Abstract
Aims
We prospectively examined the relationship between site-specific peak plantar pressure (PPP) and ulcer risk. Researchers have previously reported associations between diabetic foot ulcer and elevated plantar foot pressure, but the effect of location-specific pressures has not been studied.
Methods
Diabetic subjects (n = 591) were enrolled from a single VA hospital. Five measurements of in-shoe plantar pressure were collected using F-Scan. Pressures were measured at 8 areas: heel, lateral midfoot, medial midfoot, first metatarsal, second through fourth metatarsal, fifth metatarsal, hallux, and other toes. The relationship between incident plantar foot ulcer and PPP or pressure–time integral (PTI) was assessed using Cox regression.
Results
During follow-up (2.4 years), 47 subjects developed plantar ulcers (10 heel, 12 metatarsal, 19 hallux, 6 other). Overall mean PPP was higher for ulcer subjects (219 vs. 194 kPa), but the relationship differed by site (the metatarsals with ulcers had higher pressure, while the opposite was true for the hallux and heel). A statistical analysis was not performed on the means, but hazard ratios from a Cox survival analysis were nonsignificant for PPP across all sites and when adjusted for location. However, when the metatarsals were considered separately, higher baseline PPP was significantly associated with greater ulcer risk; at other sites, this relationship was nonsignificant. Hazard ratios for all PTI data were nonsignificant.
Conclusions
Location must be considered when assessing the relationship between PPP and plantar ulceration.
Keywords: Plantar pressure, Diabetic foot ulcer, Hallux, Heel, Metatarsal, Epidemiology
1. Introduction
Diabetes mellitus, while affecting more than 8% of the U.S. population, has been implicated in over 60% of all non-traumatic lower extremity amputations (CDCP, 2011). The development of diabetic foot ulcers is a multi-factorial process that has been associated with, among other factors, diabetic neuropathy, minor foot trauma and foot deformities (Reiber et al., 1999). Elevated plantar pressure has also been associated with ulceration, but the relationship between pressure at a specific location and ulceration at that location remains unclear.
In a retrospective study of barefoot subjects, Boulton et al. quantified the peak pressure of four groups of feet: non-diabetic control feet (n = 82), diabetic feet without neuropathy (n = 81), diabetic feet with neuropathy but no history of ulcers (n = 59), and diabetic feet with neuropathy and a history of ulcers (n = 22) (Boulton et al., 1983). Using a threshold of 11 kg/cm2 (1080 kPa), these authors found that feet with aberrant plantar pressure ranged from 7% for the controls, to 17% for the diabetic feet, to 31% for the diabetic feet with neuropathy, to 100% for the diabetic feet with a history of ulcers. However, this study was retrospective and did not examine the effect of location.
A cross-sectional analysis on 251 diabetic patients by Frykberg et al. examined the relationship between high foot pressures (>6 kg/cm2 or 588 kPa) and ulceration in patients walking in stockings (Frykberg et al., 1998). Of the patients enrolled, 99 had a current (n = 33) or past (n = 66) ulcer. After accounting for age, sex, diabetes duration and race, patients with higher plantar pressure were more than twice as likely to have an ulcer. However, as with Boulton et al.’s work, this study was retrospective and did not examine the effect of location.
Veves et al. prospectively followed 86 diabetic non-neuropathic (n = 28) and neuropathic (n = 58) patients for an average period of 30 months (Veves, Murray, Young, & Boulton, 1992). At follow-up, 35% (n = 15) of the patients had developed a plantar ulcer. All 15 ulcer patients had an elevated baseline barefoot peak pressure (>12.3 kg/cm2 or 1206 kPa) and 14 of the 15 patients were neuropathic. However, this study did not examine the effect of high baseline pressure and subsequent ulceration at a specific location.
Pham et al. conducted a prospective foot screening study and collected unshod plantar pressure data at baseline (Pham et al., 2000). They followed 248 patients over a mean period of 30 months and ulcers developed in 95 feet (19%) or 73 subjects (29%); of those ulcers, 76 (80%) were in the plantar forefoot. In a multi-regression analysis, high plantar pressure (>6 kg/cm2 or 588 kPa) and several measures of neuropathy were found to be statistically significant predictors of foot ulcer. However, as with Veves et al., this study did not examine the effect of high baseline pressure and subsequent ulceration at a specific location.
