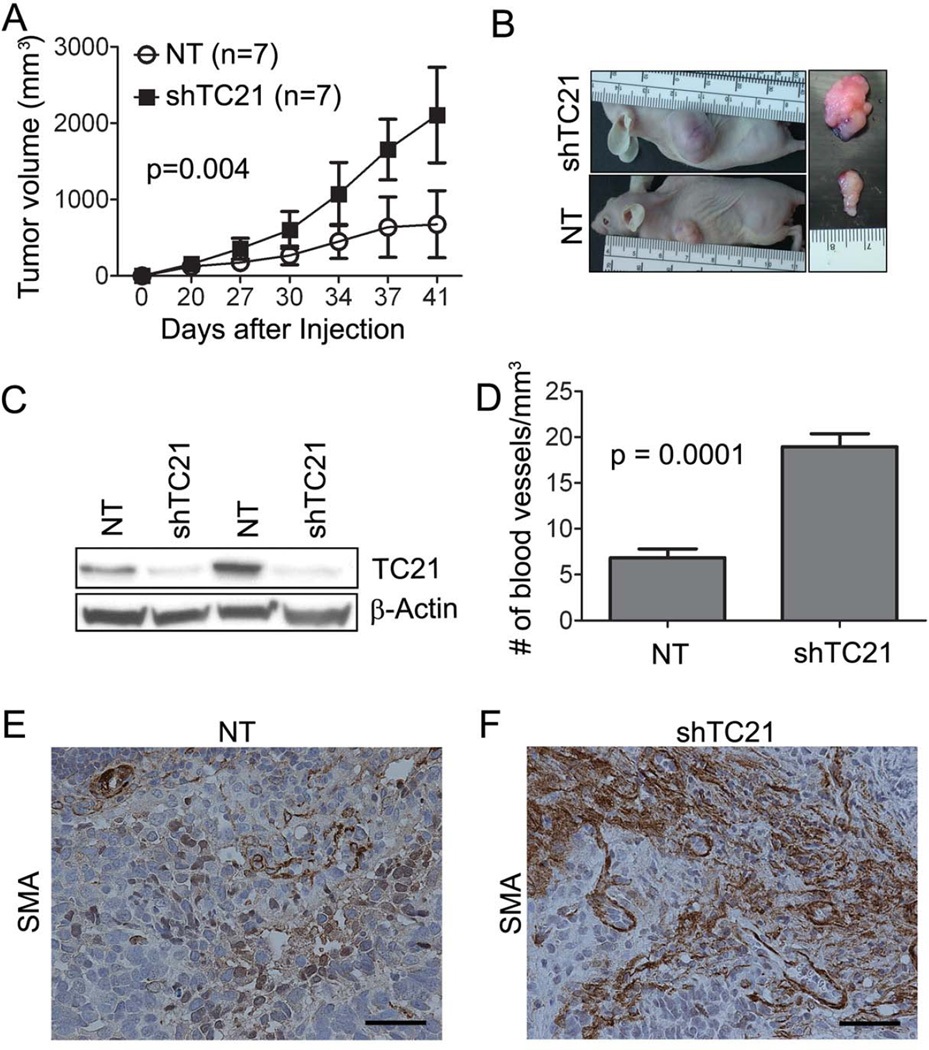
In the NPCis model of GEM-sarcoma and GEM-GBM, loss of TC21 caused early lethality (Figure 1B). Brain tumors killed mice before the normal onset of sarcoma formation in the NPCis model. We demonstrated that TC21 activity is high in human MPNST S462TY cells that are NF1−/− in comparison to NF1 WT 26T cells, using a Ras pull-down assay and blotting with anti-TC21 antibody (Supplemental Figure 5A). To investigate the role of TC21 in sarcomas, we infected the S462TY cells with a stably expressing doxycycline-inducible TRIPZ lentivirus encoding shRNA targeting TC21 (shTC21), or control TRIPZ non-target shRNA (NT). shTC21 cells were injected subcutaneously into the right flank of nu/nu mice and NT cells into the left flank and tumor growth measured. Tumors with shTC21 cells were larger than tumors with NT cells (Figures 5A, 5B). Western blot analysis confirmed low TC21 expression in excised tumors (Figure 5C). Thus, loss of TC21 in a human sarcoma xenograft increases tumor growth.
Figure 5. Loss of TC21 in MPNST cells increases sarcoma growth.
