Abstract
Biocatalytic approaches to the synthesis of optically pure chiral amines, starting from simple achiral building blocks, are highly desirable because such motifs are present in a wide variety of important natural products and pharmaceutical compounds. Herein, a novel one-pot ω-transaminase (TA)/monoamine oxidase (MAO-N) cascade process for the synthesis of chiral 2,5-disubstituted pyrrolidines is reported. The reactions proceeded with excellent enantio- and diastereoselectivity (>94 % ee; >98 % de) and can be performed on a preparative scale. This methodology exploits the complementary regio- and stereoselectivity displayed by both enzymes, which ensures that the stereogenic center established by the transaminase is not affected by the monoamine oxidase, and highlights the potential of this multienzyme cascade for the efficient synthesis of chiral building blocks.
Keywords: biocatalysis, cascade reactions, monoamine oxidase, pyrrolidines, transaminase
The exquisite chemo-, regio- and stereoselectivity displayed by enzymes has led to their widespread application as catalysts for stereocontrolled organic synthesis.1 These properties, coupled with their ability to catalyze reactions under similar conditions, has enabled the development of elegant multienzyme cascade processes, in which the product formed by the action of the first enzyme becomes the starting material for the subsequent biotransformation.2 Such tandem processes alleviate the need for protecting-group manipulations and intermediate purification steps, thus providing cost-effective routes to target molecules.
Among the most synthetically useful biocatalysts for the synthesis of chiral amines are the ω-transaminase (TA) family and variants of monoamine oxidase from Aspergillus niger (MAO-N).3 TAs are capable of mediating the selective reductive amination of prochiral ketones, thereby providing the corresponding chiral amines.3a–e MAO-N catalyzes the oxygen-dependent conversion of amines into imines and is typically selective for the (S)-enantiomer.3f–l Variants of MAO-N have been exploited for the deracemization of primary, secondary, and tertiary amines with diverse structural motifs.3a,f–j The development of several chemoenzymatic routes3c, 4 to industrially important target molecules by employing these two enzyme classes is testament to the advances in protein engineering1a, 5 that have resulted in the development of biocatalysts with the desired substrate scope, selectivity, and stability.
2,5-Disubstituted pyrrolidines are important scaffolds in pharmaceutical compounds6 and natural products,7 and considerable efforts have been devoted to developing asymmetric routes to both cis- and trans-disubstituted derivatives that show moderate to good stereoselectivity.8 The lack of stereofacial bias induced by the preexisiting C2-stereocenter means that obtaining the trans-diastereomers through reduction of the corresponding imine in high de is not straightforward. Our approach (Scheme 1) features a highly selective TA-mediated reductive amination of an achiral 1,4-diketone to generate an optically active pyrroline followed by diastereoselective chemoenzymatic conversion into the corresponding pyrrolidine by MAO-N/NH3⋅BH3.
Scheme 1.

A chemoenzymatic approach for the synthesis of 2,5-disubstituted pyrrolidines by employing an ω-transaminase (TA) and a monoamine oxidase (MAO-N).
Initially, we examined the ω-TA-mediated selective monoamination of commercially available 1,4-diketone 1 a, which bears a small methyl substituent and a large phenyl substituent (Scheme 2). The first example of the asymmetric bioamination of 1,5-diketones was recently reported with excellent regio- and stereoselectivity achieved.9 We found the commercially available (S)-selective transaminase ATA113 to be highly regioselective in mediating the reductive amination of 1 a exclusively on the methyl ketone at a substrate concentration of 25 mm with l-alanine as the amine donor. The resulting 1,4-amino ketone (S)-2 a subsequently underwent spontaneous cyclization to provide pyrroline (S)-3 a in high yield (91 %) and excellent ee (>99 %). The lactate dehydrogenase (LDH)/glucose dehydrogenase (GDH) system was used to drive the equilibrium towards the product and recycle the NAD+ cofactor (see the Supporting Information). The (R)-selective transaminase ATA117 also catalyzed the regio- and stereoselective monoamination of 1 a to afford pyrroline (R)-3 a in 65 % yield and >99 % ee.
Scheme 2.

Preparative-scale (25 mm 1 a) reductive amination of diketone 1 a mediated by (S)-selective ATA113 or (R)-selective ATA117, followed by spontaneous cyclization.