Thus, while peak pressure has been associated both retrospectively and prospectively with increased risk of ulceration, we are unaware of a study that has examined baseline plantar pressure at a specific location with a subsequent ulcer at that location. The primary purpose of this study was to address that specific issue, with an emphasis on the metatarsals. The work presented hereafter represents a subset of patients from the Seattle Diabetic Foot Study, which has been discussed elsewhere (Adler, Boyko, Ahroni, & Smith, 1999; Ahroni, Boyko, & Forsberg, 1998; Ahroni, Boyko, & Forsberg, 1999; Boyko et al., 1999; Boyko, Ahroni, Cohen, Nelson, & Heagerty, 2006; Cowley, Boyko, Shofer, Ahroni, & Ledoux, 2008), who had baseline plantar pressure measured when they were enrolled. The subjects were followed over time to see who developed an ulcer.
2. Subjects, materials, and methods
2.1. Study setting and subjects
All ambulatory General Internal Medicine Clinic patients at the Veterans Affairs Puget Sound Health Care System with diabetes between 1996 and 2002 were eligible for enrollment. The study received prior approval from the University of Washington Human Subjects Office, and informed consent was obtained from all subjects for their participation in this research. Exclusion criteria were current foot ulcer, bilateral foot amputations, wheelchair-bound or unable to walk, too sick to participate, or psychiatric illness that prevented informed consent. Subjects with clinically apparent diabetes were identified by review of hospital computerized pharmacy data for receipt of insulin, oral hypoglycemic medication or blood or urine glucose test strips, review of laboratory data, and review of medical record problem lists for the diagnosis of diabetes mellitus. The diagnosis was then confirmed by communication with clinical providers or medical record review. The vast majority of the patients in this study (perhaps 99%) lived within 50 miles of the VA Medical Center and received all their care there.
2.2. Baseline data collection
Subjects were interviewed to collect data on demographics and diabetes characteristics, and each received a physical exam. Sensory testing was performed at 9 locations on each foot using the Semmes–Weinstein monofilament. Inability to perceive the 5.07 monofilament at one or more sites was considered to represent peripheral sensory neuropathy.
2.3. Foot pressure measurements
We performed in-shoe foot-pressure measurements with F-scan insoles (Tekscan, Boston, MA) on subjects wearing their “usual” footwear walking a specified length (20 m, i.e., back and forth over a 10 m walkway) at their self-selected velocity. Sensors were placed between the patient’s sock and shoe. The F-scan insole is a pressure sensor, 0.18 mm thick, with an embedded grid of 960 contact cells. Before use, the disposable sensors were trimmed to fit into shoes, with the resultant loss of ~5%–20% of the contact cells. We have previously reported the F-scan methodology and reliability data in our study subjects (Ahroni et al., 1998).
The first five midgait footsteps for each foot were considered as separate trials for this analysis; the first few steps (gait initiation), the steps involved in turning around, and the last few steps (gait termination) were ignored. Pressure was determined by F-scan software v3.821 running on a dedicated personal computer. For each step, we recorded the plantar pressure at 8 pre-specified locations: the heel, medial midfoot, lateral midfoot, first metatarsal head, second to fourth metatarsal heads, fifth metatarsal head, hallux and lesser toes by utilizing the ability of the software to create masks over specified locations; we were especially interested in this relationship at the metatarsals which biomechanically were hypothesized to have the strongest relationship between pressure and ulceration. Peak pressure and the pressure–time integral were calculated. Peak pressure is the highest pressure sensed within the mask. The pressure–time integral was calculated as the summation of the total peak pressure experienced in a given location per each time unit; it is a measure of pressure dosage.
2.4. Follow-up data collection
Foot ulcer was defined as a full-thickness skin defect that required more than 14 days to heal. Subjects were re-examined at 12–18 month intervals (mean interval = 13 months) to assess whether the outcome had occurred. Also, subjects were contacted quarterly by mail, and were encouraged to call study staff or drop by the research clinic if they suspected that they had a foot ulcer. Subjects who did not return mailed questionnaires were contacted whenever possible in person at their next scheduled clinic visit. In order to assure capture of incident foot ulcers that were not detected by the above means, study staff publicized the project throughout the VA Medical Center and emphasized the need for clinical providers to notify them of all incident ulcers seen in ambulatory, urgent care, surgical specialty clinics, and other clinical settings. Fluorescent orange labels were affixed to the hard copy medical record problem list reminding providers to check their patients’ feet. As an incentive for this reporting, study staff offered to expedite triage of patients with foot lesions, thereby reducing provider workload.