(A) Volume quantification of xenograft tumors over time. (B) Gross photographs of nude mice tumors. (C) Western blot showing TC21 protein in S462TY xenograft tumors. (D) Quantification of anti-meca staining and measurement of numbers of vessels/hpf. (E, F) SMA immunostaining in xenograft sections (brown). Scale bar: 50uM.
However, histological analysis of shTC21 MPNST xenografts revealed no differences in morphology (H&E) or cell proliferation (Ki67) compared to controls (not shown). As TGFβ can induce blood vessel formation we analyzed tumor vasculature. The shTC21 tumors had more meca-32+ vessels per mm3 than controls (Figure 5D). Consistent with altered tumor stroma upon TC21 loss, an antibody against smooth muscle actin (SMA) detecting myofibroblasts revealed increased immunoreactivity in xenografts expressing shTC21 (Figures 5E, 5F).
Increased MPNST growth due to loss of TC21 requires TGFβ
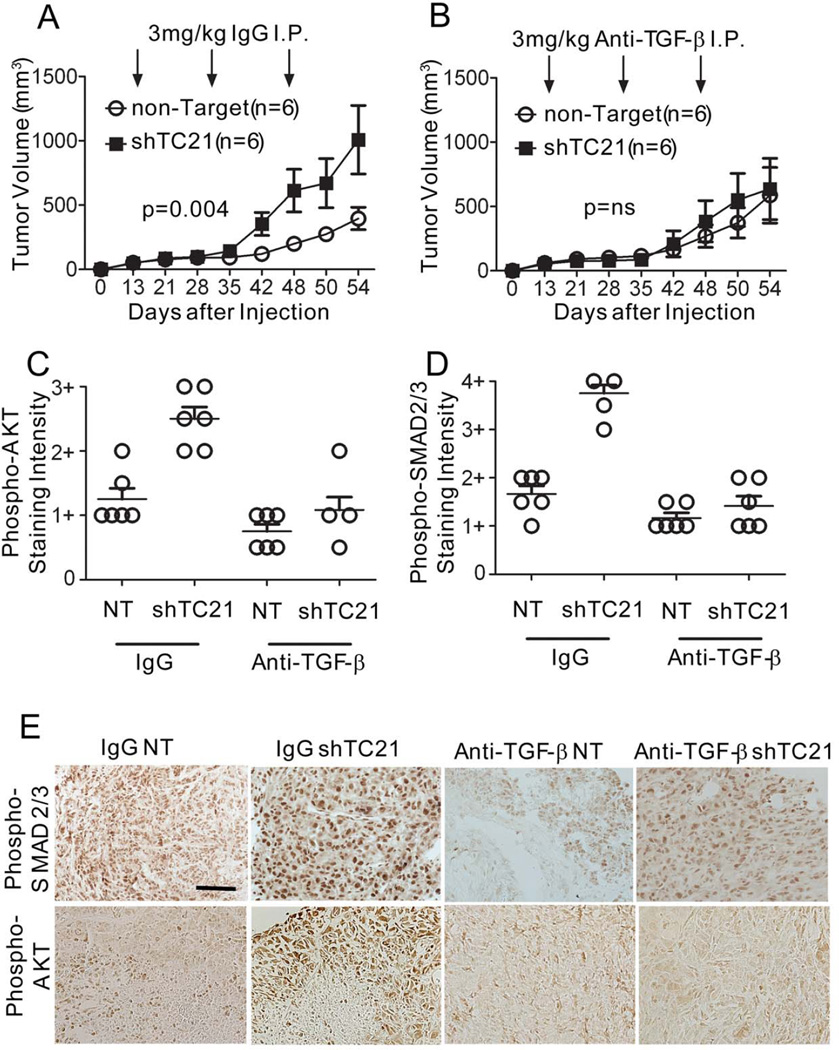
To investigate whether TGFβ plays a causal role in malignant tumor growth when TC21 is lost, we treated mice harboring MPNST xenografts with function-blocking anti- TGFβ1,2,3 in vivo (35). After tumors grew to ~250 mm3, rabbit IgG or anti-TGFβ antibody was administered by I.P. injection. Tumors expressing shTC21 were larger than those with NT (Figure 6A). Strikingly, mice injected with the anti-TGFβ antibody had tumors of similar size whether the cells were expressing shTC21 or control (Figure 6B). Western analysis of tumor xenografts expressing shTC21 confirmed sustained reduction of TC21 (not shown). Thus, loss of TC21 in NF1−/− MPNST cells increases tumor growth and requires TGFβ.