Having established an effective means of accessing optically pure pyrrolines on a preparative scale, we subsequently explored a route for the diastereoselective synthesis of 2,5-disubstituted pyrrolidine 4 a starting from 3 a (Scheme 3). 2,6-Disubstituted piperidines have been prepared through a chemoenzymatic route employing an ω-transaminase followed by diastereoselective hydrogenation using Pd/C.9 However, the same strategy is not applicable to the diastereoselective synthesis of 2,5-disubstituted pyrrolidines owing to poor diastereoselectivity in the reduction step. We envisaged using MAO-N variants in combination with NH3⋅BH3 for the asymmetric synthesis of 4 a. Two MAO-N variants (D5 and D9) were selected based on their known activity and excellent selectivity towards structurally related amine frameworks, including pyrrolidines and piperidines.3h,k Our strategy relies upon MAO-N variants displaying complete regio- and stereoselectivity to avoid stereorandomization of the C2-stereocenter generated by the (S)-selective ω-TA. Imine 3 a is in equilibrium with the open-chain amino ketone (S)-2 a and hence optimization of the MAO-N/NH3⋅BH3 oxidation/reduction cycle was necessary in order to prevent the formation of undesired amino alcohol as a side product. Ketone reduction was minimized by lowering the concentration of the MAO-N biocatalyst during the reaction while maintaining a high concentration of NH3⋅BH3. Under these conditions, rapid reduction of pyrroline 3 a occurred, thus ensuring that a minimal concentration of the amino ketone was present during the biotransformation.
Scheme 3.
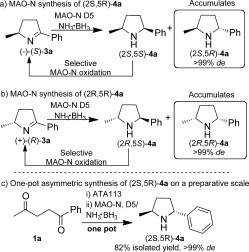
a, b) Analytical-scale synthesis of (2S,5R)- and (2R,5R)-4 a mediated by MAO-N D5. c) One-pot TA/MAO-N cascade for the preparative-scale asymmetric synthesis of (2S,5R)-4 a. Reduction of the starting diketone by NH3⋅BH3 prevented the addition of all of the reagents concurrently.
Treatment of (S)-3 a with NH3⋅BH3 afforded 4 a initially as a mixture of diastereoisomers with a slight excess of the (2S,5S)-isomer (de≈10 %; Scheme 3 a). Both MAO-N variants mediated the oxidation of the (2S,5S)-4 a diastereoisomer exclusively and displayed complete regioselectivity for the more bulky phenyl side of the pyrrolidine. Following successive rounds of selective oxidation with the MAO-N D5 variant and nonselective reduction with NH3⋅BH3, (2S,5R)-4 a was isolated in greater than 99 % de. Despite a bias towards the formation of the cis diastereoisomer upon reduction with NH3⋅BH3, the combination with MAO-N yielded solely the trans reduction product (2S,5R)-4 a. The complementary regioselectivity displayed by the ω-TA and MAO-N variants circumvents epimerization of the (S)-C2-center and provides a method for accessing optically pure 2,5-pyrrolidines.
Having developed efficient individual biocatalytic routes for the synthesis of optically pure pyrroline 3 a and the target chiral 2,5-pyrrolidine 4 a, we next sought to combine the ω-TA and MAO-N biocatalysts in a one-pot cascade (Scheme >3 c). Diketone 1 a was exposed to ATA 113 followed by MAO-N and NH3⋅BH3, and the target (2S,5R)-4 a was obtained in 82 % yield and >99 % de.
To allow access to the (2R,5R)-4 a diastereoisomer, the (R)-3 a enantiomer, derived from the use of ATA117, was treated with the NH3⋅BH3/MAO-N combination (Scheme 3b). Following nonselective reduction to give a mixture of (2R,5S)-4 a and (2R,5R)-4 a, both MAO-N variants mediated the selective oxidation of the (2R,5S)-isomer to provide (2R,5R)-4 a exclusively after successive rounds of oxidation/reduction. The stereochemistry at C2 has a minimal effect on the activity of the MAO-N enzyme and no effect on the stereoselectivity; the target (2R,5R)-3 a was isolated in >99 % de.
The generality of the TA/MAO-N cascade process was investigated by examining a series of diketones (1 b–g) using ATA113, as well as a novel transaminase (pf-ATA) from Pseudogulbenkiania ferrooxidans10 (Table 1). Pf-ATA shares 95 % sequence identity with the transaminase from Chromobacterium violaceum (cv-ATA; ATCC 12472).11 ATA113 mediated the reductive amination of diketones 1 b–g, the products of which spontaneously cyclized to yield pyrrolines 3 b–g as the sole regioisomers with excellent conversion and high ee values. Unsurprisingly, replacement of the small methyl group by a larger ethyl substituent resulted in a slightly reduced ee (entries 11 and 13). The biotransformations performed with Pf-ATA proceeded with reduced selectivity, with ee values lower than those achieved with ATA113. Interestingly, replacing the methyl substituent by an ethyl group resulted in a switch in stereoselectivity to give (R)-3 f, g as the predominant enantiomers (entries 12 and 14, see the Supporting Information for absolute configuration and ee determination). We also compared the selectivity observed with pf-ATA to that of the related cv-ATA against diketones 1 a and 1 d, e and noted comparable conversion and selectivity (see the Supporting Information). The (R)-selective ATA117 also mediated the reductive amination of diketones 1 a and 1 d, e in >99 % conversion and ee (see the Supporting Information).