2.5. Statistical methods
Differences in study participant demographic measures by ulcer outcome were assessed using t-tests (continuous measures) and Fisher’s exact test (categorical measures). As a descriptive analysis, estimates of peak pressure and pressure–time integral means and standard errors by ulcer outcome were obtained from linear mixed effects regression models. Data were analyzed across 4 levels: between subject (n = 591), within subject across foot (n = 1110, 72 pressure data were collected on only one foot), within foot across site (n = 8880, each foot had 8 sites) and within site (n = 43,912, an average of 4.95 trials per subject). To account for non-independence of observations, we utilized robust variance estimates. However, these turned out to be smaller than non-robust standard variance estimates, so we decided to use the more conservative non-robust estimates for significance testing and confidence interval estimation. Cox proportional hazards models estimated the risk for diabetic foot ulcer development in relation to both mean peak pressure and mean pressure–time integral at each plantar foot location (8880 sites). Age was modeled as the time axis in the Cox proportional hazards models and analyses were rerun adding BMI and the presence of neuropathy as covariates to adjust for potential confounding. Results are presented as hazard ratios representing the increase in risk of ulceration with respect to higher pressure or pressure–time integral equal to the magnitude of the interquartile range (the 25th percentile to the 75th percentile) (Harrell, 2001) with likelihood based 95% confidence intervals. Location was accounted for in the analysis, with emphasis on the metatarsal heads, which have been found to have the majority of plantar ulcers (Perell, Merrill, & Nouvong, 2006). Statistical significance was set a priori (p < 0.05). Analyses were carried out using the statistical software package R 2.11.1 (Team RDC. R, 2010).
3. Results
Of the 1522 subjects who were enrolled in the Seattle Diabetic Foot study, plantar pressure measurements were taken on a subset of 596 subjects (supplemental Figure). Across the entire study, 259 ulcers developed during follow-up, and of those, 162 were plantar ulcers. Merging these data sets resulted in 61 plantar ulcers for which baseline pressure data had been collected, but only 47 had all the necessary data for complete analysis. Further data refinement excluded 5 subjects (1 for corrupt pressure data, 4 for pressure data collected after the study censor date), which led to a total of 591 subjects with plantar pressure measurements. Of the 61 plantar ulcers, 11 ulcers occurred before pressure measurement and 3 ulcers occurred on the foot for which no pressure data were available resulting in 47 plantar ulcers available for analysis. Of the 544 study participants who did not develop plantar ulcers, there were 270 who withdrew during the study for various reasons, including non-plantar ulceration (n = 30), death (n = 112), and other unspecified causes (n = 128).
There were no significant differences in the age, sex or BMI of the non-ulcer and ulcer groups (Table 1). The non-ulcer group was followed for a significantly longer period of time (2.5 vs. 1.9 years), while the ulcer group had a significantly longer duration of diabetes (19 vs. 15 years) and a significantly higher percentage of patients with neuropathy (81% vs. 48%).
Table 1.
Study sample characteristics at baseline pressure measurement by ulcer outcome for 591 study participants: mean (range) or frequency (%).
| Non-ulcer (n = 544) | Ulcer (n = 47) | All (n = 591) | p-value | |
|---|---|---|---|---|
| Age (years) | 67 (26–94) | 68 (46–84) | 67 (26–94) | 0.300a |
| Years followed | 2.5 (0–6.2) | 1.9 (0.1–4.5) | 2.4 (0–6.2) | 0.017a |
| Female | 9 (1.7%) | 2 (4.3%) | 11 (1.9%) | 0.215b |
| BMI | 30.3 (18.8–58.9) | 30.0 (19.8–41.4) | 30.3 (18.8–58.9) | 0.659a |
| Duration of DM (years) | 15 (0–69) | 19 (1–56) | 15 (0–69) | 0.012a |
| Neuropathy on either limb | 259 (48%) | 38 (81%) | 297 (50%) | <0.001b |
t-test.
Fisher’s exact test.
The regions with most ulcers were the hallux (n = 19) the combined metatarsals (n = 12), and the heel (n = 10) (Table 2 and Fig. 1). The ulcer areas had peak pressure (mean ± standard error) of 219 ± 16 compared to the non-ulcer areas of 194 ± 2 kPa, and pressure–time integrals of 89 ± 7 compared to the non-ulcer areas of 79 ± 2 kPa s. (Note that a statistical analysis was not performed on the means.) Additionally, the heel and hallux regions actually had higher peak pressures at the non-ulcer sites, while the metatarsals had higher peak pressures at the ulcer sites.
