Abstract
The oral route is the most convenient and least expensive route of drug administration. Yet, it is accompanied by many physiological barriers to drug uptake including low stomach pH, intestinal enzymes and transporters, mucosal barriers, and high intestinal fluid shear. While many drug delivery systems have been developed for oral drug administration, the physiological components of the gastro intestinal tract remain formidable barriers to drug uptake. Recently, microfabrication techniques have been applied to create micron-scale devices for oral drug delivery with a high degree of control over microdevice size, shape, chemical composition, drug release profile, and targeting ability. With precise control over device properties, microdevices can be fabricated with characteristics that provide increased adhesion for prolonged drug exposure, unidirectional release which serves to avoid luminal drug loss and enhance drug permeation, and protection of a drug payload from the harsh environment of the intestinal tract. Here we review the recent developments in microdevice technology and discuss the potential of these devices to overcome unsolved challenges in oral drug delivery.
Keywords: Bio-MEMS, Microdevices, Microfabrication, Micropatches, Nanofabrication, Oral drug delivery
Introduction
Among the various routes of drug administration, including intravenous, intraperitoneal, intramuscular, and transdermal administration, oral administration is most preferred for its numerous advantages. Oral drug formulations are self-administrable and less invasive, leading to higher patient compliance and decreased cost of care. While oral drug administration is ideal in terms of cost and convenience, the oral bioavailability of many drugs is limited by a unique set of barriers to oral drug uptake, including low pH of the stomach, intestinal enzymes, transporter proteins expressed in intestinal epithelial cells, and the motile mucosal lining of the gastrointestinal (GI) tract. Many small molecule drugs suffer from poor oral bioavailability as a result of factors such as low permeation through the thick and hydrophobic mucosal layer and cell membrane, low drug solubility, and metabolic and transporter protein activity [1–2]. In addition, many recently developed biotherapeutics, including peptides, proteins, DNA, RNA, and macromolecules, have particularly low oral bioavailabilities [3]. This is due to their relatively large size and high complexity, causing decreased permeability and a tendency to denature under harsh conditions [4].
While there are many barriers to oral drug uptake, these barriers can be categorized into issues of 1) low drug permeation, 2) drug degradation, and 3) low drug solubility [5]. Current approaches to overcoming these barriers include enteric coating, permeation enhancers, drug modification, metabolic and transporter protein inhibitors, and micro-/nanoparticulate systems, which have been reviewed in detail before [6–12]. While these systems have made advances in improving oral bioavailability of select therapeutics, no reliable method for the oral delivery of biomolecules has been developed, and no single system has been shown to comprehensively address the three categories of barriers to drug uptake previously mentioned. Thus, more advanced approaches are required to provide sufficient systemic uptake of orally administered drugs ranging from small molecules to macromolecular structures.
In the past decade, micro-electro-mechanical systems (MEMS) technology originally developed by the semiconductor industry has been applied to many biomedical applications. Microfabrication techniques have facilitated the development of novel devices with precise control over device shape and size, allowing for the creation of devices specifically designed to address issues affecting drug uptake. Specifically, microfabricated devices for oral drug delivery, termed microdevices, are designed to maximize residence time in the GI tract, provide unidirectional drug release toward the intestinal epithelium, and release drug in a sustained manner. Together, these characteristics allow microdevices to simultaneously address issues with drug permeation, solubility, and degradation in a manner not provided by other drug delivery systems. This mini-review will highlight the unique mechanisms by which microdevices address the barriers to oral drug delivery, describe recent innovations in the design and fabrication of these devices, and review the efficacy of these devices in vitro and in vivo.
Barriers to oral drug delivery
The comprehensive set of barriers to oral drug uptake must be considered when examining the rationale behind microdevice design. Orally administered drugs face a sequential set of barriers to systemic drug uptake as outlined in Figure 1A. Following oral administration, drugs encounter pH values ranging from 1.5 to 3.5 and digestive enzymes in the stomach and are subsequently exposed to pH values of 5 to 7 and additional proteolytic and metabolic enzymes in the small intestine [13]. After entering the small intestine, the primary site of drug and nutrient uptake, drugs must then pass through a hydrophobic mucous membrane composed of a motile layer moving in contact with an underlying firmly adherent layer [14]. The motile mucus layer ranges between 100 and 500 µm in thickness, and the adherent mucus layer ranges from 0 to 20 µm in thickness [15–16]. After penetrating the mucus layer, drugs must pass through the glycocalyx, an extracellular matrix approximately 0.5 to 1 µm thick composed of negatively charged glycoproteins, proteoglycans, glycosaminoglycans, and glycolipids [17–18]. Drugs must then pass through the polarized enterocyte monolayer by either paracytosis directly through enterocytes or transcytosis through junctions between enterocytes. Paracytosis involves permeation through the apical cell membrane into enterocytes and subsequent permeation through the basal cell membrane into the interstitium. Paracytosis occurs through both passive diffusion through the cell membrane and facilitated diffusion involving transporter proteins or endocytosis [19–21]. Within the cytosol of enterocytes, drugs are exposed to influx and efflux protein transporters differentially expressed on the apical and basal cell membranes as well as metabolic enzymes [1]. The alternate pathway of transcytosis involves travel between cells through tight junctions, structures between closely associated cells composed of multiprotein complexes with pores approximately 1 to 3 nm in diameter [22–23]. This small pore size presents a significant obstacle to drug uptake, particularly for high molecular weight therapeutics. These barriers present a unique set of challenges not encountered in other routes of drug administration.
Figure 1.
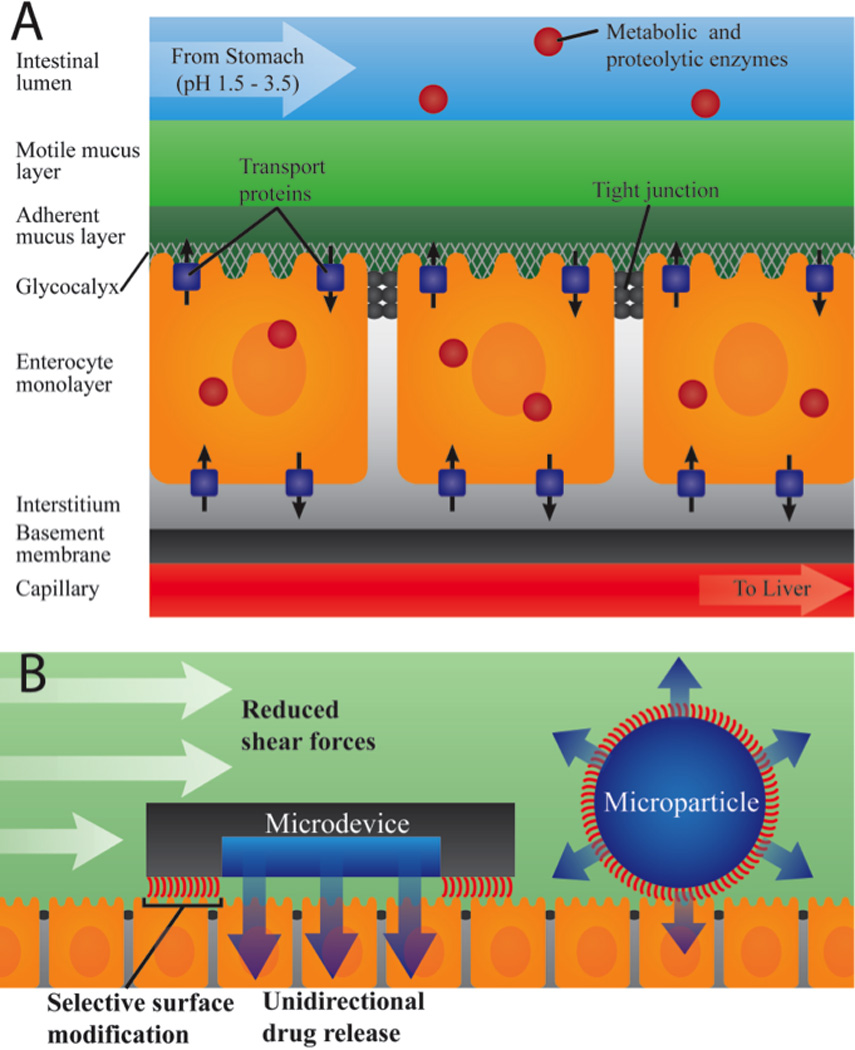
A. Physiological barriers to oral drug delivery. After encountering digestive enzymes and the low pH of the stomach, drugs enter the small intestine, the primary site of drug uptake where drugs encounter additional metabolic and proteolytic enzymes. Drugs must then pass through the motile and adherent mucus layers, the cellular monolayer through either a paracellular or transcellular route and finally pass through the interstitium and basement membrane to enter the capillary from which they are shuttled to the liver before entering systemic circulation. B. Advantages of asymmetric microdevice design for oral drug delivery include 1) reduced shear force per mass, increasing residence time, 2) unidirectional drug release toward endothelial tissue, increasing drug permeation, and 3) sustained release, reducing drug exposure to the harsh conditions of the GI tract and decreasing drug degradation.
