Abstract
Matrix metalloprotease-1 (MMP1) has been implicated in many human disease processes, however the lack of a well characterized murine homologue has significantly limited the study of MMP1 and the development of MMP-targeted therapeutics. The discovery of murine Mmp1a in 2001, the functional mouse homologue of MMP1, offers a valuable tool for modeling MMP1-mediated processes in mice. Variation in physiologic expression levels of Mmp1a in mice as compared to MMP1 in humans highlights the importance of understanding the similarities and differences between the homologues. Recent studies have demonstrated tumor growth-, invasion-, and angiogenesis-promoting functions of Mmp1a in lung cancer models, consistent with the analogous functions observed for human MMP1. Biochemical investigations have shown that point mutations in the pro-domain of mouse Mmp1a weaken docking between the pro- and catalytic domains, generating an unstable zymogen primed for activation. The difficulty to effectively maintain Mmp1a in the zymogen form may account for the tight control of Mmp1a expression and reduced expression in normal tissue as compared to inflammatory states or cancer. This discovery raises important questions about the activation mechanisms and regulation of the MMP family in general.
Since the initial discovery of MMP1 over 50 years ago in tadpoles, the matrix metalloprotease family has expanded to 24 enzymes in humans with a multitude of functions (Gross and Lapiere, 1962). MMP1 (interstitial collagenase) was initially described for its ability to degrade fibrillar collagen (types I, II, and III) with the subsequent identification of two similar enzymes, MMP8 (neutrophil collagenase) and MMP13 (collagenase 3), which make up the soluble collagenase sub-family (Murphy et al., 1977; Freije et al., 1994). In addition to their shared collagen-degrading activity, a common domain organization of pre-, pro-, catalytic, linker, and hemopexin regions unify the collagenases. Though originally defined as a collagenase, MMP1 has been shown to have activity against a broad array of extracellular matrix substrates. MMP1 can degrade the matrix proteins fibronectin, gelatin, aggrecan, laminin, perlecan, and vitronectin (Ala-aho and Kähäri, 2005).
MMP1, like the other MMPs, also has significant activity against multiple non-matrix substrates thereby modulating cell behavior and many physiologic and pathophysiologic processes. For example, MMP1 can activate pro-tumor necrosis factor alpha (pro-TNFα) into its soluble form (Gearing et al., 1994). MMP1 can also increase the bioavailability of IGF through degradation of insulin-like growth factor binding proteins (Fowlkes et al., 1994). MMP1 can dampen inflammation through inactivation of stromal cell derived factor 1 alpha (SDF1α) and monocyte chemoattractant proteins 1–4 (MCP 1–4) (McQuibban et al., 2001, 2002). Through proteolysis of these diverse substrates, MMP1 has been implicated in many pathological processes, such as tumor growth and metastasis, arthritis, atherosclerosis, and septic shock [Sukhova et al. (1999) and Tressel et al. (2011) and reviewed in Ala-aho and Kähäri (2005) and Vincenti and Brinckerhoff (2002)].
Of particular interest is the activation of protease-activated receptor-1 (PAR1) by MMP1 (reviewed in Austin et al., 2013a). PAR1 is a G-protein coupled receptor that is activated by proteolytic cleavage and has pleiotropic effects on cell proliferation, survival, migration, and gene transcription. PAR1 is classically activated by serine proteases, such as thrombin (Vu et al., 1991). However, in many disease models, including cancer, sepsis, thrombosis, and arterial restenosis, MMP1 appears to be a pathophysiologic PAR1 agonist (Boire et al., 2005; Trivedi et al., 2009; Tressel et al., 2011; Austin et al., 2013b). Interestingly, activation of PAR1 by MMP1 occurs at a slightly different site from the canonical thrombin cleavage site, generating a slightly different ligand. Recent work has demonstrated that this ligand difference modulates the PAR1 signaling phenotype in different ways, making it essential to understand the PAR1 activation cascade by different protease systems (Blackburn and Brinckerhoff, 2008; Austin et al., 2013b). However, the delayed characterization of the MMP1 homologue in mice has made it difficult to query the role of MMP1-PAR1 signaling, as well as other MMP1-mediated processes in general.
Murine Collagenases
Though MMP1 was the first MMP described in 1962, the mouse genetic homologue for MMP1 was the last murine collagenase homologue discovered in 2001 (Balbín et al., 2001). The first collagenase cloned in mice was Mmp13 (Henriet et al., 1992). The discovery of mouse MMP13 occurred 2 years prior to the discovery of human MMP13 (Freije et al., 1994), leading to the initial presumption that mouse Mmp13 was a divergent homologue of human interstitial collagenase. Three independent groups described mouse Mmp8 in 1998 (Balbín et al., 1998; Iwama et al., 1998; Lawson et al., 1998). Finally, in 2001 the two murine genes homologous to MMP1 were identified, murine collagenase-like A (Mcol-A/Mmp1a) and murine collagenase-like B (McolB/Mmp1b) (Balbín et al., 2001).