Table 2.
Number of ulcers and mean and [standard error (SE)]a of peak pressure or PTI for each region (n = 43,912 measurements on 8880 sites).
| Region | Number of ulcers per site | Peak pressure (kPa): mean [SE]
|
PTI (kPa s): mean [SE]
|
||
|---|---|---|---|---|---|
| Non-ulcer | Ulcer | Non-ulcer | Ulcer | ||
| Heel | 10 | 266 [3] | 241 [27] | 118 [2] | 147 [17] |
| Lateral midfoot | 1 | 141 [2] | 267 [85] | 70 [1] | 119 [52] |
| Medial midfoot | 0 | 109 [2] | – | 51 [1] | – |
| Fifth metatarsal | 4 | 177 [3] | 220 [43] | 77 [2] | 127 [26] |
| Second to fourth metatarsal | 5 | 308 [5] | 362 [38] | 124 [3] | 142 [23] |
| First metatarsal | 3 | 242 [4] | 383 [50] | 93 [2] | 130 [30] |
| Toes | 5 | 112 [2] | 78 [38] | 38 [1] | 26 [23] |
| Hallux | 19 | 200 [4] | 172 [20] | 56 [1] | 44 [12] |
| All regions | 47 | 194 [2] | 219 [16] | 79 [2] | 89 [7] |
Estimated using linear mixed effects models of pressure or PTI on ulcer occurrence on 43,912 measurements, and random effects for subject, foot within subject and site within foot.
Fig. 1.
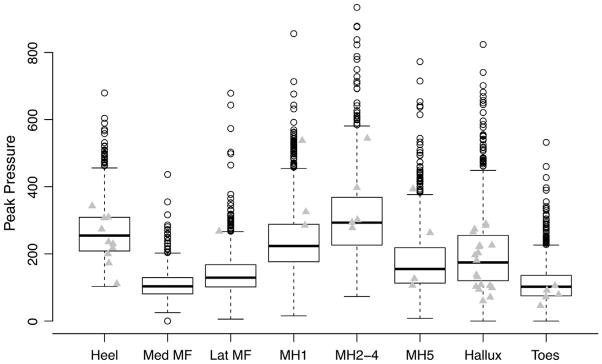
Box plot of mean pressure by plantar measurement location (n = 8880) displaying the minimum, 25th percentile, median, 75th percentile, and maximum values at each location. Grey triangles indicate sites where ulcers developed and the peak pressure for that site in the subject who developed the foot ulcer. (Site abbreviations: Calc, heel; Hal, hallux; Lat.MF, lateral midfoot; Med.MF, medial midfoot; MH1, first metatarsal head; MH2_4, second through fourth metatarsal heads; MH5, fifth metatarsal head; Toe, other toes).
Hazard ratios for risk of diabetic foot ulcer development were estimated (Table 3). When risk of foot ulcer at any site was analyzed in relation to peak pressure, the peak pressure magnitude increment between the 75th and 25th percentiles was nonsignificant (Table 3, Model 1). Adjustment for pressure measurement location further diminished the magnitude of this nonsignificant risk elevation (Table 3, Model 2). However, when hazard ratios were estimated by site, a statistically significant elevation in risk was observed in relation to peak pressure under the metatarsal heads, while the elevation in risk associated with peak pressure at other sites was nonsignificant (Table 3, Models 3a and 3b).
Table 3.
Cox proportional hazards analysis of ulcer occurrence by plantar foot location (n = 8880 sites) in relation to peak pressure or the pressure–time integral (PTI).