Table 1.
TA-mediated reductive amination of 1 a–g. 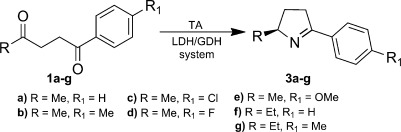
| Entry | Substrate | ω-TA | Conv. [%] | ee [%] |
|---|---|---|---|---|
| 1 | 1 a | ATA113 | >99 | >99 (S) |
| 2 | P. ferrooxidans | >99 | 75 (S) | |
| 3 | 1 b | ATA113 | >99 | >99 (S) |
| 4 | P. ferrooxidans | >99 | >78 (S) | |
| 5 | 1 c | ATA113 | >99 | >99 (S) |
| 6 | P. ferrooxidans | >99 | 68 (S) | |
| 7 | 1 d | ATA113 | >99 | >99 (S) |
| 8 | P. ferrooxidans | >99 | 76 (S) | |
| 9 | 1 e | ATA113 | >99 | >99 (S) |
| 10 | P. ferrooxidans | >99 | 78 (S) | |
| 11 | 1 f | ATA113 | 60 | 96 (S) |
| 12 | P. ferrooxidans | >99 | 76 (R) | |
| 13 | 1 g | ATA113 | >99 | 94 (S) |
| 14 | P. ferrooxidans | 75 | 46 (R) |
The efficiency of the MAO-N/NH3⋅BH3 step with the isolated pyrrolines 3 b–g was next examined (Table 2). In general, the D9 variant showed higher selectivity and employing either the D5 or D9 MAO-N variants allowed access to all of the Me/Ar and Et/Ar substituted pyrrolidines in excellent de. We have also extended the one-pot TA/MAO-N cascade for the synthesis of (2S,5R)-4 b, (2S,5R)-4 d, and (2S,5R)-4 e in >99 % conversion and >99 % de when starting from the corresponding diketones (Table 3), thus demonstrating the generality of this one-pot approach.
Table 2.
MAO/NH3⋅BH3-mediated asymmetric synthesis of (2S,5R)- 4 a–g 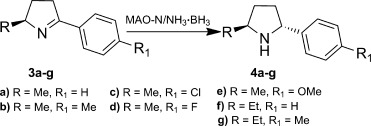
| Entry | Substrate | MAO-N variant | de [%] (2S,5R) |
|---|---|---|---|
| 1 | 3 a | D5 | >99 |
| 2 | D9 | 96 | |
| 3 | 3 b | D5 | 88 |
| 4 | D9 | 98 | |
| 5 | 3 c | D5 | >99 |
| 6 | D9 | >99 | |
| 7 | 3 d | D5 | >99 |
| 8 | D9 | >99 | |
| 9 | 3 e | D5 | 68 |
| 10 | D9 | >99 | |
| 11 | 3 f | D5 | 64 |
| 12 | D9 | >99 | |
| 13 | 3 g | D5 | 56 |
| 14 | D9 | 96 |
Table 3.
ATA113/MAO-N one-pot cascade for the synthesis of (2S,5R)-4 a, (2S,5R)-4 b, (2S,5R)-4 d, and (2S,5R)-4 e
| Ketone | ω-TA | MAO-N | Conv. [%] | de [%] |
|---|---|---|---|---|
| 1 a[a] | ATA113 | D5 | >99 | >99 (2S,5R)-4 a |
| 1 b[b] | ATA113 | D9 | >99 | >99 (2S,5R)-4 b |
| 1 d[b] | ATA113 | D9 | >99 | >99 (2S,5R)-4 d |
| 1 e[b] | ATA113 | D9 | >99 | >99 (2S,5R)-4 e |
[a] 25 mm substrate; [b] 5 mm substrate.
