Abstract
Osteopontin (OPN) is expressed by various immune cells and modulates both innate and adaptive immune responses. However, the molecular mechanisms that control opn gene expression, especially at the chromatin level, remain largely unknown. We have previously demonstrated many specific cis- and trans-regulatory elements that determine the extent of endotoxin (LPS)-mediated induction of OPN synthesis in murine macrophages. In the present study, we confirm that NF-κB also plays an important role in the setting of LPS-stimulated OPN expression through binding to a distal regulatory element. Importantly, we demonstrate that LPS stimulates chromosomal loops in the OPN promoter between NF-κB binding site and AP-1 binding site using chromosome conformation capture technology. The crucial role of NF-κB and AP-1 in LPS-stimulated DNA looping was confirmed, as small interfering RNA knock-down of NF-κB p65 and AP-1 c-Jun exhibited decreased levels of DNA looping. Furthermore, we demonstrate that p300 can form a complex with NF-κB and AP-1 and is involved in DNA looping and LPS-induced OPN expression. Therefore, we have identified an essential mechanism to remodel the local chromatin structures and spatial conformations to regulate LPS-induced OPN expression.
Osteopontin (OPN) is expressed by various immune cells, including macrophages, dendritic cells, and T lymphocytes, and has been implicated in many inflammatory autoimmune diseases (1–7). OPN acts as a cytokine with both proinflammatory and anti-inflammatory functions (2, 8). In macrophages OPN was expressed and secreted constitutively. LPS can further upregulate OPN expression in macrophages through TLR4. Many specific cis- and trans-regulatory elements that determine the extent of endotoxin (LPS)-mediated induction of OPN expression have been identified. In this regard, we demonstrated a NO-dependent mechanism to control OPN transcription in LPS-stimulated macrophages through heterogeneous nuclear ribonucleoprotein (hnRNP)-A/B proteins and hnRNP-U proteins (9, 10). Recently, we identified a NO-independent mechanism to regulate LPS-induced OPN expression in macrophages through AP-1 binding to the OPN promoter (11). Although many specific cis-and trans-regulatory elements have been identified, changes in chromatin structure that regulate the extent of endotoxin (LPS)-mediated induction of OPN expression are not determined.
Precise regulation of gene transcription requires transcription factor binding to cis-acting elements and subsequent recruitment of coactivators and alterations in higher order chromatin structure (12, 13). One alteration in higher order chromatin structure is DNA looping out of the intervening DNA between cis-regulatory sequences (14). DNA looping refers to a conformation of DNA in which cis elements of a DNA strand are physically in proximity to one another. This can increase the local concentration of transcription factors, cofactors, and chromatin-modifying factors near the transcriptional start site of genes and activate transcription (15). This mechanism has been used to explain why enhancer elements located hundreds to thousands of base pairs away from the transcriptional start site can contribute to gene activation (16, 17). Chromosome conformation capture technology (3C) has been widely used to detect the in vivo DNA looping between specific DNA sequences in chromatin (15, 18).
The stimulation of TLR4 by LPS induces IκB kinase and MAPK, ultimately leading to activation of NF-κB and AP-1, resulting in the production of various inflammatory mediators (19, 20). In this study, we find that NF-κB is involved in the setting of LPS-stimulated OPN expression through binding to a distal cis-regulatory element. Chromosome conformation capture experiments demonstrate that LPS stimulates chromosomal loops in the OPN promoter through bridging NF-κB and AP-1 together. Furthermore, we demonstrate that p300 can form a complex with NF-κB and AP-1 and is involved in DNA looping and LPS-induced OPN expression.
Materials and Methods
Cells and reagents
Mouse macrophage cell line RAW264.7 was obtained from the American Type Culture Collection (Manassas, VA) and cultured as described (11). LPS (Escherichia coli, 055:B5) was purchased from Sigma-Aldrich (St. Louis, MO) and repurified as described (21). JSH-23, a specific inhibitor for NF-κB activation, was purchased from Calbiochem (San Diego, CA). Trichostatin A (TSA) was purchased from Sigma-Aldrich. Abs specific to c-Jun, c-Fos, p65, p300, actin, and HRP-coupled secondary Abs were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). An OPN Ab was obtained from R&D Systems (Minneapolis, MN).
Plasmid constructs
The OPN promoter segment from nt −1916 to nt +80 was amplified with genomic DNA from RAW264.7 macrophages and then cloned into pGL3-basic luciferase plasmid (Promega). This plasmid is labeled OPN-1916. The primers used are 5′-CCCAAGCTTCCTTGGCTGGTTTCCTCC-3′, and 5′-CCCAAGCTTCCTTGGCTGGTTTCCTCC-3′. In OPN-1916ΔNF-κB, the NF-κB site was mutated by introducing a ClaI site on the OPN-1916 construct using KOD-Plus-Mutagenesis kit (Toyobo, Osaka, Japan) with the following primers: 5′-ATCGATATATATAACAACACTGAAAACACAAACTCCA-3′ and 5′-ACAAATCCTAAGGCAGCAAGCC-3′. All constructs were confirmed by DNA sequencing. The expression vectors containing full-length p300 (p300 wt) and its acetyltransferase deletion derivative (p300 mt) were kindly provided by Paul C. Kuo (Duke University).
