Abstract
Activation of the carotid body chemoreceptors with hypoxia alters baroreceptor mediated responses. We aimed to examine whether this relationship can be translated to other chemoreceptor stimuli (i.e. hypoglycaemia) and hypothesized: 1) activation of the carotid body chemoreceptors with hypoglycaemia would reduce spontaneous cardiac baroreflex sensitivity (sCBRS) in healthy humans and, 2) desensitization of the carotid chemoreceptors with hyperoxia would restore sCBRS to baseline levels during hypoglycaemia. Ten young healthy adults completed two 180-min hyperinsulinaemic (2 mU.kg FFM−1.min−1), hypoglycaemic (~3.2 µmol.mL−1) clamps, separated by at least one week and randomized to normoxia (PaO2 122±10 mmHg) or hyperoxia (PaO2 424±123 mmHg; to blunt activation of the carotid body glomus cells). Changes in heart rate, blood pressure, plasma catecholamines, heart rate variability (HRV), and sCBRS were assessed. During hypoglycaemia, HRV and sCBRS were reduced (p<0.05) and the baroreflex working range was shifted to higher heart rates. When hyperoxia was superimposed on hypoglycaemia, there was a greater reduction in blood pressure and a blunted rise in heart rate when compared to normoxic conditions (p<0.05); however, there was no detectable effect of hyperoxia on sCBRS or HRV during hypoglycaemia (p>0.05). In summary, hypoglycaemia-mediated changes in HRV and sCBRS cannot be exclusively attributed to the carotid chemoreceptors; however, the chemoreceptors appear to play a role in resetting the baroreflex working range during hypoglycaemia.
Keywords: blood pressure, hyperoxia, heart rate variability
INTRODUCTION
The carotid chemoreceptors are known for their oxygen-sensing capabilities (von Euler et al., 1940; Biscoe & Sampson, 1967), and recent findings from both animals (Alvarez-Buylla et al., 1997; Koyama et al., 2000; Koyama et al., 2001) and humans (Ward et al., 2007; Wehrwein et al., 2010) suggest they also play an important role in glucoregulation. Chemoreceptor activation has been shown to reduce baroreflex sensitivity (Cooper et al., 2005) and/or shift the baroreflex stimulus-response curve to higher blood pressures and heart rates during hypoxia (Halliwill et al., 2003; Cooper et al., 2004; Monahan et al., 2006; Querido et al., 2011). In this context, it is reasonable to propose activation of carotid chemoreceptors via reductions in plasma glucose levels also influences baroreflex-mediated physiological responses. Consistent with this idea, antecedent hypoglycaemia has been shown to reduce heart rate variability (HRV) and baroreflex sensitivity (Adler et al., 2009). Furthermore, work from Faigus and Berne (1991) suggests the baroreflex working range is reset with exposure to acute hypoglycemia (Fagius & Berne, 1991). However, these hypoglycaemia-mediated changes have not been directly attributed to the carotid chemoreceptors.
Under hyperoxic conditions, carotid sinus nerve afferent activity is reduced (Fitzgerald & Lahiri, 1986). Furthermore, we have shown that the regulatory response to hypoglycaemia can be blunted by desensitization of the carotid chemoreceptors with normocapnic hyperoxia (Wehrwein et al., 2010) and blood pressure responses to hypoglycaemia are altered under hyperoxic conditions in humans (Wehrwein et al., 2012). Based on these observations we hypothesized: 1) hypoglycaemia would result in a reduction in spontaneous cardiac baroreflex sensitivity (sCBRS) and HRV, and 2) desensitizing the carotid chemoreceptors with normocapnic hyperoxia would improve sCBRS and HRV during hypoglycaemia in healthy humans.
METHODS
Subjects and ethical approval
Informed consent was obtained from all subjects and all experiments and procedures were approved by the Institutional Review Board at the Mayo Clinic and conformed to the Declaration of Helsinki. Twelve subjects were recruited and 10 young, healthy subjects completed the current study (data from 2 subjects were excluded due to issues with physiological monitoring). Data from five of the current subjects were included in previous publications (Wehrwein et al., 2010; Wehrwein et al., 2012). Subjects were non-smokers, normotensive, and non-obese (BMI <30). All subjects had a physical examination including detailed medical history and were excluded if they exhibited abnormal fasting glucose or lipids, a history of heart disease, diabetes, diagnosed autonomic disorders, and/or other conditions or medications that might alter metabolism. Subjects who engaged in regular physical exercise programs or were actively losing weight were excluded. Dietary advice was provided by a research dietician to ensure subjects maintained constant body weight two weeks prior to their study days. Body composition was measured using dual energy x-ray absorptiometry (DEXA, Lunar iDXA software version 6.10, GE Healthcare Technologies, Madison, WI). Subjects refrained from exercise, alcohol, and caffeine for at least 24 h prior to each study visit.
Monitoring
Preceding the start of the hyperinsulinaemic hypoglycaemic clamp, a 20-gauge, 5 cm brachial artery catheter was placed under ultrasound guidance, after local anesthesia, for blood sampling and blood pressure monitoring (TruWave Pressure Transducer; Edwards Lifesciences; Irvine, CA, USA). Two intravenous catheters were placed in the arm opposite the brachial arterial catheter for insulin and glucose infusions. Heart rate was monitored with a 5-lead electrocardiogram (ECG), respirations via a pneumobelt, and arterial oxygen saturation by a pulse oximeter (Cardiocap/5, Datex-Ohmeda,).
Hypoglycaemic clamps
Subjects were admitted to the Clinical Research Unit (CRU) of the Mayo Clinic the evening prior to each study day (at 1700 hours). A standard 10 cal.kg−1 meal (55% carbohydrate, 30% fat, and 15% protein) was eaten between 1800 and 1830 hours and the subject fasted thereafter until the end of the study. Beginning at 0900 (T0), intravenous insulin (Novolin®, Novo Nordisk Inc., Princeton, NJ, USA) was infused at a constant rate of 2.0 mU.kg FFM−1.min−1 and exogenous glucose [50% Dextrose solution (Hospira, Inc., Lake Forest, IL, USA)] was infused in amounts sufficient to maintain hypoglycaemia (~3.2 µmol glucose.mL−1). Plasma glucose was measured every 5–10 minutes (Analox Instruments USA Inc., Lunenberg, Massachusetts). For a more detailed description of hyperinsulinemic hypoglycaemic clamps, see (Basu et al., 2004; Ward et al., 2007; Wehrwein et al., 2010).