Figure 6. TGFβ mediates aggressive growth of MPNST xenografts.
Volume quantification of xenograft tumors over time. Mice injected (A) IgG or (B) anti-TGFβ antibody. Quantification of staining intensity (C) phospho-SMAD2/3 and (D) phospho-AKT in xenograft tumors. Grading defined in Figure 4 legend. (E) Immunohistochemistry staining of xenograft tumor sections. Scale bar: 25µm.
To delineate signaling pathways modulated by the TGFβ antibody, we examined expression of phospho-SMAD in tumor sections. Phospho-SMAD was reduced in tumor cells and stroma from mice treated with the anti-TGFβ antibody compared with those treated with IgG (Figures 6D, 6E). We hypothesized that, as in neurofibromas, in the malignant setting with increased TGFβ expression we might detect increased phospho- AKT. Indeed, phospho-AKT was elevated in tumor cells expressing shTC21. Phospho-AKT levels were subsequently reduced upon treatment with anti-TGFβ antibody (Figures 6C, 6E).
The shTC21 tumors treated with anti-TGFβ also had significantly fewer meca-32+ vessels than controls (Supplemental Figure 5B). Together this data suggests that in the NF1−/− setting, in transformed cells, loss of TC21 drives tumorigenesis in a TGFβ dependent manner; TGFβ produced by MPNST cells acts in a non-autonomous fashion on stromal cells to increase blood vessels and promotes myofibroblasts formation.
MPNST cells express TGFβ and lose TGFβRII
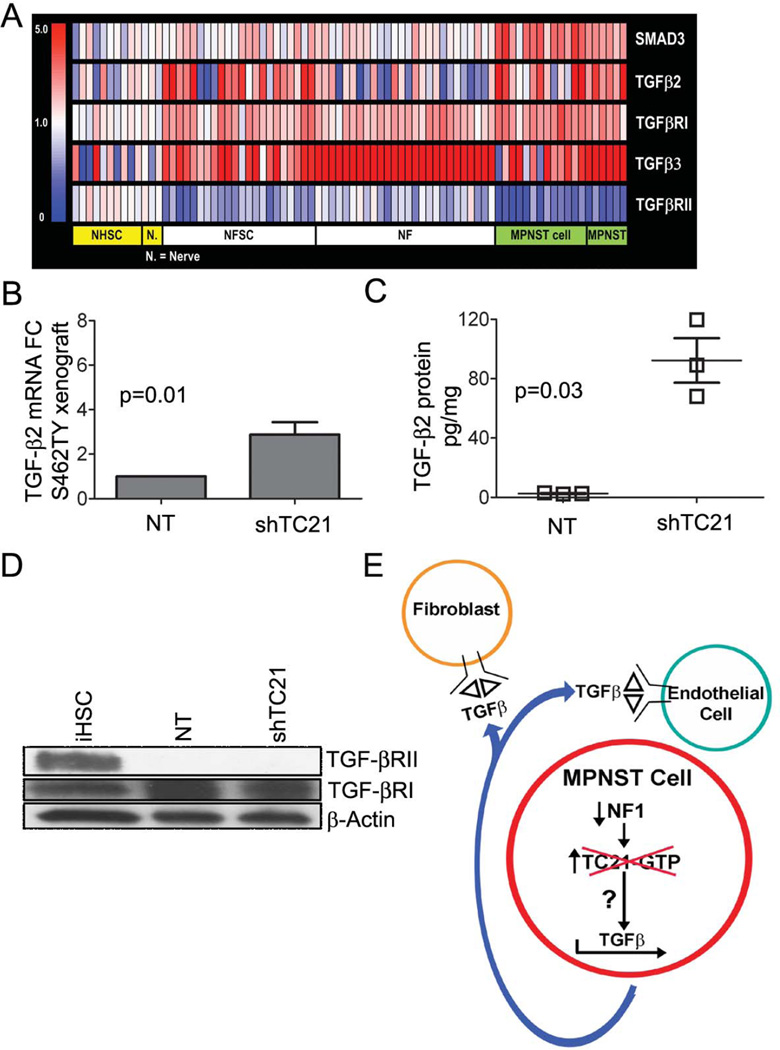
We tested if TGFβ ligands or TGFβRs are altered in neurofibromas or MPNST. We measured relative abundance of TGFβ ligands and receptor mRNAs in a panel of human neurofibromas and MPNSTs compared to cultured neurofibroma Schwann cells, MPNST cell lines, normal human Schwann cells and normal human nerves by Affymetrix gene expression analysis. The heat map (Figure 7A) shows up-regulation of TGFβ3 in most neurofibromas and MPNST, with TGFβ2 up-regulation specifically in 50% of neurofibroma Schwann cell samples, 50% MPNST cell lines and all human MPNST. TGFβRII was down regulated in all human MPNST, and in a subset (22/48) of neurofibromas and in neurofibroma Schwann cell samples (Figure 7A). TGFβRI mRNA was slightly but not significantly up-regulated. These data are consistent with findings that TGFβ ligands increase and receptors decrease in many tumor types during progression to malignancy (16).