In summary, the combination of two complementary biocatalysts has been demonstrated in a novel one-pot chemoenzymatic cascade for the regio- and stereoselective synthesis of a panel of 2,5-disubstituted pyrrolidines from the corresponding 1,4-diketones. The transaminase ω-TA is highly selective for the sterically less demanding methyl ketone while the monoamine oxidase MAO-N shows an overwhelming preference for the more bulky portion of the corresponding pyrrolidine. The compatibility of the two biocatalysts means that the reaction can be performed in one pot without the need for costly intermediate purification steps. The chemoenzymatic approach exploits four distinct biocatalytic operations and takes advantage of the complementary regioselectivity displayed by the ω-TA and MAO variants to establish two stereogenic centers. All of the biocatalysts described herein are commercially available12 and hence readily accessible for practical application.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
References
- 1a.Reetz MT. J. Am. Chem. Soc. 2013;135:12480–12496. doi: 10.1021/ja405051f. [DOI] [PubMed] [Google Scholar]
- 1b.Turner NJ, O’ Reilly E. Nat. Chem. Biol. 2013;9:285–288. doi: 10.1038/nchembio.1235. [DOI] [PubMed] [Google Scholar]
- 2a.Schrittwieser JH, Sattler J, Resch V, Mutti FG, Kroutil W. Curr. Opin. Chem. Biol. 2011;15:249–256. doi: 10.1016/j.cbpa.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2b.Oroz-Guinea I, García E. Junceda. Curr. Opin. Chem. Biol. 2013;17:236–249. doi: 10.1016/j.cbpa.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 2c.Ricca E, Brucher B, Schrittwieser JH. Adv. Synth. Catal. 2011;353:2239–2262. [Google Scholar]
- 2d.Sattler JH, Fuchs M, Tauber K, Mutti FG, Faber K, Pfeffer J, Haas T, Kroutil W. Angew. Chem. 2012;124:9290–9293. doi: 10.1002/anie.201204683. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:9156–9159. doi: 10.1002/anie.201204683. [DOI] [PubMed] [Google Scholar]
- 3a.Höhne M, Bornscheuer U. ChemCatChem. 2009;1:42–51. [Google Scholar]
- 3b.Mathew S, Yun H. ACS Catal. 2012;2:993–1001. [Google Scholar]
- 3c.Savile CK, Janey JM, Mundorff EC, Moore JC, Tam S, Jarvis WR, Colbeck JC, Krebber A, Fleitz FJ, Brands J, Devine PN, Huisman GW, Hughes GJ. Science. 2010;329:305–309. doi: 10.1126/science.1188934. [DOI] [PubMed] [Google Scholar]
- 3d.Koszelewski D, Tauber K, Faber K, Kroutil W. Trends Biotechnol. 2010;28:324–332. doi: 10.1016/j.tibtech.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 3e.Frodsham L, Golden M, Hard S, Kenworthy MN, Klauber DJ, Leslie K, Macleod C, Meadows RE, Mulholland KR, Reilly J, Squire C, Tomasi S, Watt D, Wells AS. Org. Process Res. Dev. 2013;17:1123–1130. [Google Scholar]
- 3f.Alexeeva M, Enright A, Dawson MJ, Mahmoudian M, Turner NJ. Angew. Chem. 2002;114:3309–3312. doi: 10.1002/1521-3773(20020902)41:17<3177::AID-ANIE3177>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2002;41:3177–3180. doi: 10.1002/1521-3773(20020902)41:17<3177::AID-ANIE3177>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3g.Carr R, Alexeeva M, Enright A, Eve TSC, Dawson MJ, Turner NJ. Angew. Chem. 2003;115:4955–4958. doi: 10.1002/anie.200352100. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2003;42:4807–4810. doi: 10.1002/anie.200352100. [DOI] [PubMed] [Google Scholar]
- 3h.Dunsmore CJ, Carr R, Fleming T, Turner NJ. J. Am. Chem. Soc. 2006;128:2224–2225. doi: 10.1021/ja058536d. [DOI] [PubMed] [Google Scholar]
- 3i.Rowles I, Malone KJ, Etchells LL, Willies SC, Turner NJ. ChemCatChem. 2012;4:1259–1261. [Google Scholar]