Hyperoxia and normoxia
From T0 until the end of the study (T180), subjects breathed either room air (21% oxygen; normoxia) or 100% oxygen (hyperoxia) via a face mask connected to a non-rebreathing valve and a large meteorological balloon which served as a volume reservoir. Hyperoxia was used to desensitize the carotid body chemoreceptors; hyperoxia has been shown to limit the ability of the Type I glomus cells to release neurotransmitter in response to low glucose – resulting in attenuated activation of the carotid sinus nerve afferents (Downes & Lambertsen, 1966; Lahiri & DeLaney, 1975; Wehrwein et al., 2010). Normoxic and hyperoxic trials occurred on different days separated by at least one week, and the order was randomized. Arterial blood gases (PaO2, PaCO2) were measured at baseline and throughout the clamp. Confirming the effectiveness of the hyperoxic exposure, there was a significant increase in PaO2 during hyperoxia which was not observed during normoxia (Main effect of condition, p<0.01; Interaction of condition and time, p<0.01).
Spontaneous cardiac baroreflex sensitivity (sCBRS)
sCBRS was assessed from 30-minute sections of data during euglycemic baseline [T−30 - T0 (baseline)] and steady-state hypoglycaemia [T150 – T180 (clamp)]. The distances between all R-wave peaks of the ECG recording were calculated and paired with the systolic pressure wave amplitude of the preceding beat. A computer software program (LabChart7; ADinstruments, Colorado Springs, CO) selected all sequences of three or more successive heart beats in which there were concordant increases or decreases in systolic blood pressure and R-R interval. The recordings were reviewed and non-sinus beats and segments with artifacts were removed. A linear regression was applied to each of the sequences manually and only relationships with an R2>0.80 were accepted. An average regression slope, representing the cardiac baroreflex sensitivity (ms.mmHg−1) was calculated for the acceptable sequences (average 55±8 systolic blood pressure/R-R interval pairs per segment). Responses were also evaluated by plotting the changes in systolic pressures with heart rate to take into consideration the mathematical constraint of the hyperbolic relationship between R-R interval and heart rate. The “operating points” for the relationships were determined as the average heart rate and systolic blood pressure from 5-minutes of data collected during selected timepoints, using similar methods published previously (Halliwill et al., 2003).
Heart rate variability (HRV)
Short-term data selections (Range 2–5 minutes) from a 5-lead ECG recording were analyzed during euglycemic baseline [T-30 – T0 (baseline)] and steady-state hypoglycaemia [T150–T180 (clamp)]. Physiologically stable conditions were confirmed by visual checks, ensuring only stationary segments were selected for analysis (avoiding ectopic beats, arrhythmias, missing data, and noise). A computer program (HRV Module, LabChart7, ADInstruments Pty Ltd, Australia) was used to assess both time and frequency (Fast Fourier transformation, Welsh windowing function) domains, including: Mean NN interval (time between normal cardiac cycles, reported in ms), Low Frequency normalized [LF; range: 0.04 – 0.15 Hz; reported in normalized units (nu)], High Frequency normalized [HF; range: 0.15–0.4 Hz; reported in normalized units (nu)], and Low Frequency/High Frequency ratio.
Analytical methods
Arterial blood was drawn for measures of glucose, insulin, and catecholamines during euglycemic baseline [T−30 -T0 (baseline)] and steady-state hypoglycaemia [T150–T180 (clamp)]. Arterial blood gas samples were analyzed immediately using an automatic blood gas analyzer (Radiometer ABL700; Westlake, OH, USA). All additional blood samples were immediately placed on ice and centrifuged at 4°C after which time the plasma was removed and stored at −80°C until analysis. Plasma insulin was assessed using a two-site immunoenzymatic assay performed on the DxI automated immunoassay system (Beckman Instruments, Chaska, MN). Plasma catecholamines (epinephrine, norepinephrine) were measured with reverse phase high performance liquid chromatography with electrochemical detection after extraction with activated alumina.
Data analysis and statistics
All data were collected using a PowerLab data acquisition system (analog to digital converter; ADinstruments, Inc., Colorado Springs, CO, USA), with a sampling rate of 1000 Hz. Each subject was assessed under both experimental conditions (normoxia and hyperoxia). Two-way repeated measures analysis of variance (ANOVA) was performed to compare the effect of condition (normoxia, hyperoxia) and time (baseline, clamp) on main outcome variables. Additionally, one-way repeated measures ANOVA was used to compare sCBRS and absolute changes (Δ) in main outcome variables from baseline (baseline – clamp). Post-hoc analysis was completed using the Bonferroni test. Data are reported as Mean ± Standard Deviation. Statistical significance (one-tailed) was determined a priori at the α=0.05 level and analysis was completed using SigmaPlot Version 12.0 (Systat Software, Inc.; San Jose, CA). All data are reported as Mean ± Standard Error (SE).
RESULTS
Ten young, healthy subjects completed the current study (Table 1).
Table 1.
Subject Demographics
| Characteristics | N=10 |
|---|---|
| Sex (M/F) | 7/3 |
| Age (years) | 25±1 |
| Height (cm) | 177±2 |
| Weight (kg) | 75±3 |
| Fat free mass (kg) | 56±3 |
| BMI (kg/m2) | 24±1 |
| Body fat (%) | 25±2 |
| Hemoglobin (g/dL) | 14±1 |
| Glucose (mg/dL) | 83±2 |
| Total Cholesterol (mg/dL) | 147±11 |
| Triglycerides (mg/dL) | 78±7 |
| HDL (mg/dL) | 55±6 |
| LDL (mg/dL) | 76±6 |
Mean±SE
BMI = body mass index
HDL = high density lipoproteins
LDL = low density lipoproteins
Plasma glucose, insulin and catecholamines
The hyperinsulinaemic hypoglycaemic clamp resulted in higher plasma insulin, with a concurrent reduction in plasma glucose concentration, and these changes were similar between normoxia and hyperoxia (Main effect of time, p<0.01; Main effect of condition, p>0.05). Despite similar plasma glucose levels between conditions, the glucose infusion rate required to maintain hypoglycaemia was significantly higher during hyperoxia when compared to normoxia (Table 2; Main effect of condition, p<0.01; Main effect of time, p<0.01; Interaction of condition and time, p<0.01). Plasma epinephrine and norepinephrine concentrations increased during the clamp (Main effect of time, p<0.01 and p<0.01, respectively), and both tended to be lower during hyperoxia when compared to normoxia (Table 2; Epinephrine: Main effect of condition, p=0.03; Interaction of condition and time, p=0.03; Norepinephrine: Main effect of condition, p=0.17; Interaction of condition and time, p=0.10).
Table 2.