| Hazard ratio (95% CI) | p-valuea | |
|---|---|---|
| Peak pressure (75th percentile – 25th percentile) | ||
| Model 1 | ||
| All sites | 1.27 (0.89, 1.74) | 0.171/0.181 |
| Model 2 | ||
| All sites, adjusted for pressure measurement location | 1.01 (0.65, 1.51) | 0.948/0.798 |
| Models 3a and 3b | ||
| Metatarsals | 1.75 (1.01, 2.72) | 0.046/0.034b |
| Other sites | 1.28 (0.79, 1.94) | 0.306/0.287 |
| Pressure–time Integral (75th percentile–25th percentile) | ||
| Model 4 | ||
| All sites | 1.25 (0.94, 1.52) | 0.115/0.143 |
| Model 5 | ||
| All sites, adjusted for pressure measurement location | 1.30 (0.95, 1.62) | 0.095/0.101 |
| Models 6a and 6b | ||
| Metatarsals | 1.35 (0.94, 1.74) | 0.088/0.068 |
| Other sites | 1.28 (0.84, 1.74) | 0.227/0.263 |
Likelihood ratio test, unadjusted p-value/adjusted p-value for baseline BMI and presence of neuropathy assessed as insensitivity to the 5.07 monofilament at 1 or more of 9 locations tested on each foot. Chronologic age was used as the time axis in the proportional hazards models, which represent the increase in risk of ulceration with respect to higher pressure or pressure–time integral equal to the magnitude of the interquartile range (the 25th percentile to the 75th percentile).
Note that models adjusted for BMI and not neuropathy and neuropathy but not BMI yielded nearly identical results (data not shown).
Risk of foot ulcer in relation to the pressure–time integral using proportional hazards models revealed no significant associations across all sites before and after adjustment for pressure measurement location (Table 3, Models 4 and 5). When hazard ratios were estimated by site, no statistically significant elevation in risk was observed in relation to the pressure–time integral (Table 3, Models 6a and 6b).
4. Discussion
Plantar ulceration is a frequent complication for diabetic patients. The etiology of diabetic ulceration is known to be multi-factorial, and while plantar pressure is thought to play an important role, the direct association between elevated baseline pressure and subsequent ulceration at that specific location has not been shown. This study was designed to investigate that issue.
Many investigators have explored plantar pressure and ulceration, finding higher pressure with ulcerative feet both retrospectively (Boulton et al., 1983; Frykberg et al., 1998) and prospectively (Pham et al., 2000; Veves et al., 1992). The most important novel finding of the current study is that elevated plantar pressure is prospectively associated with ulceration under the metatarsal heads. It should be stated that our results were mixed. Despite overall differences in the mean peak pressure between the ulcer and non-ulcer groups, some areas (heel and hallux) actually had higher peak pressure in the non-ulcer group, while others (metatarsals) had higher peak pressure with the ulcer group (Table 2, Fig. 1). A statistical analysis was not performed on the means, but a survival analysis indicated that peak pressure at all sites or at all sites adjusted for location was not a significant predictor of foot ulcer risk. However, when the data were separated into the metatarsals and all other sites, the former association was statistically significant, while the latter was not. Therefore, high peak pressure at the metatarsals predicts an increased risk of ulceration at the metatarsals, but this relationship does not hold elsewhere. In all instances, the pressure–time integral results were not useful predictors of plantar ulceration.
Biomechanically, of the three sites with the highest incidence of ulceration (the heel, the metatarsals and the hallux), there is a plausible explanation as to why only the metatarsals are associated with higher peak pressure. The forefoot bears loads well over body weight with each step and the anatomy of this location is heterogeneous and complex (Bojsen-Moller, 1979) with many tissue types and with thin plantar soft tissue (Gooding et al., 1986). The effect of a tight Achilles tendon, which has been significantly associated, albeit weakly, with higher plantar pressure (Orendurff, Rohr, Sangeorzan, Weaver, & Czerniecki, 2006), would also be most pronounced at the metatarsals. Conversely, the heel and the hallux have simpler anatomy (skin, plantar fat, and bone) with thicker plantar soft tissue (Gooding et al., 1986). It is possible that ulcers occurring at these locations are due to other mechanisms not quantified in this study, such as ischemia or shear stress. In addition, it has been shown previously that plantar pressure during walking is not predictive of hallux pressure during turning, where hallux pressure was much higher (Rozema, Ulbrecht, Pammer, & Cavanagh, 1996). Perhaps the critical loading of the hallux occurs when patients are not walking straight ahead. Furthermore, heel ulcers in an ambulatory population of persons with diabetes as included in this study are sometimes located on the lateral or posterior heel margins, and therefore might not be expected to develop with greater frequency in relation to plantar pressure. The question of lateral or posterior margin pressure in relation to ulceration risk was not addressed. While further investigation is necessary to determine if these speculations are correct, it is, nevertheless, plausible that ulcers at the metatarsals have an association with higher plantar pressure, while the other locations do not.