Significant redundancy in substrates and function tends to exist between closely related MMPs, leading to the postulate that the other mouse collagenases, Mmp8 and Mmp13, could be functional replacements of MMP1 in mouse pathobiology. As the three collagenases are further studied, a unique subset of functions has been identified for each of the collagenases. MMP8 is strongly expressed by neutrophils and stored in granules that can be released following activation (Hasty et al., 1990). With its high expression in neutrophils, MMP8 is an important modulator of inflammation. Studies are increasingly highlighting the role of MMP8 as an unexpected tumor suppressor gene. Mmp8-deficient male mice have a significantly increased incidence of skin tumors in experimental carcinogenesis models due to loss of neutrophil MMP8 and an abnormal inflammatory response (Balbín et al., 2003). Expression of MMP8 on breast, lung, and melanoma cancer cells also exhibits tumor suppressive functions by enhancing cell adhesion and decreasing cell invasion, migration, and metastasis (Montel et al., 2004; Gutiérrez-Fernández et al., 2008). Most strikingly, inactivating mutations in MMP8 have been identified in a subset of human melanoma tumors, strongly supporting a tumor suppressor function for MMP8 in human tumors (Palavalli et al., 2009).
Though MMP8 appears to have a distinct functional profile from the other collagenases, MMP1 and MMP13 are more biochemically and pathologically similar (Vincenti et al., 1998). MMP1 and MMP13 share 86% amino acid identity, whereas MMP8 is 57% identical to MMP1. MMP1 and MMP13 are both frequently overexpressed in inflammatory conditions and are transcriptionally regulated by many of the same inflammatory cytokines (Vincenti and Brinckerhoff, 2002; Ala-aho and Kähäri, 2005). MMP13 was originally cloned from a human breast cancer and appears to function as a tumor-promoting metalloprotease, like MMP1 (Freije et al., 1994). Mmp13-deficient mice have decreased tumor growth and metastasis of B16 melanoma allografts and decreased growth and angiogenesis of squamous cell carcinoma allografts (Zigrino et al., 2009; Lederle et al., 2010). Similarly, exogenous expression of MMP1 promotes tumorigenesis, invasion, and metastasis of human melanoma cells (Blackburn et al., 2009). While this suggests that there may be overlap in the functions ofMMP1 and MMP13, it does not exclude specific roles for MMP1 and MMP13. In many systems, MMP1 or MMP13 are expressed singularly, suggesting that even if overlap exists between these enzymes, they are likely not both simultaneously present in the same system to compensate for one another.
Mouse Matrix Metalloprotease-1
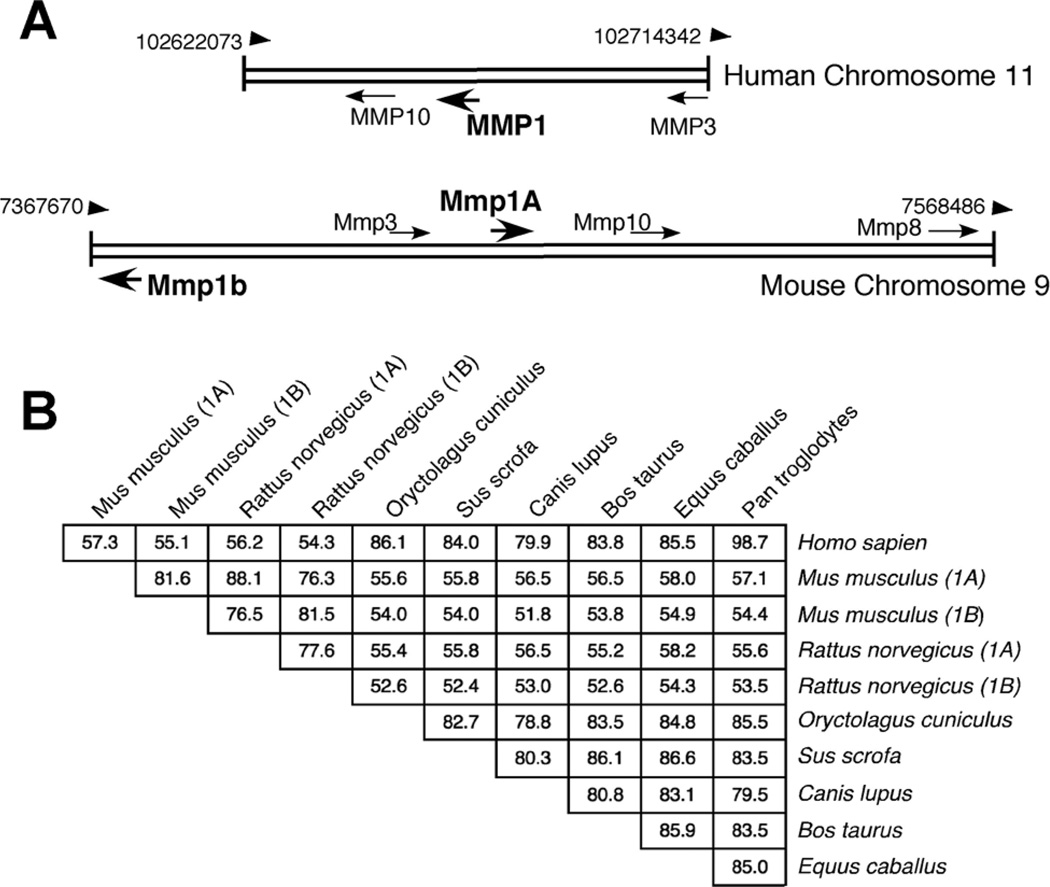
The mouse homologue of MMP1 was discovered by screening mouse cDNA libraries with a full length human MMP1 probe by Balbín et al. (2001). This strategy identified two MMP1 homologues, Mmp1a and Mmp1b. These two enzymes are a rodent-specific duplication. Mmp1a and Mmp1b are located in the MMP-rich locus of mouse chromosome 9 (Fig. 1A). The location of Mmp1a is syntenic to human MMP1, while Mmp1b is inverted and located roughly 73 kb away. Mmp1a and Mmp1b proteins are 82% identical to each other (Fig. 1B). Their genes most closely resemble human MMP1 (74% identical in nucleotides). Mmp1a has 58% amino acid identity with MMP1, making it the least identical of the known mammalian MMP1 homologues, as shown in Figure 1B. As a comparison, rabbit MMP1, which has been studied in arthritis models, is 86% identical to human MMP1 (Fini et al., 1987).