Rationale for designing oral drug delivery microdevices
Like most previously developed oral drug delivery microparticulate systems made via precipitation methods, microdevices are designed on a scale small enough to fit within the features of the intestinal wall, which is made up of micron-sized folds and pits of the intestinal villi [24]. Also, microdevices are designed to be large enough to prevent device uptake into cells through endocytosis. While microdevices are similar in size to many oral drug delivery particulate systems, conventional methods of microfabrication deliver precise and consistent dimensions of microdevices, resulting in much higher monodispersity in size and shape [25]. In addition to providing monodispersity, microfabrication also provides the ability to create devices with custom shapes and dimensions. This abililty has been utilized to design devices with a planar design that simultaneously address drug permeability barriers, drug degradation, and low drug solubility (Figure 1B).
To address issues with poor drug solubility and increase overall drug exposure, microdevices are designed to be relatively flat, providing multiple advantages for drug transfer. A flat shape increases the surface area in contact with the GI wall, improving adhesive properties of the device [26]. In addition, a flat microdevice shape decreases the shear force per mass on the devices as shown in Figure 1B, preventing detachment of the device from the intestinal epithelium and further increasing residence time [27]. To overcome a second major barrier to oral drug uptake - issues with drug permeability, microdevices are designed with reservoirs on only one side of the device, allowing drug to be released in only one direction. In addition to asymmetric shape, devices can be asymmetrically modified with targeting moieties, mucoadhesive materials, and micro- and nanotopography, providing selective binding of the device side from which drug will be selectively released [26]. Thus, microdevices are designed to adhere to the mucosal or GI epithelial layer and release drug to enterocytes in a proximal, unidirectional manner as shown in Figure 1B. Releasing drug directly toward the epithelial barrier rather than into the lumen provides a more efficient mechanism of drug release by decreasing the loss of drug downstream through the lumen and also increasing the exposure of the enterocytes to the drug. Furthermore, the unidirectional release of drug in a localized, high concentration at the device-intestinal wall interface creates a strong concentration gradient, thereby enhancing drug permeation across the intestinal enterocytes [27]. This localized release of drug in high concentrations may also increase drug uptake through a second mechanism, as high drug concentrations may saturate metabolic enzymes and protein transporters, in turn increasing the bioavailability of the drug [28].
Numerous treatments have been studied for the disruption of drug transporters, metabolic enzymes, and endothelial tight junctions to allow for increased passage of drug [10–12]. However, each of these mechanisms is accompanied by severe clinical side effects [1, 23, 29]. If the total surface area of administered microdevices is significantly smaller than the surface area of the GI tract, microdevices have the potential to deliver drug to select regions of intestinal tissue. While drugs delivered in standard tablet form will diffuse to reach enterocytes with a relatively even distribution within a region of the GI tract, larger microdevices with high residence times have potential to only deliver drug to a small subset of intestinal epithelial cells. This could be advantageous in combination therapy for increased drug bioavailability. Given their potential to target a small subset of cells with high drug concentrations, microdevices could be designed to release permeation enhancers in localized regions in which the drug of interest is also released while leaving the majority of the GI tract unaffected. Finally, to address the third major barrier to oral drug uptake - drug degradation, microdevices are fabricated to include drug reservoirs that allow for sustained release of drug, thereby decreasing the exposure of drugs to harsh conditions of the GI relative to a bolus dose [26]. With a variety of sustained drug release systems developed in the recent past for oral delivery including pH-sensitive hydrogels [30–32], enteric coating [33], and degradable polymers, well established microfabrication techniques can be effectively used to incorporate microdevice reservoirs with these drug systems [27, 34–36].
In addition to delivery of drugs for systemic uptake, oral microdevices have the potential to treat diseases local to the GI tract including Crohn’s disease, inflammatory bowel disease (IBD), and irritable bowel syndrome (IBS). While the site of action of most therapeutics for GI disorders is in intestinal tissue, many of these drugs in conventional large doses lead to severe systemic side effects [37–38]. For more efficient treatment of these diseases, microdevices could be modified to adhere to only the diseased GI tissue for localized delivery of drugs directly to the therapeutic target [39]. Direct targeting of sites of inflammation could improve drug efficacy while reducing severity of side effects associated with therapies for diseases of the GI tract.
Materials utilized for microdevice structure
To prevent toxicity and inflammation, microdevices must be made from biocompatible material. The first systems of oral microdevices used standard semiconductor materials, including silicon oxide and porous silicon as the device material [40–41]. While silicon and silicon oxide have been found to be relatively non-toxic in some studies [42–44], they have also been associated with inflammation [45–46]. In order to overcome this issue, microdevice fabrication shifted towards the use of relatively non-toxic polymers, including hydrogels and biodegradable materials. Poly(methyl methacrylate) (PMMA), an FDA-approved polymeric material [47] used in contact lenses and bone cement and also known to be stable at low pH values [26], has been utilized in numerous oral microdevice designs [34–35, 48–51]. Microdevices have also been fabricated from SU-8, an epoxy-based negative photoresist originally developed as an ultra-thick photoresist [36, 52]. While SU-8 is not currently FDA approved, studies have shown that SU-8 is non-toxic as an implantable material [53–55]. Other biocompatible polymers utilized for microdevice fabrication include chitosan [56], gelatin [57], poly(lactic-co-glycolic) acid (PLGA) [56–58], polypropylene (PP) [59], and poly(ethylene glycol) (PEG) [27, 52, 56]. The intrinsic biocompatibility, biodegradation, hydrophobicity, and structural properties of individual polymers can be tuned by adjusting the chemical structure of the monomer(s) used in polymer synthesis, the molecular weight of the polymer, and/or the crosslinking density [56, 60–62].
While materials utilized in microfabrication are typically chosen for biocompatibility and structural properties, there is a strong interest in utilizing bioresponsive or bioadhesive materials for additional properties to aid in drug delivery. The Peppas Lab has developed pH-sensitive hydrogel systems for selective insulin release in the small intestine [30–32]. These hydrogels retain insulin in low pH environments similar to that of the stomach but swell and release insulin as pH values increase to a simulated intestinal environment with a pH of 6.5 [31]. Similar pH-responsive materials could be incorporated into planar microdevices for selective drug release upon entry into the small intestine. In addition, microdevices could be surface coated with or encapsulated within enteric materials insoluble at low stomach pH values but soluble in the nearly neutral pH of the small intestine to prevent drug release and degradation [33].
Techniques utilized in the micro- and nanofabrication of oral drug delivery devices
A variety of fabrication methods, including photolithography, electron beam lithography, x-ray lithography, and soft lithography techniques are available for the fabrication of microdevices. The use of micro- and nanofabrication techniques for biological applications has been reviewed in detail elsewhere [63–65], but this review will highlight a selection of techniques that are particularly useful for oral microdevice fabrication. Many studies to this date have utilized conventional photolithography techniques originally developed by the microchip industry for the fabrication of microdevices [27, 34, 36, 40, 50–52, 62, 64–67]. Photolithography involves selective UV exposure of a photosensitive material, termed a photoresist, and is often followed by an etching step to transfer the photoresist pattern to a substrate. Typically, the substrate is spin-coated to form a thin film deposition, and a photoresist layer is spin-coated onto the substrate. The photoresist is then exposed to UV light through a mask created with custom patterns by computer-aided design (CAD), transferring the mask pattern through selective polymerization or cleavage of the photoresist in regions exposed to UV light, and non-polymerized or cleaved resist is removed by chemical development. The resist pattern can then be transferred to the substrate through either wet or dry etching processes with the resist acting to selectively protect regions of the substrate. In a straightforward fabrication technique, Tao et al. applied photolithography to fabricate microdevices from SU-8 in a two-step process [36]. A layer of SU-8 was exposed to UV light to form a device base, and then a second layer of SU-8 was UV-exposed to form the walls of drug reservoirs. The use of SU-8 photoresist as the structural component of microdevices eliminates the need for etching of a substrate material following UV exposure. To create microdevices from non-photoreactive materials, photolithography followed by reactive ion etching (RIE), a dry etching technique involving directional destruction of material by bombardment with chemically reactive plasma, has been employed [34]. As shown in Figure 2, Chirra and Desai used two series of steps each composed of photolithography followed by RIE with oxygen plasma to create microdevices with three reservoirs partially etched through the microdevice structure.