Figure 7. MPNST cells express TGFβ and lose TGFβRII.
(A) Heat map of TGFβ ligands and receptor that differ in human neurofibromas (NF (n=22)), MPNSTs (n=6), cultured human primary neurofibroma Schwann cells (NFSC (n=22)) and MPNST cell lines (MPNST cell (n=13)), compared to normal human Schwann cells (NHSC (n=10)) and normal human peripheral nerves (Nerve (n=3)). Fold change=1.5 and t-test p-value < 0.05 with FDR (Benjamini-Hochberg) correction. Scale bar: relative gene expression as colors. (B) qRT-PCR, TGFβ2 mRNA expression. (C) ELISA for TGFβ2 protein concentration. (D) Western blot of iHSC and S462TY cells. (E) Model showing effects of TC21 on TGFβ in MPNST cells.
To confirm increased expression of TGFβ in MPNST and test if TGFβ expression is regulated by TC21 in sarcoma cells (as in SCPs), we examined TGFβ mRNA and protein expressions in NF1 human MPNST cells and in sarcomas from NPCis mice. In benign neurofibromas and SCPs loss of TC21 reduced TGFβ1 mRNA levels in the Nf1−/− setting. In Nf1 mutant mouse NPCis tumors TGFβ1 was elevated on loss of TC21, but TGFβ2 mRNA was not (Supplemental Figure 5C, 5D). In NF1−/− human MPNST shTC21 xenotransplants, TGFβ2 mRNA levels were significantly elevated in tumors (Figure 7B) while TGFβ1 and TGFβ3 levels were unchanged (Supplemental Figure 5E, 5F). Thus in mouse and human, TGFβ levels are regulated by TC21, but the two species use different TGFβ ligands.
We examined the concentration of TGFβ2 protein in the media of shTC21 or control S462TY cells. Knockdown of TC21 in the NF1−/− MPNST cells caused an 80-fold increase in secreted TGFβ2 (Figure 7C). The pathway leading to up-regulation of TGFβ mRNA remains uncertain. Blockade of the MEK, AKT, p38 and ROCK kinases failed to alter TGFβ mRNA levels in MPNST cells (not shown). TP53 mutation status may be important, as no up-regulation of TGFβ2 mRNA was identified in the single existing NF1 MPNST cell line known to have WT p53 (Supplemental Figure 5G).
TGFβRs are frequently mutated or lost in malignancy (15). If TGFβRs show loss of function in NF1, then TGFβ effects would be unlikely to be cell autonomous. TGFβRII protein was undetectable by western blotting in S462TY cells compared to an immortalized human Schwann cell line (Figure 7D). In 2 of 3 additional human MPNST cell lines, TGFβRII protein was also reduced (Supplemental Figure 5H). The absence of TGFβRII in S462TY cells predicted that the cells would not respond to TGFβ. Indeed, no effects of TGFβ on MPNST survival or migration were detected (not shown). To verify this finding, we examined expression of the TGFβ target genes, p21, p15 and c-myc (19). No significant change in mRNA expression of these genes was detected after addition of TGFβ to S462TY cells in serum-free medium at 30 minutes or 8 hours, in comparison to untreated cells (not shown).
Thus, when TC21 is lost in NF1−/− MPNST cells, TGFβ mRNA and secreted protein are increased (Figure 7E). Because MPNST cells lack significant amounts of TGFβRII, TGFβ affects surrounding TGFβRII expressing endothelial and stromal fibroblasts, resulting in increased blood vessels, conversion of fibroblasts to myofibroblasts and increased tumor growth.