Changes in key variables during the hyperinsulinaemic-hypoglycemic clamp under normoxic and hyperoxic conditions.
| Study Timepoint | Change Clamp - Baseline |
||
|---|---|---|---|
| Baseline | Clamp | ||
| PaO2 (mmHg) | |||
| Normoxia | 108±3 | 122±3 | 14±3 |
| Hyperoxia | 110±6 | 424±39*a | 313±38* |
| PaCO2 (mmHg) | |||
| Normoxia | 41±1 | 41±1a | −1±1 |
| Hyperoxia* | 41±1 | 39±1a | −2±1 |
| Insulin (uU/mL) | |||
| Normoxia | 4±1 | 132±8a | 128±8 |
| Hyperoxia | 4±1 | 132±7a | 128±7 |
| Glucose (umol/mL) | |||
| Normoxia | 5.4±0.1 | 3.4±0.1a | −2.0±0.1 |
| Hyperoxia | 5.5±0.1 | 3.3±0.1a | −2.2±0.1 |
| Glucose Infusion Rate (umol/kg FFM/min) | |||
| Normoxia | ---- | 28±4a | 28±4 |
| Hyperoxia | ---- | 37±3*a | 37±3* |
| Norepinephrine (pg/mL) | |||
| Normoxia | 206±20 | 355±40a | 149±29 |
| Hyperoxia | 203±19 | 312±39a | 109±29 |
| Epinephrine (pg/mL) | |||
| Normoxia | 28±4 | 748±101a | 720±99 |
| Hyperoxia | 32±6 | 577±85*a | 545±84* |
Mean±SE, n=10
Effect of Condition: *p<0.05 vs Normoxia
Effect of Timepoint: ap<0.05 vs Baseline
Respiration, blood pressure, and heart rate
Respiratory rate increased and PaCO2 decreased with hypoglycemia (Respiratory rate Δ: ~2 breaths.min−1, Main effect of time, p<0.01; PaCO2 Δ: ~1 mmHg, Main effect of time, p<0.01). These changes were not different between conditions (Respiratory rate: Interaction of condition and time, p=0.27; PaCO2: Interaction of condition and time, p=0.12). See Tables 2 and 3.
Table 3.
Hemodynamic responses to hypoglycaemia under normoxic and hyperoxic conditions.
| Study Timepoint | Change Clamp - Baseline |
||
|---|---|---|---|
| Baseline | Clamp | ||
| Respiratory Rate (breath/min) | |||
| Normoxia | 16±1 | 18±1a | 2±1 |
| Hyperoxia | 16±1 | 17±1a | 1±1 |
| Heart Rate (beat/min) | |||
| Normoxia | 59±2 | 75±4a | 16±3 |
| Hyperoxia | 62±3* | 69±3*a | 7±2* |
| Systolic Blood Pressure (mmHg) | |||
| Normoxia | 128±4 | 131±9 | 3±6 |
| Hyperoxia | 131±5 | 126±7 | −5±3* |
| Diastolic Blood Pressure (mmHg) | |||
| Normoxia | 65±2 | 57±3a | −8±2 |
| Hyperoxia | 68±2 | 56±3a | −12±2* |
| Mean Blood Pressure (mmHg) | |||
| Normoxia | 86±3 | 82±4a | −4±2 |
| Hyperoxia | 89±3 | 79±4a | −10±2* |
| Pulse Pressure (mmHg) | |||
| Normoxia | 63±3 | 74±7a | 11±7 |
| Hyperoxia | 63±3 | 70±5a | 7±4 |
Mean±SE, n=10
Effect of Condition: *p<0.05 vs Normoxia
Effect of Timepoint: ap<0.05 vs Baseline
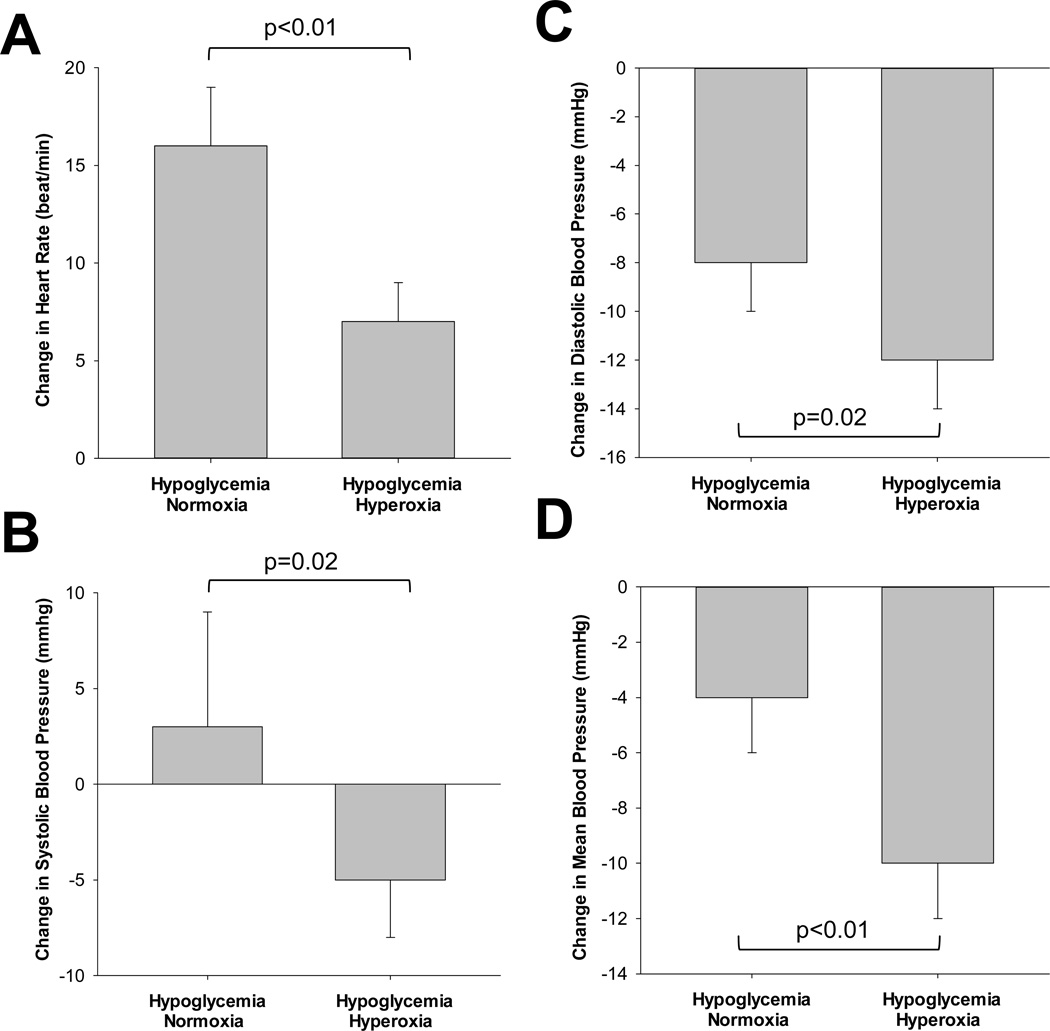
Heart rate increased and diastolic and mean blood pressures decreased during the clamp (Table 3, Main effect of time, p<0.01). No significant changes in systolic blood pressure were observed (Main effect of time, p=0.42; Main effect of condition, p=0.41). Hypoglycemia-mediated reductions in blood pressure were significantly lower during hyperoxia vs. normoxia (Figure 1; Δ: Systolic p=0.02, Diastolic p=0.02, and Mean p<0.01). Despite lower blood pressures, increases in heart rate with hypoglycaemia were blunted during hyperoxia when compared with normoxia (Interaction of condition and time, p<0.01; Table 3 and Figure 1).