Fig. 1.
Homology of MMP1 and Mmp1a. A: Chromosomal location of MMP1 (upper) and Mmp1a (lower) in the MMP rich locus of humans and mice respectively. B: Percent similarity of mammalian MMP1 homologue protein sequences. Percentage determined by Clustal sequence alignment using the Meg Align program.
Biochemistry of Mmp1a and Mmp1b
Mmp1a and Mmp1b are 464 and 463 amino acid proteins, respectively, with predicted masses of 53.5 kDa. Both enzymes contain the collagenase domain arrangement (signal peptide, pro-, catalytic, linker, and hemopexin) and a characteristic catalytic domain RGD-motif that is present in all other identified MMP1 homologues. Mmp1a contains three potential N-linked glycosylation sites, while Mmp1b contains two. A conserved catalytic-domain glycosylation site present in human MMP1 and all other collagenases is seen in Mmp1a and Mmp1b at N117.
Based on peptide sequence and structure prediction, Mmp1a and Mmp1b should both be functional enzymes. When expressed, Mmp1a demonstrates collagenase activity, generating the classic 3/4 and 1/4 length degradation fragments from fibrillar collagens. Unexpectedly, Mmp1b has not been documented to have any detectable activity against fibrillar collagen when expressed recombinantly in E. coli inclusion bodies or in mammalian overexpression systems (Balbín et al., 2001; Foley et al., 2012). However, Mmp1b does not appear to completely lack enzymatic activity. Incubation of purified, recombinant pro-Mmp1b overnight at room temperature results in autoproteolysis of the pro-domain to "active" Mmp1b (Balbín et al., 2001). It remains to be determined whether Mmp1b possesses activity against other substrates or is a functionally inactive duplication of Mmp1a. Structure-function and gain-of-function studies of Mmp1b as compared to Mmp1a have the potential to offer useful insights into structural determinants of active collagenases.
Tissue Expression of Mmp1a
The most striking difference between human MMP1 and mouse Mmp1a is the tissue expression profile. Human MMP1 has low basal expression in most human tissues and can be dynamically upregulated in response to cell activation. Mmp1a, on the other hand, has only been readily detected in days 9.5–13.5 embryos and in the placenta, uterus, and testes of physiologically normal mice (Balbín et al., 2001; Nuttall et al., 2004). Despite the low expression of Mmp1a in healthy mouse tissue, Mmp1a upregulation has been described in multiple disease models. Mmp1a mRNA is elevated in the mouse stroma of human breast cancer cell xenografts (Boire et al., 2005). Rat Mmp1a mRNA was found to be elevated >20-fold in rats with septic colonic anastamoses, recapitulating what has been observed with human MMP1 in patients who have the post-surgical complication of anastomotic dehiscence (Silva et al., 2013). Consistent with the observation that plasma MMP1 levels are a predictor of mortality in patients with septic shock, Mmp1a protein is upregulated in the plasma of mice with cecal ligation and puncture (CLP)-induced sepsis (Tressel et al., 2011). In stroke models, Mmp1a is expressed in ischemic brain and further upregulated following reperfusion, suggesting a role for Mmp1a in ischemia-reperfusion injury (Lenglet et al., 2014). Additionally, Mmp1a protein is expressed in multiple mouse cancer models, including glioblastoma, renal, kidney, and ovarian cancer xenografts (Pfaffen et al., 2010). Together, these observations suggest that Mmp1a is a relevant MMP1 homologue in mouse models.