Discussion
Using Nf1 models predicted to activate Ras proteins expressed in neurofibromin-dependent cells enabled the demonstration that the Ras-related protein TC21 critically regulates TGFβ in neurofibroma and MPNST. Loss of TC21 in the setting of Nf1 deficiency delayed formation of benign neurofibromas, accelerated formation of aggressive brain tumors and nerve sarcomas, and uniquely regulated expression of TGFβ. Our identification of a specific role for TC21 is consistent with recent studies showing differential localization of other Ras proteins (4), and different embryonic survival following knockout of the K-, H- and N-Ras genes (5).
Our in vivo studies demonstrated that TC21 functions as an oncogene in benign tumors and revealed a new role for TC21 as a tumor suppressor in nervous system malignancy. However, TC21 was identified as up-regulated in some cancers (36, 37) and activated in lymphoma in vivo (8). Therefore effects of TC21 activation are likely to be cell type dependent. Mechanistically, we showed that PI3K/AKT is a critical effector of TC21. In all NF1 models tested, TC21 regulated TGFβ, and TGFβ acted as an oncogene.
Loss of TC21 in GEM neurofibroma extended mouse survival without affecting neurofibroma number or size, suggesting effects on early stages of tumor formation. Consistent with a critical role for TC21 early in neurofibroma formation, we identified an autocrine survival loop specific to Nf1−/− SCPs, and dependent upon TGFβ, TGFβR, and the PI3K/AKT pathway. We have not excluded additional effects on more mature Schwann cells. TGFβ1 can kill mature WT Schwann cells as well as more immature cells (38, 39), and as in Nf1−/− SCPs, adult Nf1−/− nerves contained increased levels of TGFβ1 mRNA that depended on TC21. We failed to detect elevated levels of TGFβ1 mRNA in neurofibromas, but levels of TGFβ1 mRNA in Schwann cells might have been obscured by expression in neurofibroma mast cells or other cells in the tumor microenvironment (40).
Additional evidence supporting a role for TC21 early in neurofibroma formation comes from clonality assays. Numbers of Nf1−/− primary sphere-forming cells, after acute inactivation at E12.5 using the DhhCre allele, were reduced by loss of TC21. Nf1−/− primary sphere-forming cells were dependent on TGFβ they produced for their own survival. Secreted TGFβ is predicted to enhance numbers of developing Nf1−/− stem/progenitor cells and thus tumorigenic potential, leading to neurofibroma formation. Consistent with this idea, targeted loss of the TGFβRII in Schwann cells suppressed early Schwann cell death and proliferation (41). We conclude that TC21 controls TGFβ in vivo, reducing the growth and number of developing Schwann cells, and thereby acting as a brake on neurofibroma initiation/growth.
Precursor cell effects on neurofibroma initiation and/or growth through TC21 and TGFβ were not sufficient to prevent tumorigenesis. Other Ras proteins and/or Nf1 functions are likely necessary for neurofibroma growth. The idea that Nf1 regulates other Ras proteins— even in peripheral nerve cells— is supported by experiments in which farnesyl transferase inhibitors predominantly blocking H-Ras, blocks Nf1 mutant Schwann cell proliferation (42), and a report that N-Ras plays a major role in MPNST cells (43). Our finding that loss of TC21 delayed Nf1−/− embryonic lethality --but did not rescue embryos to birth-- is also consistent with roles of multiple Ras proteins in development. How each Ras protein contributes to embryonic development and tumorigenesis downstream of NF1 loss of function remains to be determined. In addition, other Ras proteins may regulate TGFβ expression or signaling in non-NF1 tumor settings. The diverse regulation of TGFβ pathways in specific cell types may result in part because specific Ras proteins are cell type specific (44).
We found that loss of TC21 enhances tumorigenesis in malignancy. In the NPCis mouse model, loss of TC21 dramatically accelerated formation of brain tumors. In a xenograft model, NF1−/− sarcoma cells showed accelerated tumor growth and increased levels of TGFβ when they expressed shTC21. Furthermore, inhibiting TGFβ blocked the elevated tumor growth caused by loss of TC21. The action of TGFβ as a growth promoter in malignancy correlated with increased TGFβ ligands in neurofibroma and MPNST and decreased expression of TGFβRII in human MPNST cell lines and tumors, as shown by gene expression and confirmed by protein analysis in cell lines. Genomic mutation or loss of one or more TGFβRs is common in many tumor types (15, 45). Despite absence of TGFβRII in S462TY cells, blocking TGFβ led to decreased expression of phospho-SMAD. This may result from use of a mutant p53 SMAD complex (18) and/or more complex activation of SMAD downstream of activin receptors. One possibility is that stromal cells secrete activins or BMPs that indirectly alter SMAD phosphorylation in tumor cells (16).