Figure 1.
Heart rate and blood pressure responses to hyperinsulinaemic-hypoglycaemia under normoxic and hyperoxic conditions. Change from baseline (Mean±SEM). A. Heart rate, B. Systolic blood pressure, C. Diastolic blood pressure, D. Mean blood pressure. When hyperoxia was superimposed on hypoglycaemia there was a greater reduction in blood pressure and a blunted rise in heart rate when compared to normoxic conditions
Spontaneous cardiac baroreflex sensitivity (sCBRS)
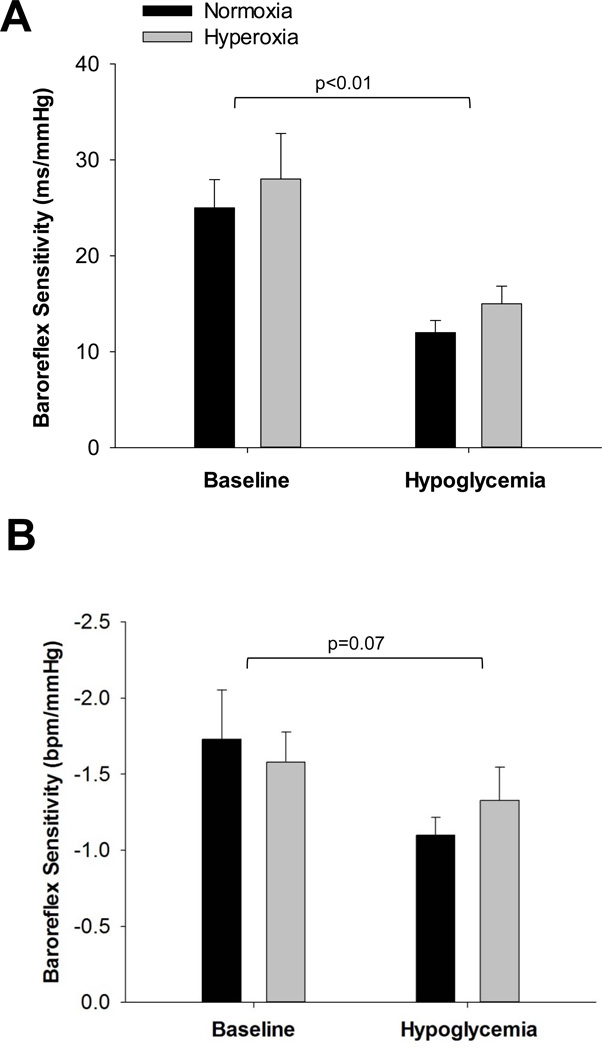
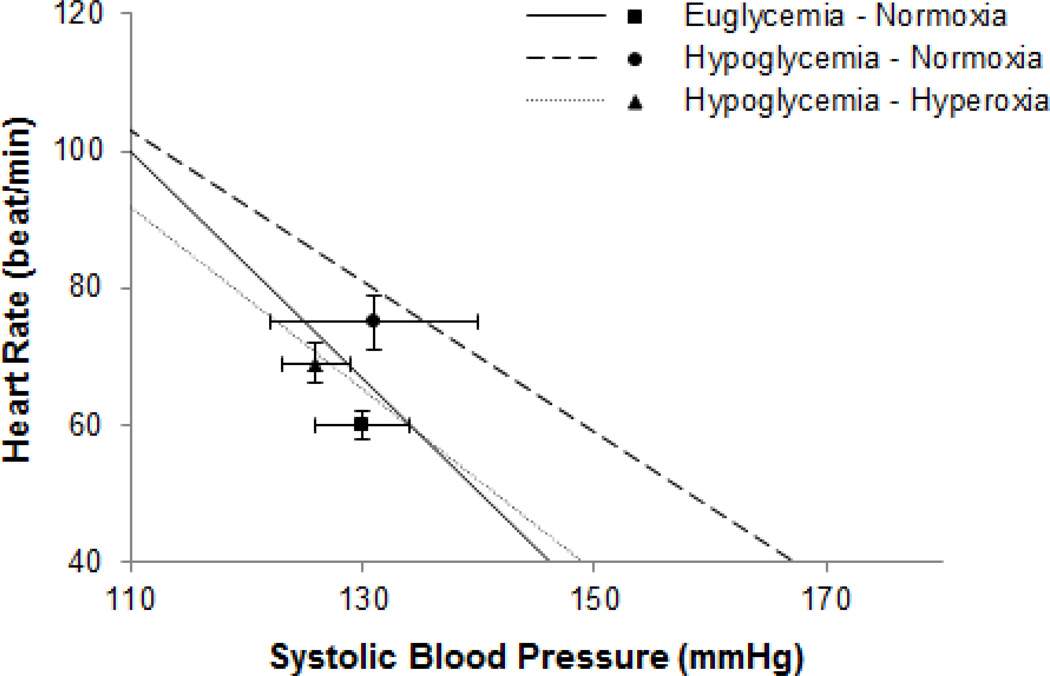
sCBRS was reduced from baseline during hypoglycaemia [Main effect of time, p<0.01 (Figure 2A) and p=0.07 (Figure 2B)]. Hypoglycemia resulted in an upward shift in the baroreflex relationship (as reflected by an increase in heart rate, Figures 1 and 3). There was no detectable effect of hyperoxia on sCBRS during hypoglycaemia [Interaction of condition and time, p=0.47 (Figure 2A) and p=0.12 (Figure 2B)]. However, when hypoglycaemia was superimposed with hyperoxia, the baroreflex stimulus-response curve shifted back toward baseline levels (as reflected by a significant change in systolic blood pressure and a blunted rise in heart rate, Figures 1 and 3).
Figure 2.
Spontaneous cardiac baroreflex sensitivity (sCBRS) during hyperinsulinaemichypoglycaemia under normoxic and hyperoxic conditions. (Mean±SEM) A. Absolute measures of baroreflex sensitivity (relationship between R-R interval and systolic blood pressure). B. Absolute measures of baroreflex sensitivity (relationship between heart rate and systolic blood pressure). sCBRS tended to be reduced from baseline during hypoglycaemia and there was no detectable effect of hyperoxia.
Figure 3.
Spontaneous cardiac baroreflex sensitivity during hyperinsulinaemichypoglycaemia under normoxic and hyperoxic conditions. Group average regressions between heart rate and systolic blood pressure are presented with operating points (Mean±SEM). Baseline sCBRS measures were not different between visits (p=0.26) and were thus averaged. sCBRS was significantly reduced from baseline during hypoglycemia and the baroreflex working range was shifted to higher heart rates. Hyperoxia did not alter sCBRS, however the baroreflex working range was shifted back toward baseline levels.