The Mmp1a Knockout Mouse
The ability to identify specific functions of Mmp1a in disease models has been significantly enhanced by the recent generation of Mmp1a knockout mice (Fanjul-Fernández et al., 2013; Foley et al., 2013). The Mmp1a−/− mouse was generated by targeted replacement of exons 4, 5, and part of exon 6 with a PGK-Neomycin cassette. These exons encode the majority of the Mmp1a catalytic domain, including the critical zinc coordination motif (H215ELGH219SLGLTH225) in exon 5. This knockout strategy is highly similar to the approach that was successfully used for the generation of Mmp8−/− mice (Balbín et al., 2003). The mutant allele disrupts all Mmp1a expression, as Mmp1a−/− placenta has no detectable Mmp1a mRNA by Northern blot. Mmp1a-deficient mice are born in Mendelian ratios from heterozygous crosses. Loss of Mmp1a has no obvious effect on growth or development, which is unsurprising given that the majority of MMP knockout animals, including Mmp8−/− and Mmp13−/− mice, have no overt defects. Mmp1a−/− mice are fertile. Given the constitutive expression of Mmp1a in the placenta and testes of adult mice, the lack of a fertility phenotype was surprising and raises interesting questions about the role of Mmp1a/MMP1 in reproduction.
Mmp1a in Lung Cancer Models
Recent studies have directly demonstrated important functional roles for Mmp1a in lung cancer models (Fanjul-Fernández et al., 2013; Foley et al., 2012, 2013). Lewis lung carcinoma (LLC1), a c57BL/6 mouse-derived, non-small cell lung cancer (NSCL) cell line, expresses high levels of Mmp1a protein (Foley et al., 2012). LLC1 cells are highly malignant, demonstrating rapid growth, invasion, and metastasis. Short-hairpin RNA (shRNA) knockdown of Mmp1a reverses the invasive phenotype of LLC1 cells in in vitro models of collagen invasion and three-dimensional stellate growth. Additionally, loss of LLC1 Mmp1a expression results in a 50% reduction in tumor growth in abdominal fat pad genograft models, demonstrating the importance of Mmp1a in promoting tumorigenesis. LLC1 Mmp1a also promotes metastasis, as silencing of Mmp1a leads to a 40% decrease in number of metastatic nodules in experimental lung metastasis (Foley et al., 2012).
The Mmp1a-deficient mouse is a highly valuable tool for studying the role of stromal Mmp1a in the tumor microenvironment. LLC1 cells implanted into the abdominal fat pads of Mmp1a−/− mice form significantly smaller tumors with impaired angiogenesis as compared to wild type littermate controls (Foley et al., 2013). The tumorigenesis defect in Mmp1a−/− mice can be rescued with co-implantation of wild-type fibroblasts, demonstrating the importance of stromal Mmp1a. On the other hand, stromal Mmp1a is dispensable in experimental lung metastasis models, as Mmp1a−/− animals do not exhibit any significant decrease in the number of metastatic foci. This observation is consistent with previous work that human MMP1 expression by the cancer cell, not the stroma, is part of the "metastatic toolkit" that enables cancer cells to metastasize to lungs (Minn et al., 2005).
The defect in LLC1 tumorigenesis in Mmp1a-deficient mice appears to be significantly mediated by Mmp1a-PAR1 signaling. RNAi mediated knockdown of PAR1 on LLC1 cells results in decrease in tumor growth in wild-type mice that is comparable to the defect seen with wild-type LLC1 cells in Mmp1a−/− mice (Foley et al., 2013). Implantation of PAR1-knockdown LLC1 cells in Mmp1a−/− mice does not have further effect on tumor growth, suggesting that Mmp1a and PAR1 are acting through the same pathway to promote tumorigenesis.
Further strengthening a role for Mmp1a in lung carcinogenesis, Fanjul-Fernández et al. (2013) examined Mmp1a levels in induced carcinogenesis models. Mmp1a was upregulated in models of chronic lung fibrosis using bleomycin treatment and in chemically induced lung carcinogenesis using urethane. When subjected to two intraperitoneal injections of urethane, Mmp1a-deficiency protected mice from lung tumorigenesis 8 months later. Mmp1a−/− male mice had a greater than 50% reduction in the number of total tumor foci, with significantly fewer large (>103 cells) lesions. Mmp1a−/− lungs had an increase in inflammatory infiltrate following urethane treatment as compared to wild type. The levels of the Th1-associated cytokines, IL-1α, IL-2, IL-27, and IFN-γ, were elevated in urethane treated Mmp1a−/− lungs, suggesting that Mmp1a may be involved in regulating the balance between Th1/Th2 inflammatory responses. The shift to a Th1 response in Mmp1a-deficient mice is hypothesized to occur via defective degradation of the Mmp1a-target protein S100a8. S100a8 is a calcium binding protein that activates the pattern recognition receptor, RAGE (receptor for advanced glycation end products), which promotes T cell Th1 differentiation (Chen et al., 2008). Thus, lack of Mmp1a is conjectured to elevate S100a8 levels, causing prolonged RAGE activation and a subsequent anti-tumorigenic, Th1 immune response. It will be interesting to see how this novel discovery from the Mmp1a−/− mouse translates into human disease.