Our data are consistent with important non-cell autonomous effects of MPNST cell produced TGFβ. This interpretation is consistent with increased blood vessels per area. SMA (myo-fibroblast) expression also increased upon down-regulation of TC21, and both phenotypes were blocked by anti-TGFβ antibody. A non-cell autonomous effect of TGFβ in malignancy has been documented previously in models of carcinoma, using genetic loss of TGFβRII (19).
In summary, TC21 has a dual effect: in developing Nf1 progenitor cells this Ras-related protein promotes cell survival by promoting TGFβ production and formation of an autocrine survival loop, so that loss of TC21 results in decreased precursors and delayed tumor formation. TC21 activation plays a role in SCP viability and growth rather than in neurofibroma growth per se. In MPNST cells loss of TC21 dramatically increases TGFβ production, increasing vascularization and tumor growth. Our results linking TC21 to regulation of TGFβ production are likely to be relevant to cancer in general, as NF1 mutations are increasingly identified in sporadic cancers (46–48).
Supplementary Material
Acknowledgements
We thank E. Ruoslahti (Sanford-Burnham Medical Research Institute) for R-Ras and TC21 mice, Luis Parada (UT Southwestern) for Nf1fl/fl mice, D. Meijer (ErasmusMC, Netherlands) for DhhCre mice, and M. Wallace (U. Florida) for immortalized human Schwann cells. We thank N. Nassar (CCHMC) for manuscript review, D. Largaespada (U Minnesota), S. Kozma and G. Thomas (U Cincinnati) for extensive discussion and comments on the manuscript.
This work was supported by NIH P50NS057531 to NR.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Mitin N, Rossman KL, Der CJ. Signaling Interplay in Ras Superfamily Function. Curr Biol. 2005;15:R563–R574. doi: 10.1016/j.cub.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Castellano E, Downward J. Role of RAS in the regulation of PI 3-kinase. Curr Top Microbiol Immunol. 2010;346:143–169. doi: 10.1007/82_2010_56. [DOI] [PubMed] [Google Scholar]
- 3.Karnoub A, Weinberg R. Ras oncogenes: split personalities. Nat Rev Mol Cell Biol. 2008;9:517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinlan M, Quatela S, Philips M, Settleman J. Activated Kras, but Not Hras or Nras, May Initiate Tumors of Endodermal Origin via Stem Cell Expansion. Mol Cell Biol. 2008;28:2659–2674. doi: 10.1128/MCB.01661-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez-Medarde A, Santos E. Ras in Cancer and Developmental Diseases. Genes Cancer. 2011;2:344–358. doi: 10.1177/1947601911411084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham S, Oldham S, Martin C, Drugan J, Zohn I, Campbell S, et al. TC21 and Ras share indistinguishable transforming and differentiating activities. Oncogene. 1999;18:2107–2116. doi: 10.1038/sj.onc.1202517. [DOI] [PubMed] [Google Scholar]
- 7.Chan A, Miki T, Meyers K, Aaronson S. A human oncogene of the RAS superfamily unmasked by expression cDNA cloning. Proc Natl Acad Sci USA. 1994;91:7558–7562. doi: 10.1073/pnas.91.16.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim R, Trubetskoy A, Suzuki T, Jenkins NA, Copeland NG, Lenz J. Genome-Based Identification of Cancer Genes by Proviral Tagging in Mouse Retrovirus-Induced T-Cell Lymphomas. J Virol. 2003;77:2056–2062. doi: 10.1128/JVI.77.3.2056-2062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohba Y, Mochizuki N, Yamashita S, Chan A, Schrader J, Hattori S, et al. Regulatory Proteins of R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3. J Biol Chem. 2000;275:20020–20026. doi: 10.1074/jbc.M000981200. [DOI] [PubMed] [Google Scholar]
- 10.Boyd K, Korf B, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1–14. doi: 10.1016/j.jaad.2008.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans D, Baser M, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis type 1. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y, Rangwala F, Fulkerson PC, Ling B, Reed E, Cox AD, et al. Role of TC21/R-Ras2 in enhanced migration of neurofibromin-deficient Schwann cells. Oncogene. 2004;23:368–378. doi: 10.1038/sj.onc.1207075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdogan M, Pozzi A, Bhowmick N, Moses HL, Zent R. Signaling Pathways Regulating TC21-induced Tumorigenesis. J Biol Chem. 2007;282:27713–27720. doi: 10.1074/jbc.M703037200. [DOI] [PubMed] [Google Scholar]