Heart rate variability (HRV)
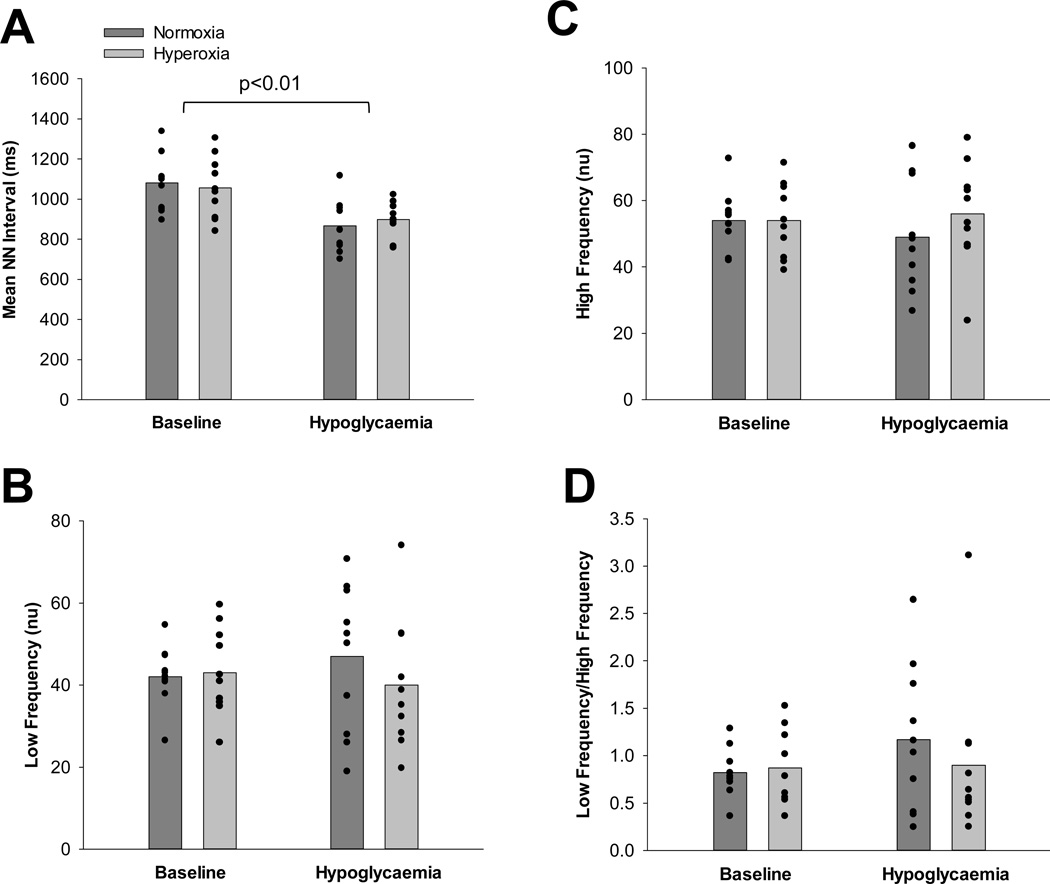
Mean NN Interval was significantly reduced from baseline during hypoglycaemia under both normoxic and hyperoxic conditions (Main effect of time, p<0.01; Δ p=0.21). Although there were trends, changes in low Frequency (nu), High Frequency (nu), and the ratio between Low Frequency and High Frequency (LF/HF) with hypoglycaemia were not detected (Main effect of condition: p=0.17, p=0.12, p=0.21, respectively). Additionally, no changes in Low Frequency (nu), High Frequency (nu), or the ratio between Low Frequency and High Frequency (LF/HF) were observed with hyperoxia (Δ p=0.21, p=0.23, p=0.21, respectively). See Figure 4. Although reductions in HRV with hypoglycaemia were not reversed with hyperoxia, this was primarily driven by the response from a single individual. When this subject was removed from the analysis, hyperoxia tended to attenuate any effect of hypoglycaemia on HRV (Mean NN Interval, Main effect of condition, p=0.08) including a reversal of the effects on cardiovagal tone (High Frequency, Main effect of condition, p=0.12) and sympathovagal balance (LF/HF, Main effect of condition, p=0.10).
Figure 4.
Heart rate variability at baseline and during hyperinsulinaemic-hypoglycaemia under normoxic and hyperoxic conditions (Mean and Individual data points). A. Mean NN Interval (time between normal cardiac cycles, reported in ms). B. Low Frequency normalized [reported in normalized units (nu)]. C. High Frequency normalized [reported in normalized units (nu)]. D. Low Frequency/High Frequency ratio. Although reductions in HRV with hypoglycaemia were not reversed with hyperoxia, this was primarily driven by the response from a single individual. When this subject was removed from the analysis, hyperoxia tended to attenuate any effect of hypoglycaemia on HRV (Mean NN Interval, p=0.08) including a reversal of the effects on cardiovagal tone (High Frequency, p=0.12) and sympathovagal balance (LF/HF, p=0.10).
DISCUSSION
Novel findings from the current study identified a reduction in HRV, sCBRS, and a shift in the baroreflex working range to higher heart rates during hypoglycemia. The changes in HRV and sCBRS during hypoglycemia cannot be exclusively attributed to the carotid body chemoreceptors, given responses could not be reversed with hyperoxia. However, the carotid body chemoreceptors appear to play a role in resetting the baroreflex working range to higher heart rates in response to a reduction in plasma glucose levels.
Changes in Autonomic Function with Hypoglycaemia
In response to a reduction in blood pressure, the baroreflex initiates reflex increases in heart rate, contractility, vascular resistance, and venous return in order to maintain blood pressure at optimal levels. The ability to adapt to challenging conditions is known as “baroreflex sensitivity (BRS)” and a reduction in BRS is often a sign of malfunction (Lanfranchi & Somers, 2002). Recently, acute antecedent hypoglycaemia was shown to result in a significant decrease in sCBRS and altered HRV (Adler et al., 2009). Along these lines, we observed a reduction in Mean NN Interval (Figure 4) and sCBRS (Figure 2) during the hyperinsulinaemic hypoglycaemic clamp. Further, we observed a shift in the baroreflex working range to higher heart rates (Figures 1 and 3) – allowing the baroreceptors to detect small fluctuations in pressure at a new level. These findings are consistent with our initial hypothesis that hypoglycaemia would result in altered sCBRS and HRV; however, the potential mechanisms behind these observations were previously unknown.