Transfection
For transient transfection, small interfering RNA (siRNA) specific for c-Jun (sc-29224), c-Fos (sc-29222), p65 (sc-29411), and control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology. siRNA duplexes were transfected into RAW264.7 macrophages using Lipofectamine 2000 transfection reagent (Invitrogen) according to the standard protocol. p300 wt or p300 mt expression vectors were transfected into RAW264.7 macrophages using JetPEI-Macrophage reagent (Polyplus) according to the standard protocol.
RNA quantification
Total RNA was extracted with TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. A LightCycler (ABI Prism 7000) and a SYBR RT-PCR kit (Takara) were used for quantitative real-time RT-PCR analysis. Specific primers used for RT-PCR assays were 5′-GCCTGTTTGGCAT-TGCCTCCTC-3′ and 5′-CACAGCATTCTGTGGCGCAAGG-3′ for OPN and 5′-TGTTACCAACTGGGACGACA-3′ and 5′-CTGGGTCATCTTT-TCACGGT-3′ for β-actin. Data are normalized to β-actin expression in each sample.
Immunoprecipitation and immunoblot
Cells were lysed with M-PER protein extraction reagent (Pierce, Rockford, IL) supplemented with a protease inhibitor “mixture,” and then protein concentrations in the extracts were measured with a bicinchoninic acid assay (Pierce). Fifty micrograms of protein was used either for immunoprecipitation or it was loaded and subjected to SDS-PAGE, transferred onto nitrocellulose membranes, and then blotted as described previously (11). The intensity of the protein bands were quantified by BandScan software.
Chromatin immunoprecipitation assay
RAW264.7 macrophages were stimulated with 100 ng/ml LPS for the indicated time periods. Chromatin from macrophages was fixed and immunoprecipitated using the chromatin immunoprecipitation (ChIP) assay kit (Upstate Biotechnology) as recommended by the manufacturer. The purified chromatin was immunoprecipitated using 2 µg anti-p300, anti-p65, anti–c-Jun, anti–c-Fos, or irrelevant Ab (anti-actin). The presence of the selected DNA sequence was assessed by PCR. The primers were 5′- ACCAGAGGAGGAAGTGTAGGAGC-3′ and 5′-ACTGCAAACCCAAGCAAGGAT-3#x02032; for OPN promoter (−127 to +42), 5′-TCCAGCAAAATCTATTCCTATACCTC-3′ and 5′-CCCTTTAAGCACAACACCCACT-3′ for OPN promoter (−1926 to 21759), and 5′-GTCCAAATAGAACATCTTACTC-3′ and 5′-TAAAGCAACGAACTACCATGAG-3′ for OPN promoter (−309 to 2136). The average size of the sonicated DNA fragments subjected to immunoprecipitation was 500 bp as determined by ethidium bromide gel electrophoresis.
3C assay
3C assays were performed according to the method described by Dekker et al. (15). Briefly, RAW264.7 cells (1 × 107) were cultured in 10-cm dishes and fixed with 1% formaldehyde for 10 min at room temperature. The reaction was quenched by the addition of 0.125 M glycine for 5 min at room temperature and 10 min at 4°C. The cells were washed with PBS and lysed in cold lysis buffer (10 mM Tris-HCI [pH 8.0], 10 mM sodium chloride, 0.2% Igepal, 0.8 µM aprotinin, 50 µM bestatin, 20 µM leupeptin, 10 µM pepstatin A, 25 µM p-bromotetramisole oxalate, 5 µM cantharidin, and 5 nM microcystin-leucine arginine). The nuclei were harvested and suspended in digestion buffer with 0.1% SDS and 1% Triton X-100. The DNA was digested with restriction enzyme PstI overnight at 37°C. Samples were then diluted with ligase buffer with 0.1% SDS and 1% Triton X-100. T4 DNA ligase was added with incubation at 16°C overnight, followed by overnight incubation at 65°C in the presence of 10 µg/ ml proteinase K to reverse the cross-links. The DNA was isolated by phenol-chloroform extraction and ethanol precipitation. The purified DNA concentration was determined and was used as a PCR template with the following primer pair: primer A, 5′-AGGCAGCAAGCCGTCCA-3′; primer B, 5′-AACACATCTATCAAGAGATAACCCAA-3′; primer C, 5′-TTAAATTCTGGGAGGTCTGAGCC-3′; primer D, 5′-ATTTCAGCTCCTTGCCTCTGC-3′. The sizes of the products from the 3C assay were as follows: primer A plus B (270 bp), primer A plus C (144 bp), primer A plus D (236 bp). As a loading control, PCR was conducted with primers for the AP-1 binding site (−127 to +42) of genomic OPN.
Assay of luciferase reporter gene expression
Assay of luciferase reporter gene expression was performed as previously described (11). Briefly, RAW264.7 cells (1 × 105 cells/well) were seeded onto 48-well plates 24 h before cotransfection with 100 ng OPN reporter plasmids and 10 ng Renilla-TK plasmid using JetPEI-Macrophage trans-fection reagent (Polyplus). Twenty-four hours after transfection, the cells were left untreated or treated with LPS for 6 h. Luciferase activities were measured using Dual-Luciferase reporter assay system (Promega) on a microplate luminometer (Centro LB 960; Berthold, Wildbad, Germany) according to the manufacturer’s instructions. Firefly luciferase activity was normalized against Renilla luciferase activity.