- 14.Delgado P, Cubelos B, Calleja E, Martínez-Martín N, Ciprés A, Mérida I, Bellas C, et al. Essential function for the GTPase TC21 in homeostatic antigen receptor signaling. Nat Immunol. 2009;10:880–888. doi: 10.1038/ni.1749. [DOI] [PubMed] [Google Scholar]
- 15.Massagué J. TGFβ in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Pang Y, Moses HL. TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahimi R, Leof E. TGF-beta signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- 18.Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, et al. A Mutant-p53/Smad Complex Opposes p63 to Empower TGFβ-Induced Metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 19.Lu S, Herrington H, Reh D, Weber S, Bornstein S, Wang D, et al. Loss of transforming growth factor-beta type II receptor promotes metastatic head-and-neck squamous cell carcinoma. Genes Dev. 2006;20:1331–1342. doi: 10.1101/gad.1413306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFb/Smad signaling by oncogenic Ras. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M, Pardoux C, Hall M, Lee P, Warburton D, Qing J, et al. TGF-b activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO. 2007;26:3957–3967. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grusch M, Petz M, Metzner T, Ozturk D, Schneller D, Mikulits W. The Crosstalk of RAS with the TGF-β Family During Carcinoma Progression and its implications for Targeted Cancer Therapy. Curr Cancer Drug Targets. 2010;10:849–857. doi: 10.2174/156800910793357943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geiser A, Kim S, Roberts A, Sporn M. Characterization of the mouse transforming growth factor-beta 1 promoter and activation by the Ha-ras oncogene. Mol Cell Biol. 1991;11:84–92. doi: 10.1128/mcb.11.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue J, Mulder K. Requirement of Ras/MAPK Pathway Activation by Transforming Growth Factor beta for Transforming Growth Factor beta 1 Production in a Smad-dependent Pathway. J Biol Chem. 2000;275:30765–30773. doi: 10.1074/jbc.M000039200. [DOI] [PubMed] [Google Scholar]
- 25.Cordenonsi M, Montagner M, Adorno M, Zacchigna L, Martello G, Mamidi A, et al. Integration of TGF-beta and Ras/MAPK signaling through p53 phosphorylation. Science. 2007;315:840–843. doi: 10.1126/science.1135961. [DOI] [PubMed] [Google Scholar]
- 26.Wu J, Williams J, Rizvi T, Kordich J, Witte D, Meijer D, et al. Plexiform and Dermal Neurofibromas and Pigmentation Are Caused by Nf1 Loss in Desert Hedgehog-Expressing Cells. Cancer Cell. 2008;13:105–116. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brannan C, Perkins A, Vogel K, Ratner N, Nordlund M, Reid S, et al. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8:1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- 28.Reilly K, Loisel D, Bronson R, McLaughlin M, Jacks T. NF1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat Genet. 2000;26:109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- 29.Williams J, Wu J, Johansson G, Rizvi T, Miller S, Geiger H, et al. Nf1 Mutation Expands an EGFR-Dependent Peripheral Nerve Progenitor that Confers Neurofibroma Tumorigenic Potential. Cell Stem Cell. 2008;3:658–669. doi: 10.1016/j.stem.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghadimi M, Young E, Belousov R, Zhang Y, Lopez G, Lusby K, et al. Survivin is a viable target for the treatment of malignant peripheral nerve sheath tumors. Clinical Cancer Res. 2012;18:2545–2557. doi: 10.1158/1078-0432.CCR-11-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frahm S, Mautner V, Brems H, Legius E, Debiec-Rychter M, Friedrich R, et al. Genetic and phenotypic characterization of tumor cells derived from malignant peripheral nerve sheath tumors of neurofibromatosis type 1 patients. Neurobiol Dis. 2004;16:85–91. doi: 10.1016/j.nbd.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 32.Miller SJ, Rangwala F, Williams JP, Ackerman P, Kong S, Jegga A, et al. Large-Scale Molecular Comparison of Human Schwann Cells to Malignant Peripheral Nerve Sheath Tumor Cell Lines and Tissues. Cancer Res. 2006;66:2584–2591. doi: 10.1158/0008-5472.CAN-05-3330. [DOI] [PubMed] [Google Scholar]