Figure 2.
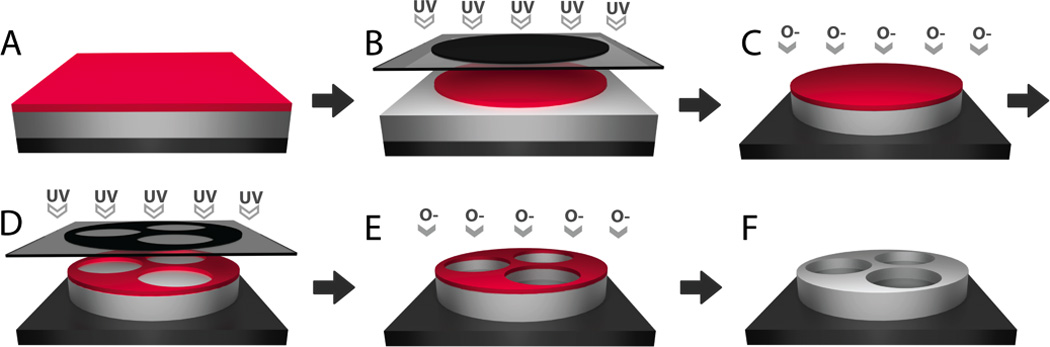
Photolithography-based techniques for microfabrication of multi-reservoir PMMA devices. A. PMMA and, subsequently, photoresist are spin-cast onto a silicon wafer. B. A circular pattern is transferred from a UV-blocking photomask to the photoresist through UV-induced cleavage. C. Reactive ion etching with oxygen plasma directionally destroys PMMA not protected by the photoresist pattern. D. Following photoresist removal and re-coating of a fresh resist layer, a reservoir-containing pattern is transferred to the photoresist by UV-exposure. E. Reactive ion etching is used to partially etch the PMMA layer to form drug reservoirs. F. Photoresist is chemically removed. Adapted with permission from [34].
While photolithography techniques are often expensive and require access to cleanroom facilities, soft lithography allows for replication of a hard patterned substrate to create an inverse pattern with a soft elastomer such as polydimethylsiloxane (PDMS). The patterned elastomer can then be used as a master mold to repeatedly pattern a wide range of materials under standard laboratory settings. These patterned elastomers can be used as either a mold, to create devices from recessed regions, or as a stamp, which can be coated with material to create devices or patterned surface modifications in regions of contact. Guan et al. have demonstrated a variety of soft lithography techniques that can be utilized to fabricate microdevices (Figure 3) [56, 59–60, 68]. In one study, a micropillar PDMS stamp was coated with PPMA before bringing the stamp into contact with a glass slide coated with polyvinyl alcohol (PVA), creating PPMA microdevices in regions of contact (Figure 3A) [59]. In contrast, Guan et al. also used a microwell stamp to collect PPMA within recessed regions before bringing the stamp into contact with PVA-coated glass, creating microdevices from the wells of the microstamp (Figure 3B) [59]. In later studies, a mixture of PEGMA and PEGDMA was applied to a PDMS microwell stamp, allowing for microdevice formation through discontinuous dewetting (Figure 3C) [56]. As a result of the interactions at the interface of the polymer solution and the PDMS, the PEGMA/PEGDMA resin selectively collected in the microwells before UV exposure induced polymerization via a photoinitiator [56, 69]. A number of other studies have utilized similar soft lithography techniques in the fabrication of microdevices [65, 70–74].
Figure 3.
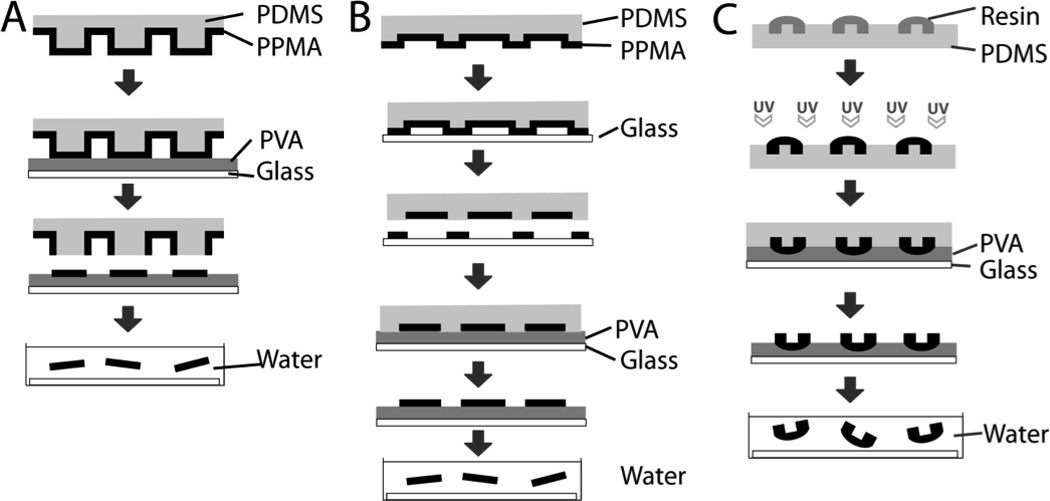
Soft lithography-based techniques for microdevice fabrication. A. Microcontact printing can be utililized for fabrication of microdevices in regions of contact of PVA with micropillar stamp with subsequent dissolution of PVA in water for device release [59]. B. Fabrication of microdevices from recessed regions of microwell stamp. The stamp was brought into contact with glass to remove PPMA from non-microwell regions before bringing the remaining PPMA into contact with PVA [59]. C. Discontinuous dewetting utilized to selectively collect resin before UV-induced polymerization. Microdevices were then brought into contact with PVA with subsequent dissolution in water for device release. [56]. Adapted with permission.
Currently, only a select number of microparticulate drug delivery systems have incorporated nanostructures to enhance oral drug delivery [75–77]. As microdevice design advances, future studies may apply a wide range of nanofabrication techniques to microdevice design as nanotopography has been shown to enhance muco- and cytoadhesion and interact with epithelial cells to enhance drug permeability [77–78]. However, resolutions below 100 nm are difficult to achieve with UV-based photolithography due to the diffraction limit of light [79]. For fabrication of devices with nanoscale features, nanofabrication techniques are required. One such technique, X-ray lithography, uses electromagnetic radiation with wavelengths ranging from 0.5 to 4 nm and is capable of achieving resolutions approaching 20 nm [80–82]. Similar to UV lithography, X-ray lithography uses an X-ray source such as a synchrotron or laser-induced plasma generator to irradiate X-ray-sensitive material through an X-ray absorbing mask [80, 82]. Maskless lithography techniques, including electron beam, ion beam, and dip-pen lithography are also available for nanofabrication. Electron beam lithography directs a beam of electrons to create a pattern on a material sensitive to electron irradiation, which is later developed or etched to form features on the irradiated material or an underlying substrate [83]. Similarly, ion beam lithography utilizes a focused beam of ions to either remove a substrate material or deposit a dissociated precursor material onto the substrate [84]. Dip-pen lithography adapts a scanning atomic force microscopy probe to direct inorganic or biological ink molecules across a substrate where they subsequently adsorb [85]. Because maskless techniques require low throughput de novo pattern creation, they are often used to create a master mold, which is then used to transfer the inverse pattern to other materials through nanoimprinting [86–89]. In addition, templating of polymeric material with nanoporous membranes provides high-throughput fabrication of nanowire arrays [90–91]. In template synthesis, a polymer is exposed to a nanoporous membrane at a temperature greater than the polymer’s glass transition temperature. The polymer is incorporated into the membrane, and the membrane is then selectively dissolved, leaving the polymer with the inverse nanowire array. Membrane selection provides control over the nanowire diameter and spacing, and templating time and temperature are adjusted to control nanowire length.