Another interesting discovery gleaned from the Mmp1a−/− mouse is that Mmp1a-deficient female mice had a less dramatic, non-significant reduction of approximately 20% in the number of tumor foci in urethane-induced carcinogenesis. This sex discrepancy of tumor burden in MMP-deficient mice has been previously described in Mmp8−/− mice, as discussed above. These sex specific phenotypes in Mmp1a−/− and Mmp8−/− mice suggest an interaction between collagenases, sex hormones, and tumorigenesis adding additional layers of complexity to our understanding of MMP signaling networks.
Mmp1a in Other Cancer Models
Given the recent success of utilizing the Mmp1a−/− mouse in lung cancer models, the question becomes how to predict which cancer models have relevant expression of Mmp1a. Thus far, Mmp1a expression has been detected in LLC1, urethane-induced lung cancer, B16 melanoma cells, and ovarian and breast cancer xenografts, as discussed previously (Boire et al., 2005; Foley et al., 2012; Fanjul-Fernández et al., 2013). Additionally, Mmp1a expression drives migration and invasion in primary cutaneous melanoma tumors with constitutive BRAF activity from V600E BRAFV600E/p19ARF−/− mice (Foley et al., 2012). Tissues from other models of chemically induced carcinogenesis also exhibit upregulation of Mmp1a, including 4-nitroquinoline 1-oxide (4-NQO)-induced oral squamous carcinoma, 7,12-dimethylbenzantracene (DMBA)-induced squamous papilloma, and 3-methylcholanthrene (MCA)-induced fibrosarcoma (Fanjul-Fernández et al., 2013).
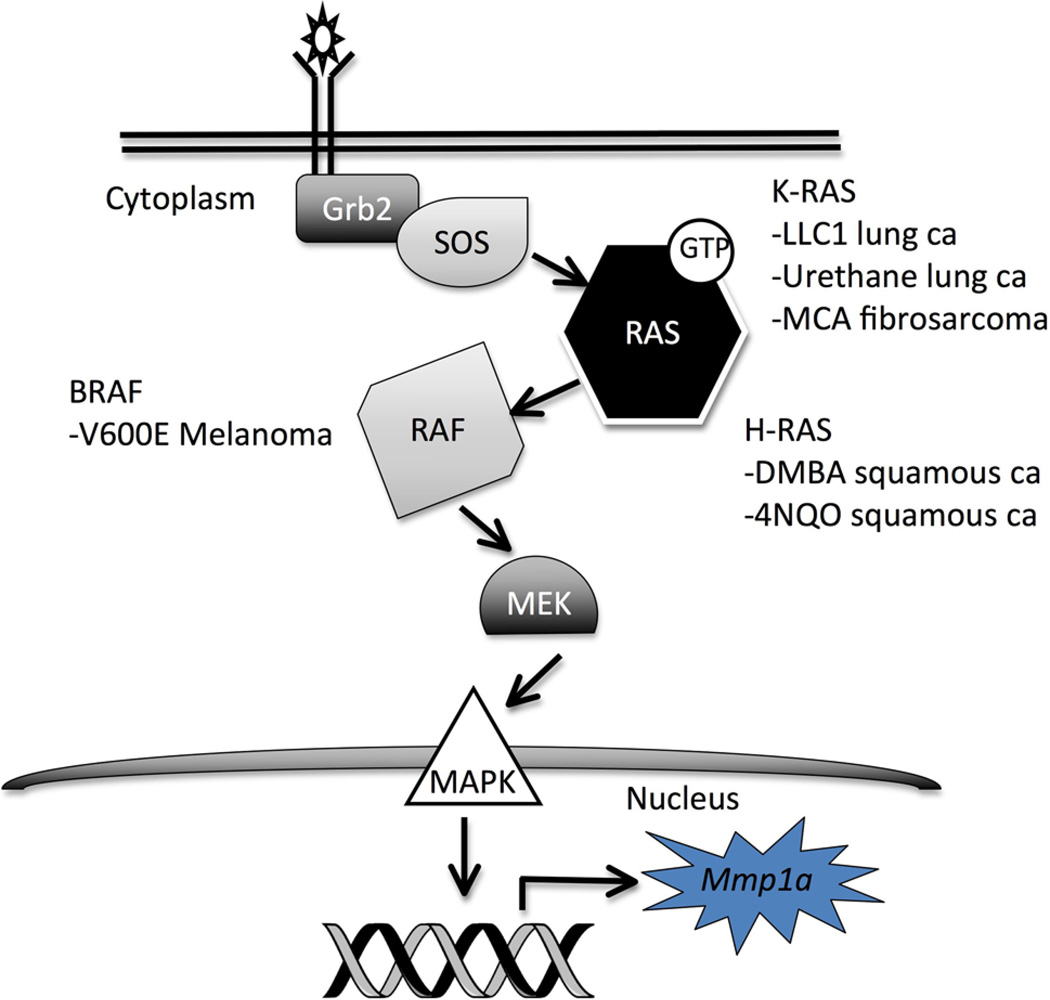
A unifying theme from these diverse cancer models is aberrant RAS activation/MAP kinase (MAPK) signaling (Fig. 2). LLC1 cells exhibit hyperactivation of K-Ras, through a mechanism potentially involving loss of p66Shc (Ma et al., 2010). In human melanomas, V600E is the most common point mutation in BRAF, a serine-threonine kinase activated by Ras, and results in constitutive BRAF activation (Davies et al., 2002). The chemical carcinogenesis models, though associated with multiple DNA mutations, are also associated with mutations in the Ras isoforms. Urethane-induced lung tumors and MCA-induced fibrosarcomas often harbor activating mutations in K-Ras (You et al., 1989; Watanabe et al., 1999). Treatment with DMBA or 4-NQO causes H-Ras mutations in skin papillomas and oral squamous carcinomas, respectively (Quintanilla et al., 1986; Yuan et al., 1994). We postulate that it is this activation of the MAPK cascade that leads to transcription of Mmp1a, potentially through the activation of AP1 or Ets, transcription factors shown to regulate human MMP1 expression (Vincenti and Brinckerhoff, 2002). While there are certainly multiple common pathways activated in these diverse cancer models and likely many pathways drivingMmp1a upregulation in inflammation, systems with aberrant MAPK signaling are obvious initial models to further explore Mmp1a function in vivo.
