Abstract
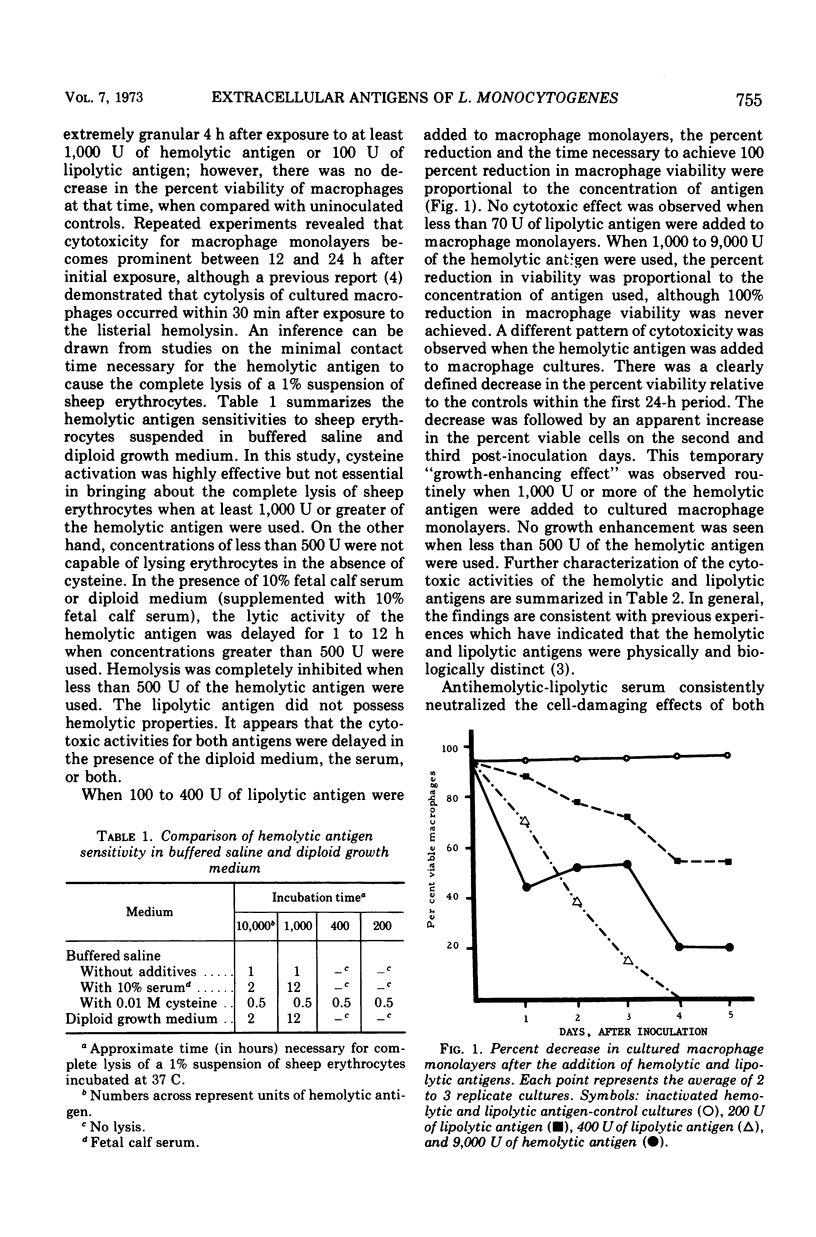
Purified preparations of hemolytic and lipolytic antigens were shown to be cytotoxic for tissue cultures of mouse peritoneal macrophage monolayers. The percent reduction in viability was proportional to the concentration of antigen used although, for a brief period, the hemolytic antigen stimulated the growth of cultured macrophages. Hemolytic and lipolytic antigens were synthesized by an hemolysin-producing strain (7973), but were deficient or totally lacking in nonhemolysin-producing strains (9037-7, 4219). The inoculation of strain 7973 onto macrophage monolayers at a bacterium to macrophage ratio of 10:1 resulted in a complete loss of viable phagocytic cells. The decrease in viable phagocytic cells was accompanied by an increase in viable listeria. Pronounced cytopathic activity with proliferation of listeria was also observed when 200 U of lipolytic antigen were added to macrophage monolayers that had been infected with the smooth type nonhemolysin-producing strain (4219) on the previous day. On the other hand, a high percentage of macrophages survived when exposed to nonhemolysin-producing strains only. In this case, listeria were not recovered by routine culture methods. The data presented do not establish that phagocytic cell destruction with accompanying microbial proliferation is the direct result of hemolysin elaboration; however, they suggest that there is need for further exploration of the role of the hemolysin in listeriosis.