Structural and chemical modifications to improve microdevice muco- and cytoadhesion
Because microdevices rely on adhesion to increase residence time and prolong drug exposure in the GI tract, many designs to increase device adhesion have been developed, including the utilization of mucoadhesive materials, targeted molecular interactions, gecko-inspired micro/nano topography, and mechanical interactions as shown in Figure 4. Conjugation of lectins to microdevices and other drug delivery systems has proven effective in increasing both adhesion to Caco-2 cell monolayers and intestinal mucosa (Figure 4A) [34–35, 50, 92–93]. Conjugation of tomato lectin to PMMA microdevices resulted in a two- to six-fold increase binding of microdevices to a Caco-2 cell monolayer relative to unmodified microparticles [50]. Furthermore, upon binding to a cell monolayer under physiologically relevant shear stress levels, only a small fraction of lectin-conjugated devices detach, and the majority of the detached devices reattach [94]. However, given the sensitivity of lectins and other peptide targeting molecules to the low pH values and enzymes of the GI tract, incorporation of bioadhesive materials and topographies that are less prone to degradation may provide more stable mechanisms for bioadhesion. Features on the micron-scale such as microposts and microneedles have also been developed to aid in transdermal drug delivery [18, 95–96]. These features have also been incorporated into planar oral drug delivery microdevices (Figure 4B) to penetrate the mucous membrane in order to increase both drug permeation and device residence time [57]. Utilizing topographical features at a smaller scale, gecko-inspired nanotopography can be incorporated into microdevices to promote adhesion by providing dramatically increased surface area, resulting in increased vaan der Waals interactions. The Desai Lab has created hierarchical nanoengineered microparticles (NEMPs) composed of silicon oxide microspheres coated with silicon nanowires (Figure 4C) that demonstrated a 100-fold increase in lift-off force in vitro and a ten-fold increase in residence time in vivo relative to uncoated silica microspheres [34, 90]. Fabrication conditions have been altered to produce planar NEMPs, resulting in significant increases in drug uptake both in vitro and in vivo [76]. Nanostructured surfaces are also capable of loosening the epithelial barrier and providing increased permeability to protein therapeutics [78]. Therefore, incorporation of nanotopography into microdevices has potential to simultaneously increase device adhesion to the epithelial barrier and interact with epithelial cells to increase drug permeability, and studies regarding this concept are currently underway in our laboratory. Given the mucoadhesive properties of chitosan [94, 97–99], chitosan-based hydrogels show great promise as materials for oral drug delivery microdevices. Hydrogels also have dynamic interactions with water, providing swelling properties that can be utilized to incorporate folding properties into microdevices [60]. Guan et al. created self-folding bilayered microdevices composed of layers of crosslinked chitosan and poly(PEGMA-co-PEGDMA) [56]. These devices were fabricated with arms that folded upon contact with water as a result of differential swelling of the two polymer layers (Figure 4D), resulting in mechanical attachment of the devices to excised pig intestinal mucosa [56].
Figure 4.
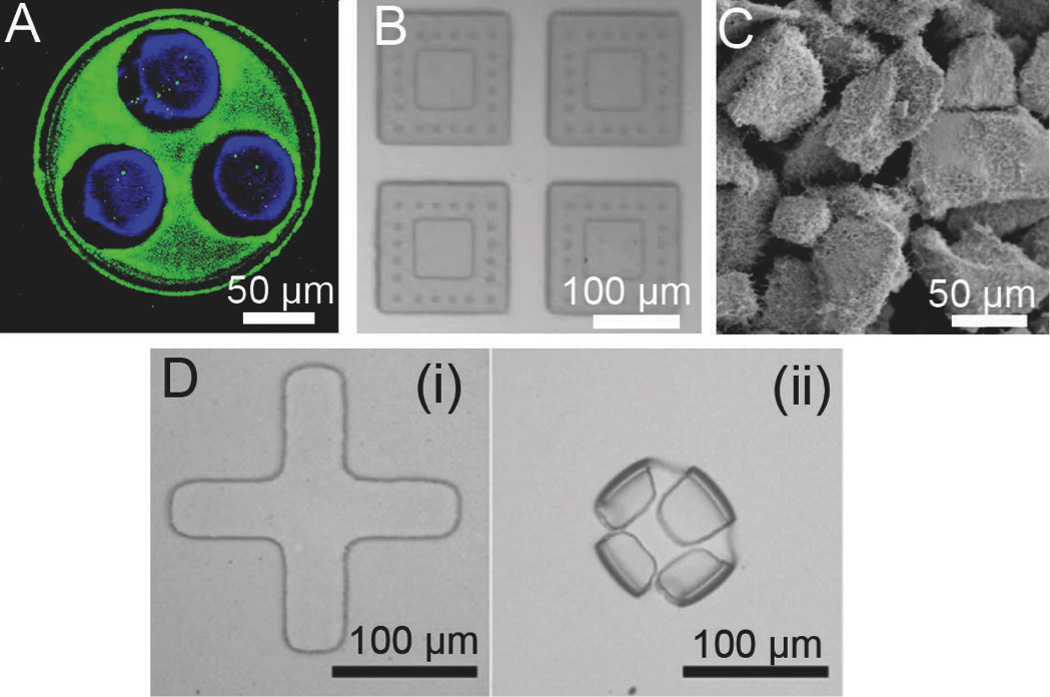
Chemical and structural modifications to enhance microdevice adhesion and increase residence time. A. Fluorescence micrograph of FITC-lectin (green) asymmetrically coated onto the drug-releasing side of microdevices for targeted bioadhesion to the intestinal mucosa with fluorescently labeled BSA shown in blue [34]. B. Microdevices with microposts designed to penetrate the mucus membrane surrounding a drug reservoir [57]. C. Planar nanowire-coated microparticles dramatically increase surface area and enhance microdevice adhesion through increased non-covalent interactions [76]. D. Self-folding microdevices shown before (i) and after (ii) exposure to water are designed to mechanically attach to intestinal tissue [60]. Reproduced with permission.
Approaches to drug loading of microdevices
Drug loading of microdevices is a difficult challenge with the ultimate goal of loading drug in a high-throughput, low-waste, and precise manner. Initial studies utilized microinjection of drug into the device reservoir with subsequent drying and crystallization of the drug [40]. For higher throughput drug loading and custom release kinetics, drug-laden PEGDMA hydrogels were spin-coated onto devices and selectively polymerized by photolithography (Figure 5A) [52]. The ability to lithographically pattern drug-loaded hydrogels was utilized by Ainslie et al. to layer drugs into reservoirs for sequential drug release kinetics [52]. In addition, Chirra and Desai loaded PMMA hydrogels crosslinked with PEGDMA each containing a differing model drug into three device reservoirs [34]. While photolithographically based drug loading allows for high precision and incorporation of hydrogels with custom release kinetics, drug is lost in the process of spin-coating and developing the drug-hydrogel solution, which is a major drawback when utilizing expensive biotherapeutics. To load drugs in a low-waste manner, discontinuous dewetting properties have been utilized. In one study, microdevices were brushed with a solution of sodium chloride, and surface interactions with drug solvent resulted in collection and crystallization of the model drug in the device reservoirs to provide a high-throughput method of drug loading with minimal drug waste (Figure 5B) [56]. In a more precise drug loading method, Marizza et al. recently developed inkjet printing for loading of drug solutions into microdevice reservoirs (Figure 5C) [100]. While this method requires sequential loading of each microdevice in a semi-automated manner and is not currently as high-throughput as discontinuous dewetting or photolithographic drug loading techniques, it is capable of precise, quasi-zero-waste performance. Marizza et al. went on to combine this method with supercritical fluid impregnation, allowing for the loading of hydrophobic drugs without the use of an organic solvent (Figure 5D) [101]. Other techniques for loading drug over the entire microdevice surface rather than into reservoirs have been utilized. NEMPs, with a large surface area as a result of nanowire coating, were loaded with insulin via a solvent-evaporation-induced capillary effect, in which drug crystallized over the microparticle surface at the base of the nanowires [76, 102].
Figure 5.