Fig. 2.
Generalized schematic of MAP kinase signaling demonstrating the points of pathway activation for cancer models shown to overexpress Mmp1a.
Mmp1a Zymogen Stability and Activation
Mmp1a functions as an important human homologue in cancer models. However, there remain fundamental differences between mouse Mmp1a and human MMP1 expression profiles in quiescent tissue. MMPs are regulated on several levels: expression, trafficking, zymogen activation, and enzyme inhibition/deactivation (Sternlicht and Werb, 2001). Mmp1a appears to be very tightly regulated at the gene expression level, given the lack of physiologic expression in healthy tissue and the selective expression in disease models.
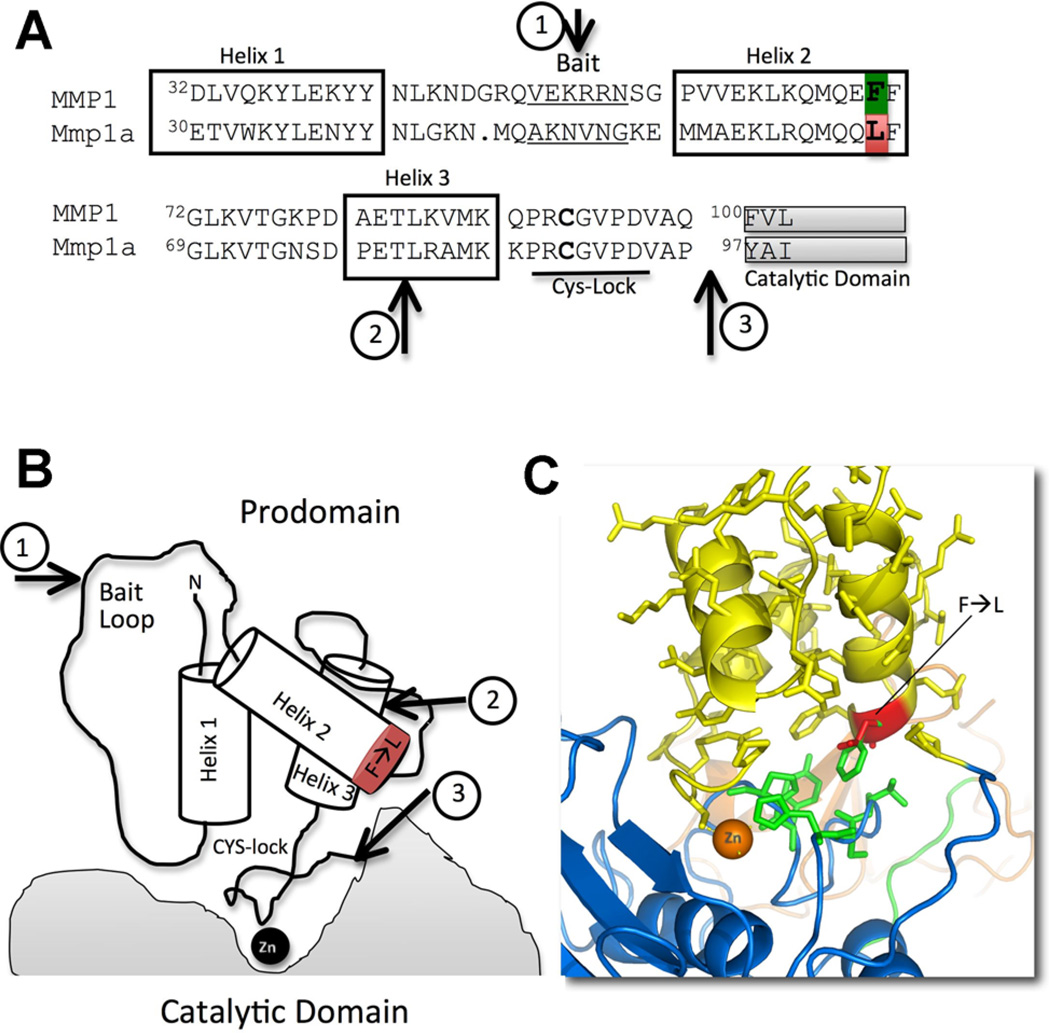
Recent biochemical studies suggest that this tight transcriptional control may be due to a propensity of spontaneous zymogen activation due to alterations in the procatalytic domain interactions (Foley et al., 2013). As compared to human MMP1 and the other collagenases, Mmp1a is secreted at low levels in heterologous expressions systems. Of the Mmp1a that is produced, it is more rapidly converted to the active form than the other collagenases. Chimeric exchange of the Mmp1a pro-domain for that of human MMP1 dramatically improves expression and stability of pro-Mmp1a, suggesting that the lack of stability of pro-Mmp1a is an inherent property of the prodomain. The prodomain of MMP1/Mmp1a is a bundle of three helices separated by flexible loop regions (Fig. 3). These three helices have multiple interactions with each other and the catalytic and hemopexin domains to stabilize the prodomain structure (Jozic et al., 2005). Homology modeling of pro-Mmp1a on the human pro-MMP1 crystal structure reveals the substitution of a highly conserved phenylalanine in human MMP1 at position 70 (F70-green) for a leucine in Mmp1a (L67-red) (Fig. 3A). In the human pro-MMP1 crystal structure, the F70 residue is an important interface between the catalytic domain and the pro-domain (Fig. 3C). Leucine is a smaller, hydrophobic residue and is predicted to result in a weaker pro-catalytic domain interaction in Mmp1a.
Fig. 3.
The prodomain of MMP1/Mmp1a. A: Sequence alignment of the human MMP1 (upper) and mouse Mmp1a (lower) prodomain (excluding the first 12 non-helix residues). The F70-L67 substitution is highlighted. Arrows illustrate cleavage sequence of pro-MMP1 activation. B: Cartoon representation of the MMP1/Mmp1a prodomain interaction with catalytic domain. Arrows illustrate cleavage sequence of pro-MMP1 activation. C: Structural model representing the prodomain (yellow) and catalytic domain (blue) of MMP1/Mmp1a. The hydrophobic interaction of human prodomain F70 (green) and mouse L67 (red) with catalytic domain H225S226S227 and F234P235 (green) are highlighted.