Statistical analysis
All data are presented as means ± SE of three or four experiments. Analysis was performed using a Student t test. The p values <0.05 were considered significant.
Results
NF-κB is involved in LPS-induced OPN expression
The stimulation of TLR4 by LPS induces IκB kinase and MAPK, ultimately leading to activation of NF-κB and AP-1, resulting in the production of various proinflammatory mediators (19, 20). Previously, we have shown that AP-1 activation plays an important role in LPS-stimulated OPN expression in macrophages (11). Whether NF-κB, another important transcription factor downstream of LPS/TLR4 signaling, also plays a very important role in the setting of LPS-stimulated OPN expression is not well defined.
To investigate the role of NF-κB on LPS-induced OPN expression, we first used NF-κB activation inhibitor II, JSH-23, in the setting of LPS-stimulated macrophages. Inhibition of LPS-induced NF-κB p65 nuclear translocation was confirmed by immunoblotting (data not shown). As reported, LPS stimulation greatly increased both OPN mRNA and protein expression in RAW264.7 macrophages (Fig. 1). In contrast, with pretreatment of JSH-23, both OPN mRNA and protein expression were greatly attenuated after LPS stimulation (Fig. 1).
FIGURE 1.
NF-κB is involved in LPS-induced OPN expression. A and B, RAW264.7 macrophages were pretreated with DMSO or 30 µM JSH-23 for 40 min and then stimulated with 100 ng/ml LPS for the indicated periods. The levels of OPN mRNA and protein were examined by quantitative PCR and Western blot respectively. C, RAW264.7 macrophages were transfected with control small RNA (Ctrl siRNA) or p65 siRNA. After 36 h, p65 expression in the cells was detected by Western blot. D and E, RAW264.7 macro-phages were transfected with Ctrl siRNA or p65 siRNA and then treated with 100 ng/ml LPS for indicated period. Expression level of OPN was examined by quantitative PCR and Western blot, respectively. Similar results were obtained in three independent experiments. Data are shown as mean ± SD (n = 3) of one representative experiment. **p < 0.01.
To further demonstrate that NF-κB is required for LPS-induced OPN expression, NF-κB p65-specific siRNA was transfected into RAW264.7 macrophages. NF-κB p65 protein was decreased ~60% by transfection of NF-κB p65-specific siRNA as measured by immunoblotting (Fig. 1C). After NF-κB p65 siRNA transfection, LPS-induced OPN expression was greatly decreased at both the mRNA level and protein level, compared with control siRNA-transfected macrophages (Fig. 1). Collectively, these data suggest that NF-κB activation is required for OPN expression in LPS-stimulated macrophages.
NF-κB binding to OPN promoter
To investigate the NF-κB binding to the OPN promoter, a 3-kb OPN promoter segment including the transcription start site was submitted to the TRANSFAC 6.0 database to determine potential NF-κB binding sites. This analysis identified a putative NF-κB binding sequence GGAATTCCC (nt −1817 to nt −1808). To confirm the function of this NF-κB binding sequence in LPS-induced OPN expression, OPN-1916 and OPN-1916ΔNF-κB reporter were constructed and transiently transfected into RAW264.7 macrophages. LPS stimulation demonstrated a significant ~4-fold increase in luciferase activity for OPN-1916 as compared with that of pGL3 basic plasmid. In contrast, LPS-induced luciferase activity in OPN-1916ΔNF-κB was greatly decreased (Fig. 2A). Importantly, LPS-induced luciferase activity was decreased ~2-fold in the presence of JSH-23 for OPN-1916. However, JSH-23 treatment did not impair the luciferase activity in OPN-1916ΔNF-κB plasmid (Fig. 2B). These results indicate that the GGAATTCCC sequence is a functional NF-κB binding site and is required for LPS-induced OPN expression.
FIGURE 2.
NF-κB binding to OPN promoter. A, RAW264.7 macrophages were transfected with the OPN-1916, the OPN-1916ΔNF-κB, or the pGL3 basic plasmids. After 24 h culture, the cells were stimulated with 100 ng/ml LPS for 6 h. The cell lysates were assayed for luciferase activity. Relative luciferase activity was calculated taking pGL3 vector activity as 1. The ratio of stimulated versus unstimulated activities is shown. B, RAW264.7 macrophages were transfected with the OPN-1916, the OPN-1916ΔNF-κB, or the pGL3 basic plasmids. The cells were pretreated with DMSO or 30 µM JSH-23 for 40 min and then stimulated with 100 ng/ ml LPS for 6 h. The cell lysates were assayed for luciferase activity. Relative luciferase activity was calculated taking pGL3 vector activity as 1. The ratio of stimulated versus unstimulated activities is shown. C, RAW264.7 macrophages were stimulated with 100 ng/ml LPS or vehicle (water) for indicated period, and the ChIP assay was used to assess the binding of p65 to the NF-κB binding sites within the −1926 to −1759 region of the murine OPN promoter. Total extract was used as a loading control, and immunoprecipitation with irrelevant Ab (anti-actin) was used as a negative control. PCR products from the amplication of a NF-κB site-free region within −309 to −136 of the murine OPN promoter were used as specificity controls. Similar results were obtained in three independent experiments. **p < 0.01, ▲ p > 0.05.