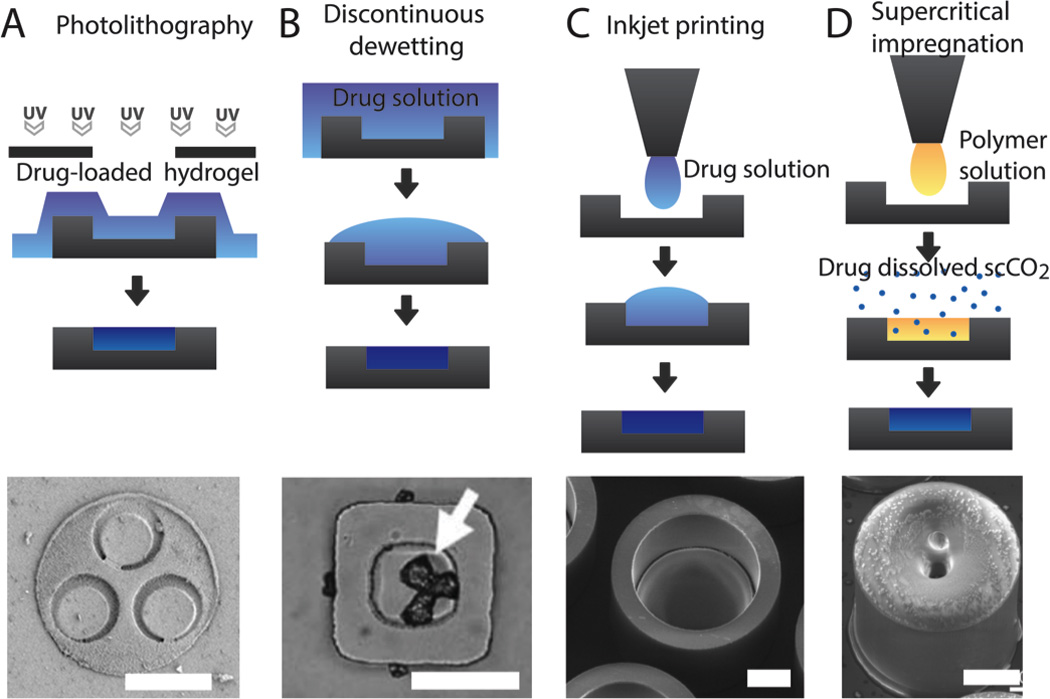
Current techniques for drug loading of microdevices with images of drug-loaded devices for each approach. A. Photolithography can be utilized to selectively induce crosslinking of drug-laden hydrogels in device reservoirs [34]. B. Discontinuous dewetting utilizes a hydrophilic material to selectively collect drug solution in device reservoirs before the drying the solvent to load drug into the device reservoir [56]. C. Inkjet printing can be utilized to deposit droplets of drug solution into device reservoirs, which later dries, leaving solidified drug [100]. D. Supercritical impregnation first utilizes inkjet printing to deposit a polymer solution into device reservoirs. After drying, the polymer is exposed to drug dissolved in supercritical carbon dioxide gas, allowing for drug incorporation of hydrophobic drugs without the use of organic solvent [101]. All scale bars are 100 µm. Adapted and reproduced with permission.
Efficacy of microdevices in vitro and in vivo
Within the last few years, the utilization of semi-conductor industry principles to fabricate oral microdevices has advanced leaps and bounds. Yet, the testing of these devices in improving the overall efficacy of most therapeutics is still at its relative infancy. Recent studies related to in vitro drug release and permeation have been done over monolayers of Caco-2 epithelial cells using Transwell® inserts. Ainslie et al. showed that the localized high concentration of drug at the device-cell interface resulted in an enhancement of drug permeation across the Caco-2 monolayer under physiological fluid flow, with a ten-fold increase in fluorescein permeation when released from microdevices relative to fluorescein free in solution [27]. Also, a sequential release of different sized drugs, insulin and camptothecin was achieved with the use of a dual layered hydrogel system that was present in microdevices made up of a single reservoir [103]. While sequential release can be harnessed to improve drug bioavailability by first releasing a permeation enhancer followed by the drug of interest, the release kinetics of the drug are co-dependent on the release kinetics of the permeation enhancer from its respective top hydrogel layer. To overcome this co-dependence issue, Chirra et al. used multiple reservoirs that can be filled with different drugs using different hydrogel/biodegradable polymeric systems [34]. Figure 6B shows the independent release of multiple model fluorophore-tagged BSAs from respective reservoirs as shown in Figure 6A. Such a device system can be used to release permeation enhancers, proteolytic enzyme inhibitors, and drugs of interest at independent rates and release times, thereby making oral microdevices effective for increasing drug efficacy as well as for combinatorial therapy. The Desai Lab also used Caco-2 monolayer coated parallel plate flow chambers to study the extent of oral microdevice retention under GI flow conditions. They have shown that 93% of tomato lectin microdevices remain attached to the cell surface under one hour of physiological shear conditions after initial binding, indicating that microdevices are capable of remaining attached to GI tissue for extended periods of time under physiological conditions [103].
Figure 6.
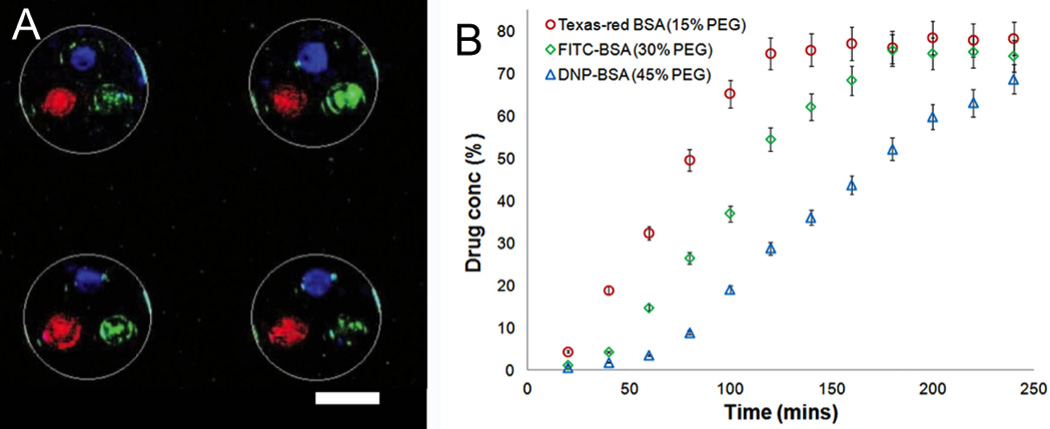
Microdevices loaded with multiple drugs with separate release profiles. A. Fluorescent image demonstrating separate drug loading of each microdevice reservoir with device shape outlined in white. Scale bar is 100 µm. B. Custom release profiles for each drug controlled by hydrogel crosslinking density. Reproduced with permission from [34].
The several advantages of using asymmetric planar oral microdevices including unidirectional release to avoid luminal drug loss, increased contact surface area and reduced shear stress with a planar design, and selective modification of reservoir side of device to introduce muco- or cytoadhesive properties were recently tested in vivo. Our lab observed that upon oral administration to mice, PMMA microdevices having the same contact surface area as that of symmetric PMMA microparticles have a 27% retention in the proximal small intestine after 2 hours due to the relatively low shear stress experienced by the thin device side walls, as compared to a retention of 12% for the curvilinear microparticles [66]. Also, a further enhancement of microdevice retention to 41% was observed after conjugation of the bioadhesive protein lectin, which targeted the intestinal epithelial cell wall. We have shown that with the help of microdevices, even the poorly permeable drug Acyclovir had a five-fold increase in oral bioavailability in mice as compared to that of a conventional solution of Acyclovir of same dosage [66]. This enhancement of oral bioavailability drastically reduces the overall dosage needed for effective therapy. Such a reduction in dosage with improved bioavailability proves vital in significantly alleviating issues of systemic side effects, thereby opening up oral administration to an array of toxic and expensive therapeutics. While most of the recent in vivo work was done using small molecule drugs, microdevices can be applied to the oral delivery of macromolecules and high-efficacy low-dosage drugs (e.g. Leuprolide, human growth hormone, etc.). Detailed studies on improving drug loading, dosage optimization, improved protection against GI environment, sustained release for systemic delivery, targeted attachment, and GI pathology oriented localized delivery are currently underway and are of much interest for future work. Therefore, the use of microfabricated planar oral devices holds promise in augmenting the range of oral therapeutics used, while solving pharmacokinetic issues associated with low permeability and avoiding systemic side effects.
Conclusion
Microfabrication techniques allow for the creation of devices with properties not easily attainable through other methods of conventional particle synthesis. This advanced control over device properties allows microdevices to be designed to simultaneously address many barriers to oral drug uptake. Specifically, planar device shape, asymmetric modifications for cyto- and mucoadhesion, and unidirectional drug release are utilized to overcome barriers to oral drug delivery. These devices can be modified with targeting moieties and tunable drug release profiles to address specific drug delivery applications. Future developments in microdevice fabrication may involve the incorporation of additional biocompatible and bioresponsive materials for smart drug delivery and mucoadhesion. Also, new micro- and nanofabrication techniques may be developed to provide device topography in order to improve cellular permeability and enhance device adhesion. Challenges in the application of microdevices to oral drug delivery include a limited drug loading capacity and the high cost of some microfabrication techniques. These potential drawbacks can be mitigated by selecting highly potent drugs to reduce the number of devices required per dosage and by optimizing fabrication and drug loading techniques to minimize cost and maximize drug capacity. Microdevices have demonstrated promising results for oral drug delivery both in vitro and in vivo. However, the exact mechanisms by which these devices interact with the mucous and epithelial layers are yet to be fully determined. Further trials will determine whether the devices function primarily through either mucoadhesion or cytoadhesion and which drugs and drug combinations are best suited for use in planar microdevices. Multi-drug carrying microdevices may allow for combination therapy involving a drug of interest and a disruptive drug for increasing bioavailabilty through localized and transient disruption of digestive enzymes, transporter proteins, and epithelial tight junctions. In addition to applications in increasing systemic drug bioavailability, microdevices could be modified for targeted and localized drug delivery to diseased tissue in the GI tract. As new materials, fabrication methods, and drug combinations are developed, microdevice technology has the potential to significantly improve the oral delivery of a wide range of therapeutics including small molecule drugs, mid-size peptide therapeutics including insulin and antigens for immunization, and larger protein-based therapeutics.