Previous studies have suggested elevated pressure thresholds of >6 kg/cm2 (Frykberg et al., 1998; Pham et al., 2000) or much larger, up to 11 kg/cm2 (Boulton et al., 1983) or 12.3 kg/cm2 (Veves et al., 1992). Expressed in these units, our average non-ulcer pressure was 1.98 kg/cm2 while the average ulcer pressure was 2.23 kg/cm2, both of which are well below those in other studies. Most likely, the differences seen were due to the non-shod data collection of the previous pressure/ulcer studies, whereas our data were all collected in shoes. A recent study of diabetic neuropathic patients with healed plantar ulcers found that peak pressures were higher when measured barefoot (566 ± 316 kPa) as compared to two in-shoe systems (207 ± 68 and 291 ± 132 kPa) (Owings et al., 2009). Our mean values corresponded to other shod studies with same sensor system (F-Scan) (Hosein & Lord, 2000) or with different sensor systems (Pedar, Novel, Munich, Germany) (Putti, Arnold, Cochrane, & Abboud, 2007). Moreover, we would suggest that in-shoe studies in the patient’s usual footwear generate useful pressure measurement values, as they represent the pressure to which the subject will frequently be exposed to, and in this analysis these pressure measurements were related to risk of developing foot ulceration. Another possible explanation is that peak pressure values are dependent on sensor size, with larger sensors resulting in smaller peak pressures due to the intrinsic averaging effect. Boulton et al. and Veves et al., who found the highest thresholds, used optical pedobarographs with resolutions presumably on the order of 1 mm2 (Boulton et al., 1983; Veves et al., 1992), while Frykberg et al. and Pham et al., who had lower thresholds, both used the F-Scan Mat (Tekscan, Boston, MA) with a 5 mm2 sensor size (Frykberg et al., 1998; Pham et al., 2000). The F-scan in-shoe sensor used in the present study had a similar sensor size (3.9 sensors per cm2).
We found no overall association between plantar pressure and foot ulcer risk in contrast to reports from two other prospective studies (Pham et al., 2000; Veves et al., 1992). This discrepancy may be due to major differences in study methodology. First, the above mentioned differences in measurement techniques (in-shoe v. pressure platform). Second, we included patients with diabetes receiving primary care as compared to the patients studied by Pham et al. and Veves et al., who were referred for either foot care or diabetes specialty care. Our population therefore probably had less severe or complicated diabetes, but those with a more complicated clinical course were eligible for and had available specialty resources for podiatric, orthopedic, and endocrinologic care.
Concerning the presence of neuropathy — it was noted that fully 19% of the patients who developed plantar ulcers were non-neuropathic at baseline. In two prospective studies on this topic, prevalence of monofilament insensitivity among persons who developed foot ulcer was 69% (Monteiro-Soares & Dinis-Ribeiro, 2010) and 91% (Pham et al., 2000). Thus our data do not seem out of line with other reports in the literature demonstrating a similar proportion of persons who develop plantar ulceration without having neuropathy. Moreover, the frequency of ulceration at each location was very similar with or without ulceration. For example, of the 47 ulcers, 9 occurred in non-neuropathic subjects. These 9 ulcers occurred at similar frequencies at the heel (n = 2, 22% of ulcers) and hallux (n = 3, 33%) as in the neuropathic subjects at the heel and hallux (n = 8, 21% and n = 16, 42%, respectively).