Next, NF-κB p65 binding to the OPN promoter was measured by ChIP assays. PCR analysis showed that NF-κB p65 Ab precipitated the OPN promoter region (nt −1926 to nt −1759) from RAW264.7 macrophages activated with LPS for 1 and 2 h (Fig. 2C), whereas unstimulated controls did not demonstrate this DNA binding. Control Abs (anti-actin Ab) did not exhibit this DNA binding activity. NF-κB p65 Ab did not precipitate the OPN promoter region (nt −309 to nt −136) from the same setting of LPS-stimulated macrophages, indicating specific binding of NF-κB p65 to the promoter region from nt −1926 to nt −1759. Taken together, these results demonstrate that NF-κB binds to OPN promoter and regulates OPN expression in LPS-stimulated macrophages.
NF-κB and AP-1 mediate DNA looping in the OPN promoter: 3C assays
We have confirmed that NF-κB and AP-1 are essential transcription factors for LPS-mediated OPN transactivation. However, the relationship between AP-1 and NF-κB in this setting is not well studied. OPN protein level was greatly decreased by transfection of either c-Jun siRNA or p65 siRNA. Importantly, OPN protein level can be completely ablated by cotransfection of c-Jun siRNA and p65 siRNA, indicating a synergistic effect of AP-1 and NF-κB on LPS-stimulated OPN expression (Fig. 3A).
FIGURE 3.
AP-1 and NF-κB mediate DNA looping in the OPN promoter: 3C assays. A, RAW264.7 macrophages were transfected with control siRNA (Ctrl siRNA), c-Jun siRNA, p65 siRNA, or p65 siRNA plus c-Jun siRNA and then treated with 100 ng/ml LPS for the indicated time period. Expression level of OPN was examined by Western blot. B, RAW264.7 macro-phages were stimulated with 100 ng/ml LPS for periods of 0, 30, and 60 min, and the 3C assays restricted by PstI were performed in the OPN locus. Input represents products of PCR using primers specific for the conserved AP-1 binding site of OPN performed to establish that equal amounts of template DNA were present in the samples. C, RAW264.7 macrophages were transfected with Ctrl siRNA or c-Jun siRNA or c-Fos siRNA. After 36 h, c-Jun or c-Fos expression in the cells was detected by Western blot. D, RAW264.7 macrophages were transfected with siRNA for p65, c-Jun, c-Fos, or control siRNA and then stimulated with LPS. The 3C assay was performed with PstI restriction for OPN. Similar results were obtained in three independent experiments.
We hypothesize that the changes of the local structures and spatial conformations of the chromosome in the OPN promoter mediated by AP-1 and NF-κB binding are intimately associated with LPS-induced OPN expression. To determine the extent of potential changes of the local structures and spatial conformations in the chromosome between AP-1 and NF-κB binding sites, DNA looping was measured by 3C assays using the relevant restriction enzyme PstI, since it can cut appropriate restriction sites in the OPN promoter (see Fig. 6). The presence of a PCR band at ~144 bp with primers A and C indicates the presence of DNA looping (see Fig. 6). RAW264.7 macrophages were treated with LPS for 30 and 60 min, and 3C assays were performed. In the 3C assay, PCR products can be obtained only when the restriction sites are close to one another. As a result, regardless of LPS stimulation, PCR analysis demonstrated the DNA products (270 bp) using primers A and B, presumably due to their physical proximity in the primary DNA sequence (Fig. 3B). There is no PCR product obtained with the more distant A and D primers under the same conditions (Fig. 3B). In contrast, a 144-bp PCR product with the A and C primers was obtained only in the LPS-stimulated macrophages (Fig. 3B). The amount of input DNA was the same in both untreated and LPS-treated macrophages. The identity of all of the products was confirmed by DNA sequencing (data not shown). In the absence of ligase, none of the PCR products was detected (Fig. 3B). These data suggest that DNA looping in the OPN promoter between AP-1 and NF-κB binding sites occurred in LPS-stimulated macrophages.
FIGURE 6.
Proposed model illustrating how NF-κB and AP-1 cooperate to regulate LPS-induced OPN expression. In unactivated macrophages, there is no NF-κB and AP-1 binding to the OPN promoter. Upon LPS stimulation, NF-κB and AP-1 bind to their respective cis-acting sequences and p300 is recruited. This leads to the formation of DNA loop in the OPN promoter and subsequent OPN expression. The NF-κB and AP-1 sites in the murine OPN promoter are indicated. The black vertical arrows denote the PstI restriction site. The black horizontal arrows denote the 3C primers used for 3C assays. The empty vertical arrows indicate the segment constructed in the reporter plasmid OPN-1916. TSS, transcriptional start site.
To elucidate the role of AP-1 and NF-κB in the DNA looping, AP-1 c-Jun and NF-κB p65 expression were knocked down using specific siRNAs. Transfection of AP-1 c-Jun and NF-κB p65 siRNA decreased c-Jun and p65 protein expression in RAW264.7 macrophages (Fig. 3C). In a 3C assay, both AP-1 c-Jun and NF-κB p65 depletion greatly decreased the efficiency of DNA looping assessed by the PCR analysis with A and C primers (Fig. 3D). Taken together, these data indicate that AP-1 and NF-κB play a crucial role in DNA looping and subsequent OPN expression in LPS-stimulated macrophages.