- 33.Miller SJ, Jessen WJ, Mehta T, Hardiman A, Sites E, Kaiser S, Jegga AG, et al. Integrative genomic analyses of neurofibromatosis tumours identify SOX9 as a biomarker and survival gene. EMBO Mol Med. 2009;1:236–248. doi: 10.1002/emmm.200900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stemmer-Rachamimov A, Louis D, Nielsen G, Antonescu C, Borowsky A, Bronson R, et al. Comparative Pathology of Nerve Sheath Tumors in Mouse Models and Humans. Cancer Res. 2004;64:3718–3724. doi: 10.1158/0008-5472.CAN-03-4079. [DOI] [PubMed] [Google Scholar]
- 35.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, et al. Losartan, an AT1 Antagonist, Prevents Aortic Aneurysm in a Mouse Model of Marfan Syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arora S, Matta A, Shukla N, Deo S, Ralhan R. Identification of differentially expressed genes in oral squamous cell carcinoma. Mol Carcinog. 2005;42:97–108. doi: 10.1002/mc.20048. [DOI] [PubMed] [Google Scholar]
- 37.Sharma R, Sud N, Chattopadhyay T, Ralhan R. TC21/R-Ras2 upregulation in esophageal tumorigenesis: potential diagnostic implications. Oncology. 2005;69:10–18. doi: 10.1159/000087283. [DOI] [PubMed] [Google Scholar]
- 38.Hagedorn L, Suter U, Sommer L. P0 and PMP22 mark a multipotent neural crest-derived cell type that displays community effects in response to TGF-beta family factors. Development. 1999;126:3781–3794. doi: 10.1242/dev.126.17.3781. [DOI] [PubMed] [Google Scholar]
- 39.Parkinson D, Dong Z, Bunting H, Whitfield J, Meier C, Marie H, et al. Transforming Growth Factor b (TGFb) Mediates Schwann Cell Death in vitro and in vivo: Examination of c-Jun Activation, Interactions with Survival Signals, and the Relationship of TGFb-mediated death to Schwann cell differentiation. J Neurosci. 2001;21:8572–8585. doi: 10.1523/JNEUROSCI.21-21-08572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang FC, Chen S, Clegg T, Li X, Morgan T, Estwick SA, et al. Nf1+/− mast cells induce neurofibroma like phenotypes through secreted TGF- signaling. Hum Mol Genet. 2006;15:2421–2437. doi: 10.1093/hmg/ddl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Antonio M, Droggiti A, Feltri ML, Roes J, Wrabetz L, Mirsky R, et al. TGFbeta Type II Receptor Signaling Controls Schwann Cell Death and Proliferation in Developing Nerves. J Neurosci. 2006;26:8417–8427. doi: 10.1523/JNEUROSCI.1578-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H, DeClue J, Ratner N. cAMP-dependent protein kinase A is required for Schwann cell growth: interactions between the cAMP and neuregulin/tyrosine kinase pathways. J Neurosci Res. 1997;49:236–247. [PubMed] [Google Scholar]
- 43.Kraniak J, Sun D, Mattingly R, Reiners JJ, Tainsky M. The role of neurofibromin in N-Ras mediated AP-1 regulation in malignant peripheral nerve sheath tumors. Mol Cell Biochem. 2010;344:267–276. doi: 10.1007/s11010-010-0551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rahimi R, Leof E. TGFbeta versatility: PI3K as a critical mediator of distinct cell type and context specific responses. Cell Cycle. 2009;8:1813–1817. doi: 10.4161/cc.8.12.8868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akhurst R, Derynck R. TGF-beta signaling in cancer-a double-edged sword. Trends Cell Biol. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 46.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Network CGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hölzel M, Huang S, Koster J, Øra I, Lakeman A, Caron H, et al. NF1 Is a Tumor Suppressor in Neuroblastoma that Determines Retinoic Acid Response and Disease Outcome. Cell. 2010;142:218–229. doi: 10.1016/j.cell.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.