Our study had several potential limitations. We only considered vertical plantar pressure. The importance of shear stress has been suggested elsewhere (Delbridge, Ctercteko, Fowler, Reeve, & Le Quesne, 1985; Yavuz et al., 2007), but there is still no commercially available in-shoe (or platform) shear stress sensor. Our analysis did not include a measure of total pressure dosage, other than pressure–time integral, and we made no attempt to quantify activity level (e.g., steps per day) with the subject population. The second to fourth metatarsals were grouped together, thus we are unable to isolate second metatarsal ulcers, arguably one of the more important areas beneath the foot. Our results, which were obtained primarily from a mostly male, Veteran population, may not be generalizable to the population as a whole. Additionally, velocity during plantar pressure data collection was not quantified. Also, footwear was not controlled, but rather, subjects were asked to wear their “usual” shoes. It is possible that ulcers developed during barefoot walking at home or when the subjects were wearing some other pair of shoes that were not tested. Furthermore, the follow-up time between baseline assessment and ulceration was up to 4.5 years and it is certainly possible that the foot or footwear characteristics changed over time. Finally, there are five potential statistical issues for consideration. First, due to the relatively low number of ulcer cases and potential informative censoring, our analysis has limited statistical power, (i.e., nonsignificant findings may not be negative findings due to a Type 2 error). Second, given the small number of metatarsal head ulcers, the site-specific models in Table 3 that include 3 covariates may be considered to violate a rule of thumb regarding the number of outcomes needed for each independent variable included in the model (Peduzzi, Concato, Feinstein, & Holford, 1995). This rule of thumb, though, has been found to be too conservative in more recent simulation studies (Vittinghoff & McCulloch, 2007). Furthermore, as mentioned in a Table 3 footnote, models that include either BMI or neuropathy alone and therefore have a higher ratio of events per covariate result in similar results to the model shown that includes both of these covariates. Third, since the data were censored with different durations of follow-up by subject, p-values were not very meaningful when comparing the means between the groups (Table 2), hence our use of the Cox models, which permit valid comparisons of survival times in the presence of censoring. Fourth, there was a difference in follow-up time between the two groups — since ulcerated subjects were followed for a shorter period of time, it is possible that ulcers on the contralateral limb would have developed had the subjects remained in enrolled, resulting in some feet being inappropriately classified as non-ulcerated. Lastly, a larger number of patients died than those who developed foot ulcers, which could potentially lead to informative censoring as risk of ulceration may not be independent of mortality risk, and might lead to biased associations toward null findings due to the occurrence of death prior to the development of foot ulcer.
In summary, we have conducted a prospective study examining the association between baseline plantar pressure at a specific location beneath the foot and the subsequent development of a plantar ulcer at that same location. Our study has shown a significantly higher peak pressure at baseline for diabetic subjects who developed metatarsal head ulcers, but this relationship did not hold for other locations beneath the foot (i.e., the heel and hallux), where higher baseline plantar pressure was not predictive of ulceration.
Supplementary Material
Acknowledgments
The authors would like to thank Ruby Forsberg from the VA Puget Sound for the pressure data collection and Peter R. Cavanagh, PhD DSc, from the University of Washington for his careful read of the manuscript and for his editorial suggestions.
Footnotes
Funding: This work was supported in part by the Department of Veterans Affairs, RR&D service grant numbers A2661C, A4843C and A99-1499A.
A preliminary version of this analysis was presented in the proceedings of the 65th Annual Scientific Sessions of the American Diabetes Association, San Diego, CA: 2005.
The authors have no conflict of interest.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jdiacomp.2013.07.004.
References
- Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999;22(7):1029–1035. doi: 10.2337/diacare.22.7.1029. [DOI] [PubMed] [Google Scholar]
- Ahroni JH, Boyko EJ, Forsberg R. Reliability of F-scan in-shoe measurements of plantar pressure. Foot & Ankle International. 1998;19(10):668–673. doi: 10.1177/107110079801901004. [DOI] [PubMed] [Google Scholar]
- Ahroni JH, Boyko EJ, Forsberg RC. Clinical correlates of plantar pressure among diabetic veterans. Diabetes Care. 1999;22(6):965–972. doi: 10.2337/diacare.22.6.965. [DOI] [PubMed] [Google Scholar]