P300 is involved in OPN expression and DNA looping
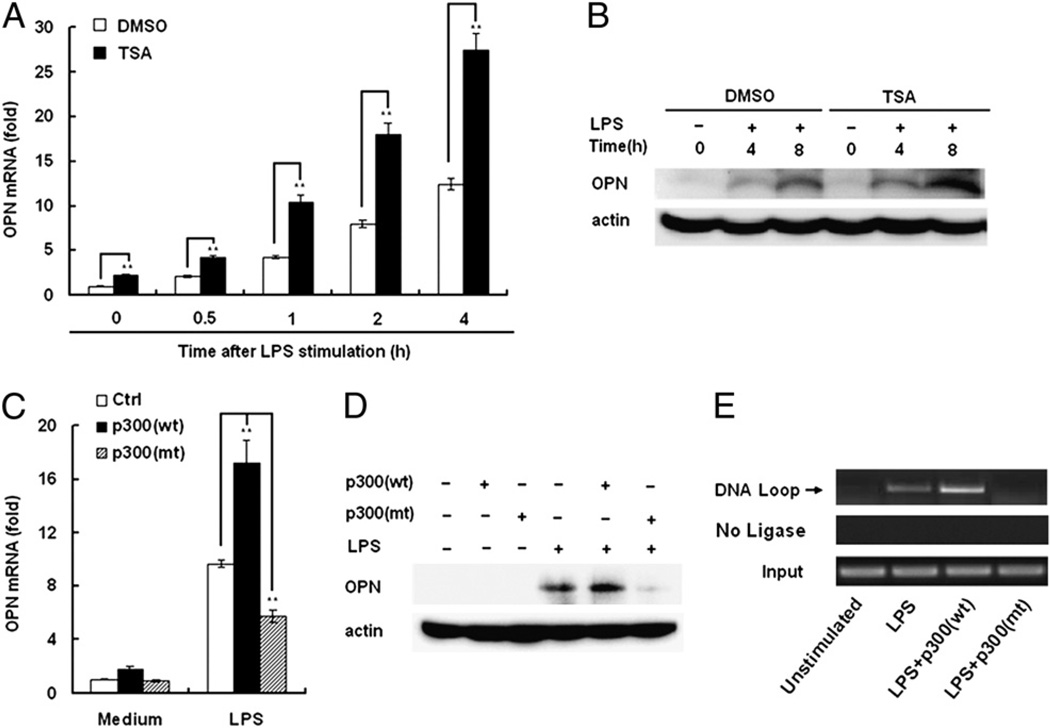
Histone epigenetic modifications, such as acetylation and methylation play, an essential role in gene expression (22, 23). To investigate the possible functions of histone acetylation in LPS-induced OPN expression, TSA, a histone deacetylase inhibitor, was used in the setting of LPS-induced macrophages. TSA treatment greatly increased LPS-induced OPN expression at both the mRNA and protein levels (Fig. 4), which indicates that histone acetylation may positively regulate OPN expression in LPS-stimulated macrophages. CBP/P300, a transcriptional coactivator, is required for histone acetylation and gene expression in the living cells (24). To further confirm that the histone acetylation is involved in LPS-induced OPN expression, p300 expression plasmid was transfected into RAW264.7 macrophages, then OPN mRNA and protein level was measured by quantitative PCR and immunoblot analysis, respectively. Transfection of p300 expression plasmid greatly increased both LPS-induced OPN mRNA expression and protein expression (Fig. 4). Importantly, transfection of a mutant p300, containing an acetyltransferase deletion, greatly decreased LPS-induced OPN expression (Fig. 4). Without LPS stimulation, p300 could not induce OPN expression (Fig. 4D). These data indicate that LPS-induced OPN expression requires p300 intact acetyltranferase activity. Finally, the function of p300 in the DNA looping in the OPN promoter was measured in LPS-stimulated macrophages. In this case, p300 expression plasmid and mutant p300 with an acetyltransferase deletion were transfected into RAW264.7 macrophages, then macrophages were treated with LPS and 3C assays were performed. Transfection of p300 expression plasmids resulted in a great increase of DNA looping in the OPN promoter of LPS-stimulated RAW264.7 macrophages compared with control vector-transfected cells (Fig. 4E). In contrast, transfection of mutant p300 with an acetyltransferase deletion resulted in the loss of DNA looping (Fig. 4E). These data suggest that p300 with intact acetyltransferase activity is required for DNA looping and subsequent OPN expression in LPS-stimulated RAW264.7 macrophages.
FIGURE 4.