The F→L67 substitution in proMmp1a occurs at the distal end of helix 2 (Fig. 3). Helix 2 is virtually perpendicular to the catalytic domain. In the human pro-MMP1 structure, a highly conserved F70F71 at the distal end of helix 2 interacts directly with catalytic domain H228S229T230 and Y237P238 (Fig. 3C). F70F71 is conserved in every other known MMP1 homologue and is in the other secreted collagenases, MMP8 and MMP13. In pro-Mmp1a, the corresponding residues are L67F68 interacting with catalytic domain H225S226S227 and F234P235. The F→L67 substitution reduces the hydrophobic interactions of this highly conserved pro-catalytic domain interaction, resulting in a zymogen with a propensity for facile activation. Though the exact mechanism for the spontaneous activation of pro-Mmp1a has not been completely delineated, it appears to be heavily dependent on auto-catalysis as opposed to exogenous proteolysis. This hypothesis is supported by the observation that catalytically inactive Mmp1a is robustly expressed and maintained in zymogen form. Additionally, Mmp1b, which lacks potent enzyme activity and also contains the L66F67 motif of Mmp1a, is strongly produced in the zymogen form. Thus, the destabilizing effect of this mutation on zymogen maintenance is dependent on catalytic domain activity.
MMPs are maintained in the zymogen state through a conserved cysteine switch motif (PRCxxPD) in the pro-domain (Van Wart and Birkedal-Hansen, 1990). In this motif, the free thiol of the cysteine acts as a fourth coordinating residue for the zinc moiety in the active site. This interaction enables the pro-domain to essentially cover the active site and prevent a water molecule from entering the catalytic pocket (Van Wart and Birkedal-Hansen, 1990). Disruption of the thiol–zinc interaction leads to zymogen activation. This disruption can occur through proteolysis of the prodomain or non-proteolytic mechanisms. Non-proteolytic activation occurs through reduction of the free thiol to disrupt the thiol–zinc interaction by oxidants, heavy metals, or alkylating agents. Following loss of thiol–zinc coordination, water can enter the catalytic pocket and the pro-domain is subsequently removed through an intramolecular, autolytic cleavage (Stricklin et al., 1983).