Acknowledgements
This work was supported by Z Cube Zambon Research Venture. CBF was supported by an NIH Training Grant (5T32GM007175-37).
Footnotes
Conflict of interest
None declared.
References
- 1.International Transporter C, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nature reviews. Drug discovery. 2010;9(3):215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thanki K, Gangwal RP, Sangamwar AT, Jain S. Oral delivery of anticancer drugs: challenges and opportunities. Journal of controlled release : official journal of the Controlled Release Society. 2013;170(1):15–40. doi: 10.1016/j.jconrel.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nature reviews. Drug discovery. 2003;2(4):289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Jain A, Chakraborty M, Sahni JK, Ali J, Dang S. Oral delivery of therapeutic proteins and peptides: a review on recent developments. Drug delivery. 2013;20(6):237–246. doi: 10.3109/10717544.2013.819611. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal U, Sharma R, Gupta M, Vyas SP. Is nanotechnology a boon for oral drug delivery? Drug discovery today. 2014 doi: 10.1016/j.drudis.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Felton LA, Porter SC. An update on pharmaceutical film coating for drug delivery. Expert opinion on drug delivery. 2013;10(4):421–435. doi: 10.1517/17425247.2013.763792. [DOI] [PubMed] [Google Scholar]
- 7.Kriegel C, Attarwala H, Amiji M. Multi-compartmental oral delivery systems for nucleic acid therapy in the gastrointestinal tract. Advanced drug delivery reviews. 2013;65(6):891–901. doi: 10.1016/j.addr.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Hunter AC, Elsom J, Wibroe PP, Moghimi SM. Polymeric particulate technologies for oral drug delivery and targeting: a pathophysiological perspective. Nanomedicine : nanotechnology, biology, and medicine. 2012;8(Suppl 1):S5–S20. doi: 10.1016/j.nano.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Dahan A, Khamis M, Agbaria R, Karaman R. Targeted prodrugs in oral drug delivery: the modern molecular biopharmaceutical approach. Expert opinion on drug delivery. 2012;9(8):1001–1013. doi: 10.1517/17425247.2012.697055. [DOI] [PubMed] [Google Scholar]
- 10.Zee YK, Goh BC, Lee SC. Pharmacologic modulation strategies to reduce dose requirements of anticancer therapy while preserving clinical efficacy. Future oncology. 2012;8(6):731–749. doi: 10.2217/fon.12.53. [DOI] [PubMed] [Google Scholar]
- 11.Kuppens IE, Breedveld P, Beijnen JH, Schellens JH. Modulation of oral drug bioavailability: from preclinical mechanism to therapeutic application. Cancer investigation. 2005;23(5):443–464. doi: 10.1081/cnv-58823. [DOI] [PubMed] [Google Scholar]
- 12.Lemmer HJ, Hamman JH. Paracellular drug absorption enhancement through tight junction modulation. Expert opinion on drug delivery. 2013;10(1):103–114. doi: 10.1517/17425247.2013.745509. [DOI] [PubMed] [Google Scholar]
- 13.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharmaceutics & drug disposition. 1995;16(5):351–380. doi: 10.1002/bdd.2510160502. [DOI] [PubMed] [Google Scholar]
- 14.Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastroduodenal mucosal protection. Physiological reviews. 1993;73(4):823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- 15.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. American journal of physiology. Gastrointestinal and liver physiology. 2001;280(5):G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson JK, Ermund A, Johansson ME, Schutte A, Hansson GC, Sjovall H. An ex vivo method for studying mucus formation, properties, and thickness in human colonic biopsies and mouse small and large intestinal explants. American journal of physiology. Gastrointestinal and liver physiology. 2012;302(4):G430–G438. doi: 10.1152/ajpgi.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Archiv : European journal of physiology. 2000;440(5):653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- 18.van den Berg BM, Nieuwdorp M, Stroes ES, Vink H. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacological reports : PR. 2006;58(Suppl):75–80. [PubMed] [Google Scholar]
- 19.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced drug delivery reviews. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 20.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nature reviews. Molecular cell biology. 2007;8(8):603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 21.Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nature reviews. Molecular cell biology. 2005;6(2):112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- 22.Pantzar N, Lundin S, Wester L, Westrom BR. Bidirectional small-intestinal permeability in the rat to some common marker molecules in vitro. Scandinavian journal of gastroenterology. 1994;29(8):703–709. doi: 10.3109/00365529409092497. [DOI] [PubMed] [Google Scholar]
- 23.Salama NN, Eddington ND, Fasano A. Tight junction modulation and its relationship to drug delivery. Advanced drug delivery reviews. 2006;58(1):15–28. doi: 10.1016/j.addr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Tang R, Li WX, Huang W, Yan F, Chai WM, Yang GY, Chen KM. CO2-based in-line phase contrast imaging of small intestine in mice. Scientific reports. 2013;3:2313. doi: 10.1038/srep02313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sant S, Tao SL, Fisher OZ, Xu Q, Peppas NA, Khademhosseini A. Microfabrication technologies for oral drug delivery. Advanced drug delivery reviews. 2012;64(6):496–507. doi: 10.1016/j.addr.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chirra HD, Desai TA. Emerging microtechnologies for the development of oral drug delivery devices. Advanced drug delivery reviews. 2012;64(14):1569–1578. doi: 10.1016/j.addr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ainslie KM, Lowe RD, Beaudette TT, Petty L, Bachelder EM, Desai TA. Microfabricated devices for enhanced bioadhesive drug delivery: attachment to and small-molecule release through a cell monolayer under flow. Small. 2009;5(24):2857–2863. doi: 10.1002/smll.200901254. [DOI] [PubMed] [Google Scholar]
- 28.Tachibana T, Kato M, Takano J, Sugiyama Y. Predicting drug-drug interactions involving the inhibition of intestinal CYP3A4 and P-glycoprotein. Current drug metabolism. 2010;11(9):762–777. doi: 10.2174/138920010794328922. [DOI] [PubMed] [Google Scholar]
- 29.Konig J, Muller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacological reviews. 2013;65(3):944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- 30.Besheer A, Wood KM, Peppas NA, Mader K. Loading and mobility of spin-labeled insulin in physiologically responsive complexation hydrogels intended for oral administration. Journal of controlled release : official journal of the Controlled Release Society. 2006;111(1–2):73–80. doi: 10.1016/j.jconrel.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Kim B, Peppas NA. In vitro release behavior and stability of insulin in complexation hydrogels as oral drug delivery carriers. International journal of pharmaceutics. 2003;266(1–2):29–37. doi: 10.1016/s0378-5173(03)00378-8. [DOI] [PubMed] [Google Scholar]
- 32.Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. Journal of pharmaceutical sciences. 1999;88(9):933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 33.Al-Hilal TA, Alam F, Byun Y. Oral drug delivery systems using chemical conjugates or physical complexes. Advanced drug delivery reviews. 2013;65(6):845–864. doi: 10.1016/j.addr.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Chirra HD, Desai TA. Multi-reservoir bioadhesive microdevices for independent rate-controlled delivery of multiple drugs. Small. 2012;8(24):3839–3846. doi: 10.1002/smll.201201367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao SL, Desai TA. Micromachined devices: the impact of controlled geometry from cell-targeting to bioavailability. Journal of controlled release : official journal of the Controlled Release Society. 2005;109(1–3):127–138. doi: 10.1016/j.jconrel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 36.Tao SL, Popat K, Desai TA. Off-wafer fabrication and surface modification of asymmetric 3D SU-8 microparticles. Nature protocols. 2006;1(6):3153–3158. doi: 10.1038/nprot.2006.451. [DOI] [PubMed] [Google Scholar]