P300 is involved in OPN expression and DNA looping. A and B, RAW264.7 macrophages were pretreated with DMSO or 1 µM TSA for 40 min and then stimulated with 100 ng/ml LPS for the indicated time periods. The levels of OPN mRNA and protein were examined by quantitative PCR and Western blot, respectively. C and D, RAW264.7 macrophages were transfected with p300 (mt), a p300 containing an acetyltransferase deletion, or p300 (wt). After 24 h of culture, the cells were stimulated with 100 ng/ml LPS for the indicated time periods. Expression level of OPN was examined by quantitative PCR and Western blot, respectively. E, RAW264.7 macrophages were transfected with p300 (mt) or p300 (wt). After 24 h culture, the cells were stimulated with LPS. The 3C assay was performed for OPN promoter using primer A and C. Similar results were obtained in three independent experiments. Data are shown as mean ± SD (n = 3) of one representative experiment. **p < 0.01.
NF-κB, AP-1, and p300 co-occupy OPN promoter
It has been reported that p300 interacts with NF-κB and that AP-1 and is a key scaffolding protein in the formation of enhanceosomes (24). Domain examination of p300 indicates that the NF-κB p65 binding site is present at the CREB-binding domain and AP-1 c-Jun is present at the N-terminal zinc finger domain (24). We hypothesize that after binding to their consensus sequence, NF-κB and AP-1 can be bridged together by p300 to form a complex and mediate DNA looping and LPS-induced OPN expression. Therefore, p300, NF-κB, and AP-1 will co-occupy the NF-κB and AP-1 binding sites in the OPN promoter. To confirm our hypothesis, ChIP assays were performed with p300, p65, c-Jun, and c-Fos Abs. PCR analysis showed that all Abs precipitated the OPN promoter region (nt −1926 to nt −1759) that covers the NF-κB binding site from LPS-stimulated RAW264.7 macrophages (Fig. 5). At the same time, all Abs precipitated the OPN promoter region (nt −156 to nt +6) that covers the AP-1 binding site from LPS-stimulated RAW264.7 macrophages (Fig. 5). All Abs did not precipitate the OPN promoter region (nt −309 to nt −136) from the same setting of LPS-stimulated macrophages, indicating specific binding of the Abs to these promoter regions. Collectively, these results support the engagement of the NF-κB p65, AP-1, c-Jun/c-Fos, and p300 complex in LPS-mediated OPN gene transcription (Fig. 6).
FIGURE 5.
NF-κB, AP-1, and p300 co-occupy OPN promoter. A and B, RAW264.7 macrophages were stimulated with 100 ng/ml LPS or vehicle (water) for indicated time period, and the ChIP assay was used to assess the binding of p65, c-Jun, c-Fos, and p300 to the NF-κB binding sites within the −1926 to −1759 region and to the AP-1 binding sites within the −127 to +42 region of the murine OPN promoter. Total extract was used as a loading control, and immunoprecipitation with irrelevant Ab (anti-actin) was used as a negative control. PCR products from the amplification of a NF-κB or AP-1 site-free region within −309 to −136 of the murine OPN promoter were used as specificity controls. Similar results were obtained in three independent experiments.
Discussion
OPN contributes to diverse physiological and pathological processes, including immune responses, inflammation, tumor growth and metastasis, bone formation, and remodeling (25). Its expression is tissue-specific and subject to regulation by many transcription factors (26). Macrophages constitutively express and secrete low levels of OPN. Its expression can be further upregulated by LPS stimulation (9). However, the molecular mechanisms of LPS-mediated induction of OPN expression are not well defined.
Previously, we demonstrated a NO-dependent mechanism to control OPN transcription in LPS-stimulated macrophages through hnRNP-A/B and hnRNP-U proteins (9, 10). In the absence of NO, hnRNP-A/B as a constitutive trans-repressor inhibits OPN transcription by binding to the OPN promoter. In the presence of NO, hnRNP-A/B is S-nitrosylated and dissociates from its OPN promoter site with subsequent derepression of OPN promoter activity. Subsequently, hnRNP-U binds to the same site to further augment OPN promoter activation. Recently, we identified an NO-independent mechanism to regulate LPS-induced OPN expression in macrophages. TLR stimulation can induce intracellular form of OPN expression through TLR-induced AP-1 activation. Mutation analysis showed that the cis-element for AP-1 binding (TGACACA between nt −69 and nt −75) is essential for LPS-induced OPN expression (11).
LPS induces activation of transcription factors NF-κB and AP-1, resulting in the production of various proinflammatory mediators through TLR4 (19, 20). NF-κB and AP-1 are the two most important transcription factors downstream of LPS/TLR4 signaling. Expression of various cytokines induced by LPS stimulation is mediated by NF-κB and AP-1 (27–31). In this study, we provide evidence that NF-κB also plays an important role in the setting of LPS-stimulated OPN expression through binding to a distal cis-regulatory element (GGAATTCCC between nt −1817 and nt −1808). Therefore, LPS-induced OPN expression in macrophages can be controlled by NF-κB and AP-1 activation.
Although OPN transcription can be regulated by varieties of transcription factors (26, 32), the contribution of chromatin structure and transcriptional coactivators is not known. In this regard, Sakata et al. (33) reported that TSA, a histone deacetylase inhibitor can increase the OPN mRNA level and OPN protein in mouse undifferentiated mesenchymal cell line through the AP-1 site in the OPN promoter. Similarly, we found that TSA treatment increased both OPN mRNA and protein expression in murine macrophages. Importantly, we found that coactivator p300 and p300 acetyltransferase activity were required for LPS-induced OPN expression.