- 3j.Znabet A, Polak MM, Janssen E, de Kanter FJJ, Turner NJ, Orru RVA, Ruijter E. Chem. Commun. 2010;46:7918–7920. doi: 10.1039/c0cc02823a. [DOI] [PubMed] [Google Scholar]
- 3k.Ghislieri D, Green AP, Pontini M, Willies SC, Rowles I, Frank A, Grogan G, Turner NJ. J. Am. Chem. Soc. 2013;135:10863–10869. doi: 10.1021/ja4051235. [DOI] [PubMed] [Google Scholar]
- 3l.Ghislieri D, Houghton D, Green AP, Willies SC, Turner NJ. ACS Catal. 2013;3:2869–2872. [Google Scholar]
- 4a.Li T, Liang J, Ambrogelly A, Brennan T, Gloor G, Huisman G, Lalonde J, Lekhal A, Mijts B, Muley S, Newman L, Tobin M, Wong G, Zaks A, Zhang X. J. Am. Chem. Soc. 2012;134:6467–6472. doi: 10.1021/ja3010495. [DOI] [PubMed] [Google Scholar]
- 4b.de Lange B, Hyett DJ, Maas PJD, Mink D, van Assema FBJ, Sereinig N, de Vries AHM, de Vries JG. ChemCatChem. 2011;3:289–292. [Google Scholar]
- 5a.Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K. Nature. 2012;485:185–194. doi: 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- 5b.Turner NJ. Nat. Chem. Biol. 2009;5:567–573. doi: 10.1038/nchembio.203. [DOI] [PubMed] [Google Scholar]
- 6a.Brenneman JB, Martin SF. Org. Lett. 2004;6:1329–1331. doi: 10.1021/ol049631e. [DOI] [PubMed] [Google Scholar]
- 6b.Xu F. Org. Lett. 2013;15:1324–1345. [Google Scholar]
- 6c.Hanessian S, Bayrakdarian M, Luo X. J. Am. Chem. Soc. 2002;124:4716–4721. doi: 10.1021/ja0126226. [DOI] [PubMed] [Google Scholar]
- 7a.Aggarwal VK, Astle CJ, Rogers-Evans M. Org. Lett. 2004;6:1469–1471. doi: 10.1021/ol049665m. [DOI] [PubMed] [Google Scholar]
- 7b.Zhang S, Xu L, Miao L, Shu H, Trudell ML. J. Org. Chem. 2007;72:3133–3136. doi: 10.1021/jo062532p. [DOI] [PubMed] [Google Scholar]
- 7c.Goti A, Cicchi S, Mannucci V, Cardona F, Guarna F, Marino P, Tejero T. Org. Lett. 2003;5:4235–4238. doi: 10.1021/ol035798g. [DOI] [PubMed] [Google Scholar]
- 7d.Trost BM, Horne DB, Woltering MJ. Angew. Chem. 2003;115:6169–6172. doi: 10.1002/anie.200352857. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2003;42:5987–5990. doi: 10.1002/anie.200352857. [DOI] [PubMed] [Google Scholar]
- 7e.Severino EA, Correia CRD. Org. Lett. 2000;2:3039–3042. doi: 10.1021/ol005762d. [DOI] [PubMed] [Google Scholar]
- 8a.Davis FA, Song M, Augustine A. J. Org. Chem. 2006;71:2779–2786. doi: 10.1021/jo052566h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b.Lemen GS, Wolfe JP. Org. Lett. 2010;12:2322–2325. doi: 10.1021/ol1006828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8c.Moloney MG, Panchal T, Pike R. Org. Biomol. Chem. 2006;4:3894–3897. doi: 10.1039/b611583g. [DOI] [PubMed] [Google Scholar]
- 8d.Enkisch C, Schneider C. Eur. J. Org. Chem. 2009:5549–5564. [Google Scholar]
- 8e.Campos KR, Kalpars A, Waldman JH, Dormer PG, Chen C-y. J. Am. Chem. Soc. 2006;128:3538–3539. doi: 10.1021/ja0605265. [DOI] [PubMed] [Google Scholar]
- 8f.Davis FA, Zhang J, Qui H, Wu Y. Org. Lett. 2008;10:1433–1436. doi: 10.1021/ol800255r. [DOI] [PubMed] [Google Scholar]
- 9.Simon RC, Grischek B, Zepeck F, Steinreiber A, Belaj F, Kroutil W. Angew. Chem. 2012;124:6817–6820. doi: 10.1002/anie.201202375. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2012;51:6713–6716. doi: 10.1002/anie.201202375. [DOI] [PubMed] [Google Scholar]
- 10.Byrne-Bailey KG, Weber KA, Coates JD. J. Bacteriol. 2012;194:2400–2401. doi: 10.1128/JB.00214-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaulmann U, Smithies K, Smith MEB, Hailes HC, Ward JM. Enzyme Microb. Technol. 2007;41:628–637. [Google Scholar]
- 12a. MAO-N Screening Kits: http://www.discovery-bc.co.uk/monoamineoxidase.php (2013)
- 12b. Transaminases: http://www.codexis.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