- 37.Harris LA, Hansel S, DiBaise J, Crowell MD. Irritable bowel syndrome and chronic constipation: emerging drugs, devices, and surgical treatments. Current gastroenterology reports. 2006;8(4):282–290. doi: 10.1007/s11894-006-0048-y. [DOI] [PubMed] [Google Scholar]
- 38.Wald A. Irritable bowel syndrome--diarrhoea. Best practice & research. Clinical gastroenterology. 2012;26(5):573–580. doi: 10.1016/j.bpg.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Orally delivered thioketal nanoparticles loaded with TNF-alpha-siRNA target inflammation and inhibit gene expression in the intestines. Nature materials. 2010;9(11):923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed A, Bonner C, Desai TA. Bioadhesive microdevices with multiple reservoirs: a new platform for oral drug delivery. Journal of controlled release : official journal of the Controlled Release Society. 2002;81(3):291–306. doi: 10.1016/s0168-3659(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 41.Foraker AB, Walczak RJ, Cohen MH, Boiarski TA, Grove CF, Swaan PW. Microfabricated porous silicon particles enhance paracellular delivery of insulin across intestinal Caco-2 cell monolayers. Pharmaceutical research. 2003;20(1):110–116. doi: 10.1023/a:1022211127890. [DOI] [PubMed] [Google Scholar]
- 42.Hiebl B, Hopperdietzel C, Hunigen H, Jung F, Scharnagl N. Influence of a silicon (Si14)-based coating substrate for biomaterials on fibroblast growth and human C5a. Clinical hemorheology and microcirculation. 2013;55(4):491–499. doi: 10.3233/CH-131785. [DOI] [PubMed] [Google Scholar]
- 43.Peng F, Su Y, Zhong Y, Fan C, Lee ST, He Y. Silicon nanomaterials platform for bioimaging, biosensing, and cancer therapy. Accounts of chemical research. 2014;47(2):612–623. doi: 10.1021/ar400221g. [DOI] [PubMed] [Google Scholar]
- 44.Santos HA, Makila E, Airaksinen AJ, Bimbo LM, Hirvonen J. Porous silicon nanoparticles for nanomedicine: preparation and biomedical applications. Nanomedicine. 2014;9(4):535–554. doi: 10.2217/nnm.13.223. [DOI] [PubMed] [Google Scholar]
- 45.Fubini B, Hubbard A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free radical biology & medicine. 2003;34(12):1507–1516. doi: 10.1016/s0891-5849(03)00149-7. [DOI] [PubMed] [Google Scholar]
- 46.Tungjai M, Whorton EB, Rithidech KN. Persistence of apoptosis and inflammatory responses in the heart and bone marrow of mice following whole-body exposure to (2)(8)Silicon ((2)(8)Si) ions. Radiation and environmental biophysics. 2013;52(3):339–350. doi: 10.1007/s00411-013-0479-4. [DOI] [PubMed] [Google Scholar]
- 47.Food; Drug Administration, H. H. S. Medical devices; reclassification of polymethylmethacrylate (PMMA) bone cement. Final rule. Federal register. 2002;67(137):46852–46855. [PubMed] [Google Scholar]
- 48.Xu Y, Xie F, Qiu T, Xie L, Xing W, Cheng J. Rapid fabrication of a microdevice with concave microwells and its application in embryoid body formation. Biomicrofluidics. 2012;6(1):16504–1650411. doi: 10.1063/1.3687399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reedy CR, Price CW, Sniegowski J, Ferrance JP, Begley M, Landers JP. Solid phase extraction of DNA from biological samples in a post-based, high surface area poly(methyl methacrylate) (PMMA) microdevice. Lab on a chip. 2011;11(9):1603–1611. doi: 10.1039/c0lc00597e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tao SL, Lubeley MW, Desai TA. Bioadhesive poly(methyl methacrylate) microdevices for controlled drug delivery. Journal of controlled release : official journal of the Controlled Release Society. 2003;88(2):215–228. doi: 10.1016/s0168-3659(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 51.Wei S, Vaidya B, Patel AB, Soper SA, McCarley RL. Photochemically patterned poly(methyl methacrylate) surfaces used in the fabrication of microanalytical devices. The journal of physical chemistry. B. 2005;109(35):16988–16996. doi: 10.1021/jp051550s. [DOI] [PubMed] [Google Scholar]
- 52.Ainslie KM, Kraning CM, Desai TA. Microfabrication of an asymmetric, multi-layered microdevice for controlled release of orally delivered therapeutics. Lab on a chip. 2008;8(7):1042–1047. doi: 10.1039/b800604k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotzar G, Freas M, Abel P, Fleischman A, Roy S, Zorman C, Moran JM, Melzak J. Evaluation of MEMS materials of construction for implantable medical devices. Biomaterials. 2002;23(13):2737–2750. doi: 10.1016/s0142-9612(02)00007-8. [DOI] [PubMed] [Google Scholar]
- 54.Voskerician G, Shive MS, Shawgo RS, von Recum H, Anderson JM, Cima MJ, Langer R. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials. 2003;24(11):1959–1967. doi: 10.1016/s0142-9612(02)00565-3. [DOI] [PubMed] [Google Scholar]
- 55.Vernekar VN, Cullen DK, Fogleman N, Choi Y, Garcia AJ, Allen MG, Brewer GJ, LaPlaca MC. SU-8 2000 rendered cytocompatible for neuronal bioMEMS applications. Journal of biomedical materials research. Part A. 2009;89(1):138–151. doi: 10.1002/jbm.a.31839. [DOI] [PubMed] [Google Scholar]
- 56.Guan J, He H, Lee LJ, Hansford DJ. Fabrication of particulate reservoir-containing, capsulelike, and self-folding polymer microstructures for drug delivery. Small. 2007;3(3):412–418. doi: 10.1002/smll.200600240. [DOI] [PubMed] [Google Scholar]
- 57.Tao SL, Desai TA. Microfabrication of multilayer, asymmetric, polymeric devices for drug delivery. Adv Mater. 2005;17(13):1625-+. [Google Scholar]
- 58.Ryu WH, Vyakarnam M, Greco RS, Prinz FB, Fasching RJ. Fabrication of multi-layered biodegradable drug delivery device based on micro-structuring of PLGA polymers. Biomedical microdevices. 2007;9(6):845–853. doi: 10.1007/s10544-007-9097-8. [DOI] [PubMed] [Google Scholar]
- 59.Guan JJ, Chakrapani A, Hansford DJ. Polymer microparticles fabricated by soft lithography. Chem Mater. 2005;17(25):6227–6229. [Google Scholar]
- 60.Guan J, He H, Hansford DJ, Lee LJ. Self-folding of three-dimensional hydrogel microstructures. The journal of physical chemistry. B. 2005;109(49):23134–23137. doi: 10.1021/jp054341g. [DOI] [PubMed] [Google Scholar]
- 61.He H, Cao X, Lee LJ. Design of a novel hydrogel-based intelligent system for controlled drug release. Journal of controlled release : official journal of the Controlled Release Society. 2004;95(3):391–402. doi: 10.1016/j.jconrel.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Ito Y, Hasuda H, Morimatsu M, Takagi N, Hirai Y. A microfabrication method of a biodegradable polymer chip for a controlled release system. Journal of biomaterials science. Polymer edition. 2005;16(8):949–955. doi: 10.1163/1568562054414621. [DOI] [PubMed] [Google Scholar]
- 63.Norman JJ, Desai TA. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Annals of biomedical engineering. 2006;34(1):89–101. doi: 10.1007/s10439-005-9005-4. [DOI] [PubMed] [Google Scholar]
- 64.Qian T, Wang Y. Micro/nano-fabrication technologies for cell biology. Medical & biological engineering & computing. 2010;48(10):1023–1032. doi: 10.1007/s11517-010-0632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziaie B, Baldi A, Lei M, Gu Y, Siegel RA. Hard and soft micromachining for BioMEMS: review of techniques and examples of applications in microfluidics and drug delivery. Advanced drug delivery reviews. 2004;56(2):145–172. doi: 10.1016/j.addr.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Chirra HD, Shao L, Ciaccio N, Fox CB, Wade JM, Ma A, Desai TA. Planar Microdevices for Enhanced In Vivo Retention and Oral Bioavailability of Poorly Permeable Drugs. Advanced healthcare materials. 2014 doi: 10.1002/adhm.201300676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tao SL, Desai TA. Gastrointestinal patch systems for oral drug delivery. Drug discovery today. 2005;10(13):909–915. doi: 10.1016/S1359-6446(05)03489-6. [DOI] [PubMed] [Google Scholar]
- 68.Guan J, Ferrell N, James Lee L, Hansford DJ. Fabrication of polymeric microparticles for drug delivery by soft lithography. Biomaterials. 2006;27(21):4034–4041. doi: 10.1016/j.biomaterials.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 69.Jackman RJ, Duffy DC, Ostuni E, Willmore ND, Whitesides GM. Fabricating large arrays of microwells with arbitrary dimensions and filling them using discontinuous dewetting. Analytical chemistry. 1998;70(11):2280–2287. doi: 10.1021/ac971295a. [DOI] [PubMed] [Google Scholar]