Precise regulation of gene transcription requires transcription factor binding to cis-acting elements and subsequent recruitment of coactivators and alterations in higher order chromatin structure (12, 13). Consistently, our ChIP experiments showed that both NF-κB and AP-1 bind to the OPN promoter after LPS stimulation. We further showed that coactivator p300 was recruited and formed a complex with NF-κB and AP-1 and co-occupied the NF-κB and AP-1 binding sites in OPN promoter. Importantly, we showed formation of DNA looping in the OPN promoter between NF-κB binding site and AP-1 biding site in LPS-stimulated macrophages. Looping formation requires the presence of the transcription factor NF-κB and AP-1 and coactivator p300 acetyltransferase activity. These data indicate that OPN expression requires a special higher order chromatin structure and cocativators such as p300. The exact mechanisms of chromatin remodeling in mediating LPS-induced OPN expression in macrophages are under investigation.
DNA looping refers to a conformation of DNA in which cis-elements of a DNA strand are physically in proximity to one another (14). This can bring a locally high concentration of transcription factors, cofactors, and chromatin-modifying factors near the transcriptional start site of genes and activate transcription (15). The formation of DNA looping mediated by NF-κB and AP-1 has been previously reported (27, 34–37). In the iNOS promoter, short-range DNA looping mediated by NF-κB and AP-1 binding has been characterized in endotoxin-stimulated macrophages (27). In another report, Yun et al. (34) reported that DNA looping mediated by physical interactions between lymphoid enhancer binding factor 1 and NF-κB and AP-1 regulates the expression of MMP13 and COX2 expression, respectively.
Based on the experimental data obtained above, we propose a model to illustrate the regulatory mechanism for LPS-induced OPN expression in macrophages (Fig. 6). After activation by LPS stimulation, NF-κB and AP-1 bind to their respective cis-acting regulatory sequences in the OPN promoter. Then, coactivator p300 is recruited and forms a complex with NF-κB and AP-1 through the NF-κB p65 binding domain and AP-1 c-Jun binding domain. The formation of this complex facilitates histone acetylation and DNA looping out of the intervening DNA between NF-κB and AP-1 binding site. This leads to the changes of the local structures and spatial conformations of the chromatin in the OPN promoter and subsequent OPN expression. Our results have identified an essential mechanism to establish higher order chromatin structure to regulate LPS-induced OPN expression.
Acknowledgments
This work was supported in part by the Taishan Scholar Program of Shandong Province, the Specialized Research Fund for the Doctoral Program of Higher Education of China (20090131110045), the National Natural Science Foundation of China (81041080), and the China Postdoctoral Science Foundation (to Z.W.).
Abbreviations used in this article
- 3C
chromosome conformation capture
- ChIP
chromatin immunoprecipitation
- hnRNP
heterogeneous nuclear ribonucleoprotein
- OPN
osteopontin
- siRNA
small interfering RNA
- TSA
trichostatin A
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, Sobel RA, Lock C, Karpuj M, Pedotti R, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 2.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 3.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc. Natl. Acad. Sci. USA. 2005;102:17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H. Osteopontin expression is essential for interferon-a production by plasmacytoid dendritic cells. Nat. Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss JM, Renkl AC, Maier CS, Kimmig M, Liaw L, Ahrens T, Kon S, Maeda M, Hotta H, Uede T, Simon JC. Osteopontin is involved in the initiation of cutaneous contact hypersensitivity by inducing Langerhans and dendritic cell migration to lymph nodes. J. Exp. Med. 2001;194:1219–1229. doi: 10.1084/jem.194.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 2007;8:74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 9.Gao C, Guo H, Wei J, Mi Z, Wai P, Kuo PC. S-nitrosylation of heterogeneous nuclear ribonucleoprotein A/B regulates osteopontin transcription in endotoxin-stimulated murine macrophages. J. Biol. Chem. 2004;279:11236–11243. doi: 10.1074/jbc.M313385200. [DOI] [PubMed] [Google Scholar]
- 10.Gao C, Guo H, Mi Z, Wai PY, Kuo PC. Transcriptional regulatory functions of heterogeneous nuclear ribonucleoprotein-U and -A/B in endotoxin-mediated macrophage expression of osteopontin. J. Immunol. 2005;175:523–530. doi: 10.4049/jimmunol.175.1.523. [DOI] [PubMed] [Google Scholar]
- 11.Zhao W, Wang L, Zhang L, Yuan C, Kuo PC, Gao C. Differential expression of intracellular and secreted osteopontin isoforms by murine mac-rophages in response to Toll-like receptor agonists. J. Biol. Chem. 2010;285:20452–20461. doi: 10.1074/jbc.M110.110312. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Fraser P, Bickmore W. Nuclear organization of the genome and the potential for gene regulation. Nature. 2007;447:413–417. doi: 10.1038/nature05916. [DOI] [PubMed] [Google Scholar]