Role of the Carotid Chemoreceptors
As shown previously (Wehrwein et al., 2010), desensitization of the carotid chemoreceptors results in a blunted regulatory response to hypoglycaemia and thus requires an increase in glucose infusion rate in order to preserve plasma glucose levels (Table 2). These results suggest the carotid chemoreceptors play an important role in glucoregulation in humans. Since chemoreflex activation with hypoxia may contribute to a change in BRS (Heistad et al., 1975; Mancia, 1975; Somers et al., 1991; Cooper et al., 2005) and/or a shift in the baroreflex operating point (Halliwill et al., 2003; Cooper et al., 2004; Monahan et al., 2006; Querido et al., 2011), we aimed to examine whether such relationships could be translated to other chemoreceptor stimuli (i.e. hypoglycaemia). Thus, we hypothesized desensitizing the carotid body chemoreceptors with hyperoxia (Downes & Lambertsen, 1966; Lahiri & DeLaney, 1975; Wehrwein et al., 2010) would reverse any effect of hypoglycaemia on HRV and sCBRS. Contrary to our hypothesis, the effect of hypoglycaemia on sCBRS and HRV was not reversed with hyperoxia (Figures 1 and 3). The inability of hyperoxia to return measures of sCBRS to baseline levels suggests the carotid body chemoreceptors may not play a primary role in the observed reduction in sCBRS during hypoglycaemia. Furthermore, when examining changes in HRV, it appears the attenuated heart rate response during hyperoxia (Figure 1) cannot be exclusively attributed to an increase in vagal control of heart rate and/or a reduction in the sympathovagal balance – as indexed by a lack of a significant change in High Frequency HRV or Low Frequency/High Frequency ratio with hyperoxia (Figure 4).
Although hypoglycaemia-mediated changes in sCBRS were not altered with hyperoxia (Figure 2), we observed a shift in the baroreflex stimulus-response curve back toward baseline levels (Figure 3). Thus, desensitization of the carotid chemoreceptors during hypoglycemia resulted in resetting of the cardiac baroreflex working range back toward baseline pressures and heart rates. It is reasonable to propose such a shift in the baroreflex working range may be attributed to an interaction within the medulla [e.g. paramedian reticular nuclei (Miura & Reis, 1972)], although such relationships were not specifically examined in the present investigation. It is important to note, spontaneous assessment of baroreflex function can provide only an estimate of the CBRS within a narrow range of systolic blood pressures, thus it is also possible an improvement in sCBRS with hyperoxia occurred which was masked by a shift in the operating point.
Individual Responses and Potential Physiological “Outliers”
Although reductions in HRV with hypoglycaemia were not reversed with hyperoxia (Figure 4), this conclusion was primarily driven by the outlying response from a single individual; during hypoglycemia+hyperoxia, HRV measures were >2 standard deviations from the mean. When this subject was removed from the analysis, hyperoxia tended to attenuate the effect of hypoglycaemia on HRV including a reversal of the negative effects on cardiovagal tone and sympathovagal balance (See Results). Importantly, such changes in HRV are unlikely to be attributed to hyperoxia alone (Francis et al., 2000; Graff et al., 2013). Thus, activation of the carotid chemoreceptors during hypoglycaemia likely contributes to altered autonomic nervous system function and the observed reductions in HRV (Figure 4). By attenuating the activity of the carotid chemoreceptors with hyperoxia, vagal control of heart rate during hypoglycaemia (High Frequency HRV) was improved, which may lower the risk of ventricular arrhythmias. While speculative, it is possible insulin sensitivity may play a role in the divergent response given the excluded individual was lean (Body Fat: 13% vs. group average 25%) and extremely sensitive to insulin – the subject required a large amount of glucose infused during the hypoglycaemic clamp to maintain plasma glucose levels (Glucose infusion rate: 41 µmol.kg FFM−1.min−1 vs. group average of 28 µmol.kg FFM−1.min−1). Along these lines, when basal measures of HRV are compared between individuals with and without insulin resistance, HRV tends to be greater in those that are most insulin sensitive (Pikkujamsa et al., 1998; Flanagan et al., 1999; Reims et al., 2004).
Experimental Considerations
Despite previous studies supporting the use of hyperoxia during experimental procedures to desensitize the carotid chemoreceptors and examine their contribution to cardiometabolic reflexes (Stickland et al., 2008; Ward et al., 2009), it is important to acknowledge hyperoxia can have complex and widespread effects. For example, hyperoxia may result in non-specific sympathoinhibition, augmented cerebral blood flow, altered brain glucose utilization, reduced myocardial contractility and relaxation, and altered central chemoreceptor sensitivity [See (Wehrwein et al., 2010) for more details]. Furthermore, in addition to sensitizing the carotid chemoreceptors, hypoglycaemia may also have widespread, systemic effects. For example, DeRosa & Cryer suggest plasma norepinephrine and hemodynamic responses to hypoglycaemia may be attributed to changes in peripheral adrenomedullary, rather than sympathetic nervous system, activity (DeRosa & Cryer, 2004). Additionally, both baroreceptor activation and hypoglycaemia evoke changes in sympathetic activity via convergent areas in the brainstem (Damanhuri et al., 2012). Thus, the effect of hypoglycaemia on changes in heart rate and blood pressure may be independent of the carotid chemoreceptor.
Insulin is known to have independent effects on sympathoexcitation and peripheral vasodilation (Vollenweider et al., 1995) and studies in animals have recently implicated the carotid chemoreceptors in the autonomic responses to hyperinsulinaemia (Ribeiro et al., 2013). In addition, systemic hyperinsulinaemia has been shown to increase BRS (as assessed by the relationship between muscle sympathetic nerve activity and diastolic blood pressure) in healthy humans (Young et al., 2010). Given the current study was conducted under hyperinsulinaemic hypoglycaemic conditions without a hyperinsulinaemic euglycemic control, it is possible the independent effect of insulin at the level of the chemoreceptors and/or baroreceptors may limit our findings. For example, Laitinen and colleagues (2003) observed no change in sCBRS with hypoglycaemia using a hyperinsulinaemic euglycemic control (Laitinen et al., 2003). However, Young and colleagues (2010) observed no effect of hyperinsulinaemia alone on sCBRS (Young et al., 2010), suggesting it is unlikely the reduction in baroreflex control of heart rate observed during the current study (Figure 2) can be attributed to hyperinsulinaemia alone.
Summary and Clinical Significance
Presently we report a reduction in sCBRS and HRV during hypoglycaemia. Significant reductions in baroreflex control of heart rate and HRV are indicative of a reduction in vagal tone – which has been shown to increase the risk of ventricular arrhythmias. From a physiological perspective, research from our laboratory supports a critical role for the carotid chemoreceptors in regulatory responses to hypoglycaemia (Wehrwein et al., 2010) and desensitization of the carotid chemoreceptors during hypoglycaemia results in an improvement in HRV and a potential shift of the baroreflex operating point back toward baseline levels.