Full text
PDF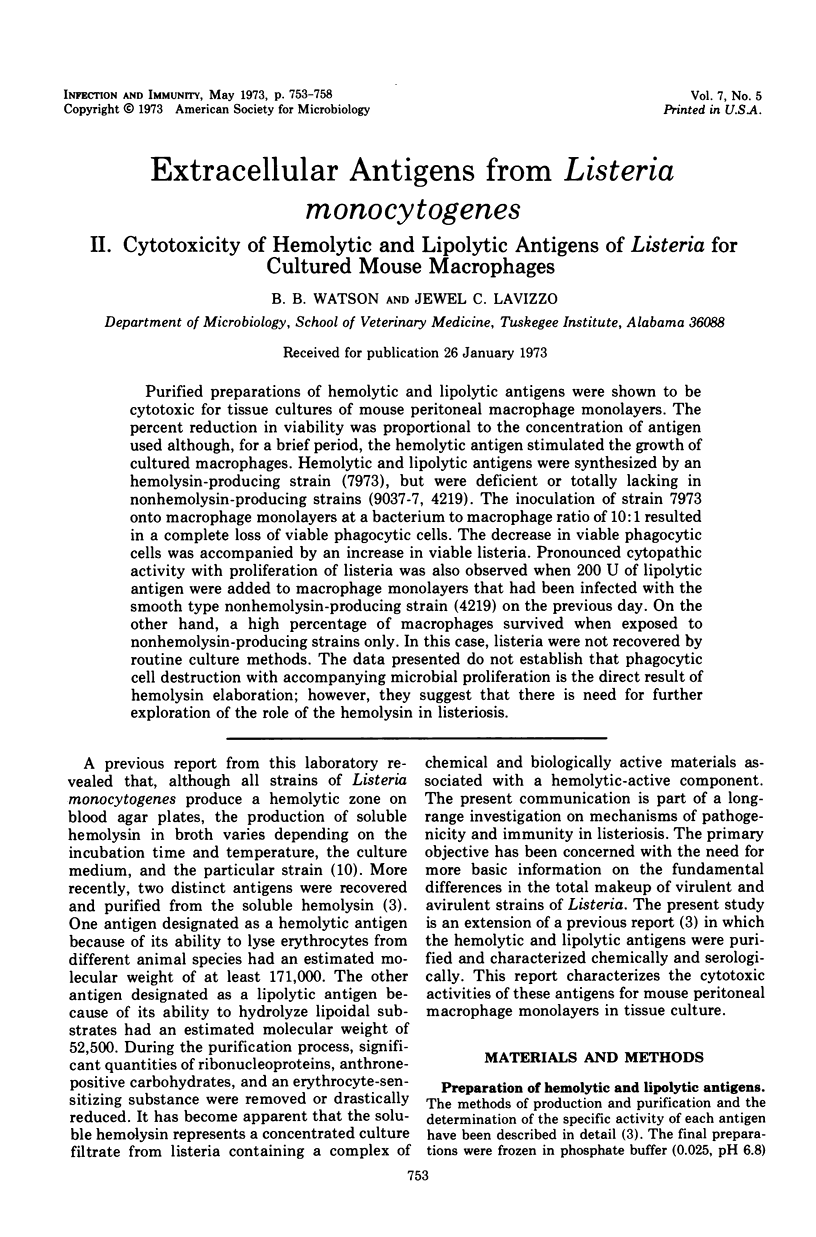



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gröschel D., Jakubowitch R. Use of ampicillin instead of streptomycin in Salmonella-infected mouse peritoneal macrophage cultures. J Bacteriol. 1967 Mar;93(3):1199–1200. doi: 10.1128/jb.93.3.1199-1200.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINS E. M., NJOKU-OBI A. N., ADAMS E. W. PURIFICATION OF THE SOLUBLE HEMOLYSINS OF LISTERIA MONOCYTOGENES. J Bacteriol. 1964 Aug;88:418–424. doi: 10.1128/jb.88.2.418-424.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins E. M., Watson B. B. Extracellular Antigens from Listeria monocytogenes I. Purification and Resolution of Hemolytic and Lipolytic Antigens from Culture Filtrates of Listeria monocytogenes. Infect Immun. 1971 Apr;3(4):589–594. doi: 10.1128/iai.3.4.589-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon G. C., Sword C. P. Biochemical and Immunological Effects of Listeria monocytogenes Hemolysin. Infect Immun. 1970 Apr;1(4):363–372. doi: 10.1128/iai.1.4.363-372.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon G. C., Sword C. P. Effects of Listeria monocytogenes Hemolysin on Phagocytic Cells and Lysosomes. Infect Immun. 1970 Apr;1(4):356–362. doi: 10.1128/iai.1.4.356-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden P., McClatchy J. K., Farr R. S. Shared antigens between heterologous bacterial species. Infect Immun. 1972 Oct;6(4):574–582. doi: 10.1128/iai.6.4.574-582.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NJOKU-OBI A. N., JENKINS E. M., NJOKU-OBI J. C., ADAMS J., COVINGTON V. PRODUCTION AND NATURE OF LISTERIA MONOCYTOGENES HEMOLYSINS. J Bacteriol. 1963 Jul;86:1–8. doi: 10.1128/jb.86.1.1-8.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzan K. R., Musher D. M., Keusch G. T., Weinstein L. Correlation of increased metabolic activity, resistance to infection, enhanced phagocytosis, and inhibition of bacterial growth by macrophages from Listeria- and BCG-infected mice. Infect Immun. 1972 Apr;5(4):499–504. doi: 10.1128/iai.5.4.499-504.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique I. H. Cytotoxic activity of hemolysin from Listeria monocytogenes onL-M strain of mouse cells. Can J Microbiol. 1969 Aug;15(8):955–957. doi: 10.1139/m69-166. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKSMAN B. H., MATOLTSY M. The effect of tuberculin on peritoneal exudate cells of sensitized guinea pigs in surviving cell culture. J Immunol. 1958 Sep;81(3):220–234. [PubMed] [Google Scholar]