- 70.Ferrell N, Woodard J, Hansford D. Fabrication of polymer microstructures for MEMS: sacrificial layer micromolding and patterned substrate micromolding. Biomedical microdevices. 2007;9(6):815–821. doi: 10.1007/s10544-007-9094-y. [DOI] [PubMed] [Google Scholar]
- 71.Geipel A, Goldschmidtboeing F, Jantscheff P, Esser N, Massing U, Woias P. Design of an implantable active microport system for patient specific drug release. Biomedical microdevices. 2008;10(4):469–478. doi: 10.1007/s10544-007-9147-2. [DOI] [PubMed] [Google Scholar]
- 72.Husler R, Schlittler FL, Kreutziger J, Streit M, Banic A, Schoni-Affolter F, Hunger RE, Constaninescu MA. Staged surgical therapy of basal cell carcinoma of the head and neck region: an evaluation of 500 procedures. Swiss medical weekly. 2008;138(49–50):746–751. doi: 10.4414/smw.2008.12424. [DOI] [PubMed] [Google Scholar]
- 73.Lu X, Kim-Han JS, O'Malley KL, Sakiyama-Elbert SE. A microdevice platform for visualizing mitochondrial transport in aligned dopaminergic axons. Journal of neuroscience methods. 2012;209(1):35–39. doi: 10.1016/j.jneumeth.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park J, Li J, Han A. Micro-macro hybrid soft-lithography master (MMHSM) fabrication for lab-on-a-chip applications. Biomedical microdevices. 2010;12(2):345–351. doi: 10.1007/s10544-009-9390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer KE, Nagaraj G, Hugh Daniels R, Li E, Cowles VE, Miller JL, Bunger MD, Desai TA. Hierarchical nanoengineered surfaces for enhanced cytoadhesion and drug delivery. Biomaterials. 2011;32(13):3499–3506. doi: 10.1016/j.biomaterials.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uskokovic V, Lee K, Lee PP, Fischer KE, Desai TA. Shape effect in the design of nanowire-coated microparticles as transepithelial drug delivery devices. ACS nano. 2012;6(9):7832–7841. doi: 10.1021/nn3019865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Uskokovic V, Lee PP, Walsh LA, Fischer KE, Desai TA. PEGylated silicon nanowire coated silica microparticles for drug delivery across intestinal epithelium. Biomaterials. 2012;33(5):1663–1672. doi: 10.1016/j.biomaterials.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kam KR, Walsh LA, Bock SM, Koval M, Fischer KE, Ross RF, Desai TA. Nanostructure-mediated transport of biologics across epithelial tissue: enhancing permeability via nanotopography. Nano letters. 2013;13(1):164–171. doi: 10.1021/nl3037799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lohmuller T, Aydin D, Schwieder M, Morhard C, Louban I, Pacholski C, Spatz JP. Nanopatterning by block copolymer micelle nanolithography and bioinspired applications. Biointerphases. 2011;6(1):MR1–MR12. doi: 10.1116/1.3536839. [DOI] [PubMed] [Google Scholar]
- 80.Marmiroli B, Amenitsch H. X-ray lithography and small-angle X-ray scattering: a combination of techniques merging biology and materials science. European biophysics journal : EBJ. 2012;41(10):851–861. doi: 10.1007/s00249-012-0843-3. [DOI] [PubMed] [Google Scholar]
- 81.Betancourt T, Brannon-Peppas L. Micro- and nanofabrication methods in nanotechnological medical and pharmaceutical devices. International journal of nanomedicine. 2006;1(4):483–495. doi: 10.2147/nano.2006.1.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y, Pepin A. Nanofabrication: conventional and nonconventional methods. Electrophoresis. 2001;22(2):187–207. doi: 10.1002/1522-2683(200101)22:2<187::AID-ELPS187>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 83.Hu WC, Sarveswaran K, Lieberman M, Bernstein GH. Sub-10 nm electron beam lithography using cold development of poly(methylmethacrylate) J Vac Sci Technol B. 2004;22(4):1711–1716. [Google Scholar]
- 84.Pulsifer DP, Lakhtakia A. Background and survey of bioreplication techniques. Bioinspiration & biomimetics. 2011;6(3):031001. doi: 10.1088/1748-3182/6/3/031001. [DOI] [PubMed] [Google Scholar]
- 85.Wu CC, Reinhoudt DN, Otto C, Subramaniam V, Velders AH. Strategies for patterning biomolecules with dip-pen nanolithography. Small. 2011;7(8):989–1002. doi: 10.1002/smll.201001749. [DOI] [PubMed] [Google Scholar]
- 86.Chou SY, Krauss PR. Imprint lithography with sub-10 nm feature size and high throughput. Microelectron Eng. 1997;35(1–4):237–240. [Google Scholar]
- 87.Chou SY, Krauss PR, Renstrom PJ. Imprint lithography with 25-nanometer resolution. Science. 1996;272(5258):85–87. [Google Scholar]
- 88.Lan H, Liu H. UV-nanoimprint lithography: structure, materials and fabrication of flexible molds. Journal of nanoscience and nanotechnology. 2013;13(5):3145–3172. doi: 10.1166/jnn.2013.7437. [DOI] [PubMed] [Google Scholar]
- 89.Truskett VN, Watts MP. Trends in imprint lithography for biological applications. Trends in biotechnology. 2006;24(7):312–317. doi: 10.1016/j.tibtech.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Fischer KE, Aleman BJ, Tao SL, Hugh Daniels R, Li EM, Bunger MD, Nagaraj G, Singh P, Zettl A, Desai TA. Biomimetic nanowire coatings for next generation adhesive drug delivery systems. Nano letters. 2009;9(2):716–720. doi: 10.1021/nl803219f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Porter JR, Henson A, Popat KC. Biodegradable poly(epsilon-caprolactone) nanowires for bone tissue engineering applications. Biomaterials. 2009;30(5):780–788. doi: 10.1016/j.biomaterials.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 92.Wood KM, Stone GM, Peppas NA. The effect of complexation hydrogels on insulin transport in intestinal epithelial cell models. Acta biomaterialia. 2010;6(1):48–56. doi: 10.1016/j.actbio.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arbos P, Arangoa MA, Campanero MA, Irache JM. Quantification of the bioadhesive properties of protein-coated PVM/MA nanoparticles. International journal of pharmaceutics. 2002;242(1–2):129–136. doi: 10.1016/s0378-5173(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 94.Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Advanced drug delivery reviews. 2010;62(1):59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 95.Bariya SH, Gohel MC, Mehta TA, Sharma OP. Microneedles: an emerging transdermal drug delivery system. The Journal of pharmacy and pharmacology. 2012;64(1):11–29. doi: 10.1111/j.2042-7158.2011.01369.x. [DOI] [PubMed] [Google Scholar]
- 96.Escobar-Chavez JJ, Bonilla-Martinez D, Villegas-Gonzalez MA, Molina-Trinidad E, Casas-Alancaster N, Revilla-Vazquez AL. Microneedles: a valuable physical enhancer to increase transdermal drug delivery. Journal of clinical pharmacology. 2011;51(7):964–977. doi: 10.1177/0091270010378859. [DOI] [PubMed] [Google Scholar]
- 97.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharmaceutical research. 1998;15(9):1326–1331. doi: 10.1023/a:1011929016601. [DOI] [PubMed] [Google Scholar]
- 98.Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chemical reviews. 2004;104(12):6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 99.Patel MP, Patel RR, Patel JK. Chitosan mediated targeted drug delivery system: a review. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2010;13(4):536–557. doi: 10.18433/j3jc7c. [DOI] [PubMed] [Google Scholar]
- 100.Marizza P, Keller SS, Boisen A. Inkjet printing as a technique for filling of micro-wells with biocompatible polymers. Microelectron Eng. 2013;111:391–395. [Google Scholar]
- 101.Marizza P, Keller SS, Mullertz A, Boisen A. Polymer-filled microcontainers for oral delivery loaded using supercritical impregnation. Journal of controlled release : official journal of the Controlled Release Society. 2014;173:1–9. doi: 10.1016/j.jconrel.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 102.Fischer KE, Jayagopal A, Nagaraj G, Daniels RH, Li EM, Silvestrini MT, Desai TA. Nanoengineered surfaces enhance drug loading and adhesion. Nano letters. 2011;11(3):1076–1081. doi: 10.1021/nl103951e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ainslie KM, Desai TA. Microfabricated implants for applications in therapeutic delivery, tissue engineering, and biosensing. Lab on a chip. 2008;8(11):1864–1878. doi: 10.1039/b806446f. [DOI] [PMC free article] [PubMed] [Google Scholar]