- 13.Sexton T, Schober H, Fraser P, Gasser SM. Gene regulation through nuclear organization. Nat. Struct. Mol. Biol. 2007;14:1049–1055. doi: 10.1038/nsmb1324. [DOI] [PubMed] [Google Scholar]
- 14.Schleif R. DNA looping. Annu. Rev. Biochem. 1992;61:199–223. doi: 10.1146/annurev.bi.61.070192.001215. [DOI] [PubMed] [Google Scholar]
- 15.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 16.Rippe K, von Hippel PH, Langowski J. Action at a distance: DNA-looping and initiation of transcription. Trends Biochem. Sci. 1995;20:500–506. doi: 10.1016/s0968-0004(00)89117-3. [DOI] [PubMed] [Google Scholar]
- 17.Majumder P, Gomez JA, Chadwick BP, Boss JM. The insulator factor CTCF controls MHC class II gene expression and is required for the formation of long-distance chromatin interactions. J. Exp. Med. 2008;205:785–798. doi: 10.1084/jem.20071843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simonis M, Kooren J, Laat Wde. An evaluation of 3C–based methods to capture DNA interactions. Nat. Methods. 2007;4:895–901. doi: 10.1038/nmeth1114. [DOI] [PubMed] [Google Scholar]
- 19.Kawai T, Akira S. TLR signaling. Semin. Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of Toll-like receptor-mediated immune responses. Nat. Rev. Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, An H, Zhou J, Xu H, Yu Y, Cao X. Hyperthermia differentially regulates TLR4 and TLR2-mediated innate immune response. Immunol. Lett. 2007;108:137–142. doi: 10.1016/j.imlet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 23.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J. Biol. Chem. 2001;276:13505–13508. doi: 10.1074/jbc.R000025200. [DOI] [PubMed] [Google Scholar]
- 25.Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J. Clin. Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wai PY, Kuo PC. The role of osteopontin in tumor metastasis. J. Surg. Res. 2004;121:228–241. doi: 10.1016/j.jss.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Guo H, Mi Z, Kuo PC. Characterization of short range DNA looping in endotoxin-mediated transcription of the murine inducible nitric-oxide synthase (iNOS) gene. J. Biol. Chem. 2008;283:25209–25217. doi: 10.1074/jbc.M804062200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Liu H, Sidiropoulos P, Song G, Pagliari LJ, Birrer MJ, Stein B, Anrather J, Pope RM. TNF-a gene expression in macrophages: regulation by NF-κB is independent of c-Jun or C/EBPb. J. Immunol. 2000;164:4277–4285. doi: 10.4049/jimmunol.164.8.4277. [DOI] [PubMed] [Google Scholar]
- 29.Peters KL, Smith HL, Stark GR, Sen GC. IRF-3-dependent, NFkB- and JNK-independent activation of the 561 and IFN-b genes in response to double-stranded RNA. Proc. Natl. Acad. Sci. USA. 2002;99:6322–6327. doi: 10.1073/pnas.092133199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ndlovu N, Van Lint C, Van Wesemael K, Callebert P, Chalbos D, Haegeman G, Vanden Berghe W. Hyperactivated NF-κB and AP-1 transcription factors promote highly accessible chromatin and constitutive transcription across the interleukin-6 gene promoter in metastatic breast cancer cells. Mol. Cell. Biol. 2009;29:5488–5504. doi: 10.1128/MCB.01657-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma W, Gee K, Lim W, Chambers K, Angel JB, Kozlowski M, Kumar A. Dexamethasone inhibits IL-12p40 production in lipopolysaccharide-stimulated human monocytic cells by down-regulating the activity of c-Jun N-terminal kinase, the activation protein-1, and NF-κB transcription factors. J. Immunol. 2004;172:318–330. doi: 10.4049/jimmunol.172.1.318. [DOI] [PubMed] [Google Scholar]
- 32.Denhardt DT, Mistretta D, Chambers AF, Krishna S, Porter JF, Raghuram S, Rittling SR. Transcriptional regulation of osteopontin and the metastatic phenotype: evidence for a Ras-activated enhancer in the human OPN promoter. Clin. Exp. Metastasis. 2003;20:77–84. doi: 10.1023/a:1022550721404. [DOI] [PubMed] [Google Scholar]
- 33.Sakata R, Minami S, Sowa Y, Yoshida M, Tamaki T. Trichostatin A activates the osteopontin gene promoter through AP1 site. Biochem. Biophys. Res. Commun. 2004;315:959–963. doi: 10.1016/j.bbrc.2004.01.152. [DOI] [PubMed] [Google Scholar]
- 34.Yun K, So JS, Jash A, Im SH. Lymphoid enhancer binding factor 1 regulates transcription through gene looping. J. Immunol. 2009;183:5129–5137. doi: 10.4049/jimmunol.0802744. [DOI] [PubMed] [Google Scholar]
- 35.Liu Z, Ma Z, Terada LS, Garrard WT. Divergent roles of RelA and c-Rel in establishing chromosomal loops upon activation of the Igk gene. J. Immunol. 2009;183:3819–3830. doi: 10.4049/jimmunol.0901781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Ye L, Christianson GJ, Yang JQ, Roopenian DC, Zhu X. NF-κB signaling regulates functional expression of the MHC class I-related neonatal Fc receptor for IgG via intronic binding sequences. J. Immunol. 2007;179:2999–3011. doi: 10.4049/jimmunol.179.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meacock S, Pescini-Gobert R, DeLamarter JF, Hooft van Huijsduijnen R. Transcription factor-induced, phased bending of the E-selectin promoter. J. Biol. Chem. 1994;269:31756–31762. [PubMed] [Google Scholar]