Increased severity of sleep apnea (i.e. carotid chemoreceptor overactivity) is associated with impaired glycemic control (Tasali et al., 2008) and increased risk of cardiac arrhythmias (Parekh, 2009); however, continuous positive airway pressure (CPAP) treatment – which decreases carotid chemoreceptor activity in adults with sleep apnea – has been shown to improve each of these factors and reduce the risk of cardio-metabolic disease (Babu et al., 2005; Hassaballa et al., 2005; Myhill et al., 2012). Thus, reducing the activity of the carotid chemoreceptors, for example, during nocturnal hypoglycaemia might lower the risk of ventricular arrhythmias and sudden death during these events. To further our understanding, it will be necessary to examine the interactions between baroreflex and chemoreflex activation during varying levels of blood glucose and insulin in both healthy adults and patients with chronically sensitized chemoreceptors such as those with sleep apnea, chronic obstructive pulmonary disease, and heart failure.
ACKNOWLEDGEMENTS
The authors would like to thank the research subjects who participated in this study. In addition, we wish to acknowledge the contributions of nursing and technical staff to this study: Cheryl Shonkwiler, Barbara Norby, Shelly Roberts, Karen Krucker, Sarah Wolhart, Jean Knutson, Betty Ann Dicke, Brent McConahey, Pamela Reich, Nancy Meyer, Pam Engrav and Christopher Johnson of the Mayo Clinic. In addition, we thank the Clinical Research Unit staff at Mayo Clinic, the Immunochemical Core Laboratory at Mayo Clinic, and in particular Hilary Blair. Thanks to Brandon Bucher and Brenton Nelson at ADinstruments for the development of the Spontaneous Cardiac Baroreflex Analysis Program.
FUNDING: NIH DK07352 (to M. J. Joyner, R. Basu), NIH NS32352 (to M. J. Joyner), NIH T32 DK07352 (to E. A. Wehrwein, J. K. Limberg), NIH F32 DK84624 (to E. A. Wehrwein), NIH 1 UL1 RR024150 (Mayo Clinic CTSA, to M. J. Joyner), NIH DK29953 (to R. Basu).
ABBREVIATIONS
- FFM
fat free mass
- HRV
heart rate variability
- PaO2
arterial partial pressure of oxygen
- sCBRS
spontaneous cardiac baroreflex sensitivity
Footnotes
CONFLICTS OF INTEREST/DISCLOSURES: There are no conflicts of interests to disclose.
Author Contributions:
- Analysis and interpretation of data
- Drafting the article and revising it critically for important intellectual content
- Final approval of the version to be published
- Data acquisition.
- Analysis and interpretation of data
- Drafting the article and revising it critically for important intellectual content
- Final approval of the version to be published
- Data acquisition.
- Analysis and interpretation of data
- Drafting the article and revising it critically for important intellectual content
- Final approval of the version to be published
- Conception and design of protocol
- Data acquisition.
- Analysis and interpretation of data
- Drafting the article and revising it critically for important intellectual content
- Final approval of the version to be published
- Conception and design of protocol
- Data acquisition.
- Analysis and interpretation of data
- Drafting the article and revising it critically for important intellectual content
- Final approval of the version to be published
- Conception and design of protocol
- Data acquisition.
- Analysis and interpretation of data
- Drafting the article and revising it critically for important intellectual content
- Final approval of the version to be published
- Conception and design of protocol
- Data acquisition.
- Analysis and interpretation of data
- Drafting the article and revising it critically for important intellectual content
- Final approval of the version to be published
LITERATURE CITED
- Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes. 2009;58:360–366. doi: 10.2337/db08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla R, Alvarez-Buylla E, Mendoza H, Montero SA, Alvarez-Buylla A. Pituitary and adrenals are required for hyperglycemic reflex initiated by stimulation of CBR with cyanide. Am J Physiol. 1997;272:R392–R399. doi: 10.1152/ajpregu.1997.272.1.R392. [DOI] [PubMed] [Google Scholar]
- Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Arch Intern Med. 2005;165:447–452. doi: 10.1001/archinte.165.4.447. [DOI] [PubMed] [Google Scholar]
- Basu R, Basu A, Johnson CM, Schwenk WF, Rizza RA. Insulin dose-response curves for stimulation of splanchnic glucose uptake and suppression of endogenous glucose production differ in nondiabetic humans and are abnormal in people with type 2 diabetes. Diabetes. 2004;53:2042–2050. doi: 10.2337/diabetes.53.8.2042. [DOI] [PubMed] [Google Scholar]
- Biscoe TJ, Sampson SR. Stimulus response curves of single carotid body chemoreceptor afferent fibres. Nature. 1967;215:654–655. doi: 10.1038/215654a0. [DOI] [PubMed] [Google Scholar]
- Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J Physiol. 2004;557:1055–1065. doi: 10.1113/jphysiol.2004.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper VL, Pearson SB, Bowker CM, Elliott MW, Hainsworth R. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia - a mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol. 2005;568:677–687. doi: 10.1113/jphysiol.2005.094151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damanhuri HA, Burke PG, Ong LK, Bobrovskaya L, Dickson PW, Dunkley PR, Goodchild AK. Tyrosine hydroxylase phosphorylation in catecholaminergic brain regions: a marker of activation following acute hypotension and glucoprivation. PLoS One. 2012;7:e50535. doi: 10.1371/journal.pone.0050535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosa MA, Cryer PE. Hypoglycemia and the sympathoadrenal system: neurogenic symptoms are largely the result of sympathetic neural, rather than adrenomedullary, activation. Am J Physiol Endocrinol Metab. 2004;287:E32–E41. doi: 10.1152/ajpendo.00539.2003. [DOI] [PubMed] [Google Scholar]
- Downes JJ, Lambertsen CJ. Dynamic characteristics of ventilatory depression in man on abrupt administration of O. J Appl Physiol. 1966;21:447–453. doi: 10.1152/jappl.1966.21.2.447. [DOI] [PubMed] [Google Scholar]
- Fagius J, Berne C. Rapid resetting of human baroreflex working range: insights from sympathetic recordings during acute hypoglycaemia. J Physiol. 1991;442:91–101. doi: 10.1113/jphysiol.1991.sp018784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald R, Lahiri S. Reflex responses to chemoreceptor stimulation. In: Fishman AP, Cherniack NS, Widdicombe JG, Geiger SR, editors. Handbook of Physiology, Section. 3, ed. Bethesda: American Physiological Society; 1986. pp. 313–362. [Google Scholar]
- Flanagan DE, Vaile JC, Petley GW, Moore VM, Godsland IF, Cockington RA, Robinson JS, Phillips DI. The autonomic control of heart rate and insulin resistance in young adults. J Clin Endocrinol Metab. 1999;84:1263–1267. doi: 10.1210/jcem.84.4.5592. [DOI] [PubMed] [Google Scholar]