- Bojsen-Moller F. Anatomy of the forefoot, normal, and pathologic. 1979 July August;142:10–8. [PubMed] [Google Scholar]
- Boulton AJ, Hardisty CA, Betts RP, Franks CI, Worth RC, Ward JD, et al. Dynamic foot pressure and other studies as diagnostic and management aids in diabetic neuropathy. Diabetes Care. 1983;6(1):26–33. doi: 10.2337/diacare.6.1.26. [DOI] [PubMed] [Google Scholar]
- Boyko EJ, Ahroni JH, Cohen V, Nelson KM, Heagerty PJ. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: The Seattle Diabetic Foot Study. Diabetes Care. 2006;29(6):1202–1207. doi: 10.2337/dc05-2031. [DOI] [PubMed] [Google Scholar]
- Boyko EJ, Ahroni JH, Stensel V, Forsberg RC, Davignon DR, Smith DG. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care. 1999;22(7):1036–1042. doi: 10.2337/diacare.22.7.1036. [DOI] [PubMed] [Google Scholar]
- CDCP. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- Cowley MS, Boyko EJ, Shofer JB, Ahroni JH, Ledoux WR. Foot ulcer risk and location in relation to prospective clinical assessment of foot shape and mobility among persons with diabetes. Diabetes Research and Clinical Practice. 2008;82(2):226–232. doi: 10.1016/j.diabres.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Delbridge L, Ctercteko G, Fowler C, Reeve TS, Le Quesne LP. The aetiology of diabetic neuropathic ulceration of the foot. British Journal of Surgery. 1985;72(1):1–6. doi: 10.1002/bjs.1800720102. [DOI] [PubMed] [Google Scholar]
- Frykberg RG, Lavery LA, Pham H, Harvey C, Harkless L, Veves A. Role of neuropathy and high foot pressures in diabetic foot ulceration. Diabetes Care. 1998;21(10):1714–1719. doi: 10.2337/diacare.21.10.1714. [DOI] [PubMed] [Google Scholar]
- Gooding GA, Stess RM, Graf PM, Moss KM, Louie KS, Grunfeld C. Sonography of the sole of the foot. Evidence for loss of foot pad thickness in diabetes and its relationship to ulceration of the foot. Investigative Radiology. 1986;21(1):45–48. [PubMed] [Google Scholar]
- Harrell FJ. Regression modeling strategies: With application to linear models, logistic regression and survival analysis. New York, NY: Springer-Verlag; 2001. [Google Scholar]
- Hosein R, Lord M. A study of in-shoe plantar shear in normals. Clinical biomechanics. 2000;15(1):46–53. doi: 10.1016/s0268-0033(98)00059-x. [DOI] [PubMed] [Google Scholar]
- Monteiro-Soares M, Dinis-Ribeiro M. External validation and optimisation of a model for predicting foot ulcers in patients with diabetes. Diabetologia. 2010;53(7):1525–1533. doi: 10.1007/s00125-010-1731-y. [DOI] [PubMed] [Google Scholar]
- Orendurff MS, Rohr ES, Sangeorzan BJ, Weaver K, Czerniecki JM. An equinus deformity of the ankle accounts for only a small amount of the increased forefoot plantar pressure in patients with diabetes. The Journal of Bone and Joint Surgery. 2006;88(1):65–68. doi: 10.1302/0301-620X.88B1.16807. British Volume. [DOI] [PubMed] [Google Scholar]
- Owings TM, Apelqvist J, Stenstrom A, Becker M, Bus SA, Kalpen A, et al. Plantar pressures in diabetic patients with foot ulcers which have remained healed. Diabetic medicine: a journal of the British Diabetic Association [Multicenter Study] 2009;26(11):1141–1146. doi: 10.1111/j.1464-5491.2009.02835.x. [DOI] [PubMed] [Google Scholar]
- Peduzzi P, Concato J, Feinstein AR, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. Journal of Clinical Epidemiology. 1995;48(12):1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- Perell KL, Merrill V, Nouvong A. Location of plantar ulcerations in diabetic patients referred to a Department of Veterans Affairs podiatry clinic. Journal of Rehabilitation Research and Development. 2006;43(4):421–426. doi: 10.1682/jrrd.2005.10.0157. [DOI] [PubMed] [Google Scholar]
- Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: A prospective multicenter trial. Diabetes Care. 2000;23(5):606–611. doi: 10.2337/diacare.23.5.606. [DOI] [PubMed] [Google Scholar]
- Putti AB, Arnold GP, Cochrane L, Abboud RJ. The Pedar in-shoe system: Repeatability and normal pressure values. Gait & Posture. 2007;25(3):401–405. doi: 10.1016/j.gaitpost.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Reiber GE, Vileikyte L, Boyko EJ, del Aguila M, Smith DG, Lavery LA, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22(1):157–162. doi: 10.2337/diacare.22.1.157. [DOI] [PubMed] [Google Scholar]
- Rozema A, Ulbrecht JS, Pammer SE, Cavanagh PR. In-shoe plantar pressures during activities of daily living: Implications for therapeutic footwear design. Foot & Ankle International. 1996;17(6):352–359. doi: 10.1177/107110079601700611. [DOI] [PubMed] [Google Scholar]
- Team RDC. R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: A prospective study. Diabetologia. 1992;35(7):660–663. doi: 10.1007/BF00400259. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. American Journal of Epidemiology. 2007;165(6):710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- Yavuz M, Erdemir A, Botek G, Hirschman GB, Bardsley L, Davis BL. Peak plantar pressure and shear locations: Relevance to diabetic patients. Diabetes Care. 2007;30(10):2643–2645. doi: 10.2337/dc07-0862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