- Francis DP, Davies LC, Willson K, Ponikowski P, Coats AJ, Piepoli M. Verylow- frequency oscillations in heart rate and blood pressure in periodic breathing: role of the cardiovascular limb of the hypoxic chemoreflex. Clin Sci (Lond) 2000;99:125–132. [PubMed] [Google Scholar]
- Graff B, Szyndler A, Czechowicz K, Kucharska W, Graff G, Boutouyrie P, Laurent S, Narkiewicz K. Relationship between heart rate variability, blood pressure and arterial wall properties during air and oxygen breathing in healthy subjects. Auton Neurosci. 2013 doi: 10.1016/j.autneu.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Morgan BJ, Charkoudian N. Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. J Physiol. 2003;552:295–302. doi: 10.1113/jphysiol.2003.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassaballa HA, Tulaimat A, Herdegen JJ, Mokhlesi B. The effect of continuous positive airway pressure on glucose control in diabetic patients with severe obstructive sleep apnea. Sleep Breath. 2005;9:176–180. doi: 10.1007/s11325-005-0033-y. [DOI] [PubMed] [Google Scholar]
- Heistad D, Abboud FM, Mark AL, Schmid PG. Effect of baroreceptor activity on ventilatory response to chemoreceptor stimulation. J Appl Physiol. 1975;39:411–416. doi: 10.1152/jappl.1975.39.3.411. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Denny JC, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Role of carotid bodies in control of the neuroendocrine response to exercise. Am J Physiol Endocrinol Metab. 2001;281:E742–E748. doi: 10.1152/ajpendo.2001.281.4.E742. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes. 2000;49:1434–1442. doi: 10.2337/diabetes.49.9.1434. [DOI] [PubMed] [Google Scholar]
- Lahiri S, DeLaney RG. Relationship between carotid chemoreceptor activity and ventilation in the cat. Respir Physiol. 1975;24:267–286. doi: 10.1016/0034-5687(75)90018-3. [DOI] [PubMed] [Google Scholar]
- Laitinen T, Huopio H, Vauhkonen I, Camaro C, Hartikainen J, Laakso M, Niskanen L. Effects of euglycaemic and hypoglycaemic hyperinsulinaemia on sympathetic and parasympathetic regulation of haemodynamics in healthy subjects. Clin Sci (Lond) 2003;105:315–322. doi: 10.1042/CS20030079. [DOI] [PubMed] [Google Scholar]
- Lanfranchi PA, Somers VK. Arterial baroreflex function and cardiovascular variability: interactions and implications. Am J Physiol Regul Integr Comp Physiol. 2002;283:R815–R826. doi: 10.1152/ajpregu.00051.2002. [DOI] [PubMed] [Google Scholar]
- Mancia G. Influence of carotid baroreceptors on vascular responses to carotid chemoreceptor stimulation in the dog. Circ Res. 1975;36:270–276. doi: 10.1161/01.res.36.2.270. [DOI] [PubMed] [Google Scholar]
- Miura M, Reis DJ. The role of the solitary and paramedian reticular nuclei in mediating cardiovascular reflex responses from carotid baro- and chemoreceptors. J Physiol. 1972;223:525–548. doi: 10.1113/jphysiol.1972.sp009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan KD, Leuenberger UA, Ray CA. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J Physiol. 2006;574:605–613. doi: 10.1113/jphysiol.2006.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhill PC, Davis WA, Peters KE, Chubb SA, Hillman D, Davis TM. Effect of continuous positive airway pressure therapy on cardiovascular risk factors in patients with type 2 diabetes and obstructive sleep apnea. J Clin Endocrinol Metab. 2012;97:4212–4218. doi: 10.1210/jc.2012-2107. [DOI] [PubMed] [Google Scholar]
- Parekh B. The mechanism of dead-in-bed syndrome and other sudden unexplained nocturnal deaths. Curr Diabetes Rev. 2009;5:210–215. doi: 10.2174/157339909789804387. [DOI] [PubMed] [Google Scholar]
- Pikkujamsa SM, Huikuri HV, Airaksinen KE, Rantala AO, Kauma H, Lilja M, Savolainen MJ, Kesaniemi YA. Heart rate variability and baroreflex sensitivity in hypertensive subjects with and without metabolic features of insulin resistance syndrome. Am J Hypertens. 1998;11:523–531. doi: 10.1016/s0895-7061(98)00035-1. [DOI] [PubMed] [Google Scholar]
- Querido JS, Wehrwein EA, Hart EC, Charkoudian N, Henderson WR, Sheel AW. Baroreflex control of muscle sympathetic nerve activity as a mechanism for persistent sympathoexcitation following acute hypoxia in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1779–R1785. doi: 10.1152/ajpregu.00182.2011. [DOI] [PubMed] [Google Scholar]
- Reims HM, Sevre K, Fossum E, Hoieggen A, Mellem H, Kjeldsen SE. Relations between insulin sensitivity, fitness and autonomic cardiac regulation in healthy, young men. J Hypertens. 2004;22:2007–2015. doi: 10.1097/00004872-200410000-00025. [DOI] [PubMed] [Google Scholar]
- Ribeiro MJ, Sacramento JF, Gonzalez C, Guarino MP, Monteiro EC, Conde SV. Carotid body denervation prevents the development of insulin resistance and hypertension induced by hypercaloric diets. Diabetes. 2013 doi: 10.2337/db12-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87:1953–1957. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol. 2008;586:1743–1754. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133:496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- Vollenweider L, Tappy L, Owlya R, Jequier E, Nicod P, Scherrer U. Insulininduced sympathetic activation and vasodilation in skeletal muscle. Effects of insulin resistance in lean subjects. Diabetes. 1995;44:641–645. doi: 10.2337/diab.44.6.641. [DOI] [PubMed] [Google Scholar]
- von Euler US, Liljestrand G, Zotterman Y. The Excitation Mechanism of the Chemoreceptors of the Carotid Body. Skandinavisches Archiv Für Physiologie. 1940;83:132–152. [Google Scholar]
- Ward DS, Voter WA, Karan S. The effects of hypo- and hyperglycaemia on the hypoxic ventilatory response in humans. J Physiol. 2007;582:859–869. doi: 10.1113/jphysiol.2007.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DS, Voter WA, Karan S. The role of the carotid bodies in the counterregulatory response to hypoglycemia. Adv Exp Med Biol. 2009;648:273–280. doi: 10.1007/978-90-481-2259-2_31. [DOI] [PubMed] [Google Scholar]
- Wehrwein EA, Basu R, Basu A, Curry TB, Rizza RA, Joyner MJ. Hyperoxia blunts counterregulation during hypoglycaemia in humans: possible role for the carotid bodies? J Physiol. 2010;588:4593–4601. doi: 10.1113/jphysiol.2010.197491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrwein EA, Curry TB, Basu A, Rizza RA, Basu R, Joyner MJ. Do the carotid bodies modulate hypoglycemic counterregulation and baroreflex control of blood pressure in humans? Adv Exp Med Biol. 2012;758:129–135. doi: 10.1007/978-94-007-4584-1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol. 2010;588:3593–3603. doi: 10.1113/jphysiol.2010.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]