While thiol-reduction is a useful laboratory technique for MMP activation, proteolytic activation by other proteases is likely the predominant activation mechanism in vivo. Proteolytic activation is a multi-step process in which a "bait" region of the pro-domain is initially cleaved, exposing previously masked residues and promoting destabilization of the helix bundle. The exact activation sequence for MMP1 has not been defined because no protease has been shown to be a definitive activator of MMP1 in vivo. However, stepwise models have been extrapolated from the proMMP1 crystal structures and in vitro studies of MMP1 activation by MMP3, trypsin, plasmin, and plasma kallikrein (Stricklin et al., 1983; Murphy et al., 1987; Suzuki et al., 1990; Jozic et al., 2005). The prodomain of MMP1 contains the "bait region," V51KERRNS57, that is, flexible and exposed on the surface (Fig. 3, arrow 1). Activating proteases initially cleave this bait region, leading to destabilization of the helix bundle and allowing for a secondary autolytic cleavage at T82L83 (Fig. 3, arrow 2). The pro- and catalytic domain junction is then exposed so that this region can be cleaved either by exogenous protease or autolytic cleavage to generate a mature, active enzyme (Fig. 3, arrow 3).
As the pro-form of Mmp1a is inherently unstable, it may be a dangerously active enzyme for tissues to express. Once produced, a significant fraction of the enzyme is rapidly activated, bypassing the regulatory step of activation and essentially behaving as a constitutively active protease. The main mechanisms remaining for cells to control Mmp1a activity are the only regulation of protein translation/trafficking and enzyme inactivation. Hence, it is expected that Mmp1a is not ubiquitously expressed but rather upregulated in response to appropriate stimuli.
Implications for Future Studies
A limitation to our knowledge of MMPs is the reliance on in vitro systems conducted under optimized conditions usually with excesses of substrate or enzyme. It is far more difficult to define how these enzymes behave in physiologic systems. In particular, little is understood about MMP activation cascades function in vivo (Ra and Parks, 2007). In vitro experiments have demonstrated a wide array of proteases that activate MMP1 under in vitro conditions. However, it is necessary to understand the activation mechanism in vivo, as this has a critical effect on the activity of the enzyme. The process by which MMP1 is activated determines the final N-terminus of the mature enzyme and the resultant catalytic efficiency. For example, MMP3 activation of MMP1 generates a mature enzyme that begins with F100. Activation of MMP1 by organomercurials/autolytic cleavage results in a mature enzyme starting at V101 or L102 (Suzuki et al., 1990). MMP3-activated F100-MMP1 has 10-fold more collagenase activity than V101- or L102-MMP1, suggesting that the mechanism of activation is an additional regulator of MMP1 activity. The in vivo activators of a given MMP offer additional targets for modulating MMP signaling and activity in disease processes.
The facile activation of pro-Mmp1a adds further complexity to the variation between the activation kinetics of all MMP family members. When and how do certain tissue milieus and disease states generate environments conducive to MMP activation? Are other MMPs "primed" for activation and under what biological conditions does this occur? What downstream signaling is modulated by this propensity for activation? While novel strategies for monitoring MMP activation in vivo will help answer these questions, the generation of animals deficient in a given MMP or MMP-activating protease will be valuable tools for advancing the field. Ideally, understanding the precise activation behavior of MMPs in vivo will identified new approaches to targeting MMPs for treatment of human disease.
Acknowledgments
Contract grant sponsor: National Institute of Heart, Lung, and Blood;
Contract grant numbers: F30-HL104835, R01-HL57905.
Footnotes
The authors declared that they have no conflict of interest.
Literature Cited
- Ala-aho R, Kähäri V-M. Collagenases in cancer. Biochimie. 2005;87:273–286. doi: 10.1016/j.biochi.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Austin KM, Covic L, Kuliopulos A. Matrix metalloproteases and PAR1 activation. Blood. 2013a;121:431–439. doi: 10.1182/blood-2012-09-355958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin KM, Nguyen N, Javid G, Covic L, Kuliopulos A. Noncanonical matrix metalloprotease-1-protease-activated receptor-1 signaling triggers vascular smooth muscle cell dedifferentiation and arterial stenosis. J Biol Chem. 2013b;288:23105–23115. doi: 10.1074/jbc.M113.467019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbín M, Fueyo A, Knäuper V, López JM, Alvarez J, Sánchez LM, Quesada V, Bordallo J, Murphy G, López-Otín C. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J Biol Chem. 2001;276:10253–10262. doi: 10.1074/jbc.M009586200. [DOI] [PubMed] [Google Scholar]
- Balbín M, Fueyo A, Knäuper V, Pendás AM, López JM, Jiménez MG, Murphy G, López-Otín C. Collagenase 2 (MMP-8) expression in murine tissue-remodeling processes. Analysis of its potential role in postpartum involution of the uterus. J Biol Chem. 1998;273:23959–23968. doi: 10.1074/jbc.273.37.23959. [DOI] [PubMed] [Google Scholar]
- Balbín M, Fueyo A, Tester AM, Pendás AM, Pitiot AS, Astudillo A, Overall CM, Shapiro SD, López-Otín C. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- Blackburn JS, Brinckerhoff CE. Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am J Pathol. 2008;173:1736–1746. doi: 10.2353/ajpath.2008.080512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JS, Liu I, Coon CI, Brinckerhoff CE. A matrix metalloproteinase-1/protease activated receptor-1 signaling axis promotes melanoma invasion and metastasis. Oncogene. 2009;28:4237–4248. doi: 10.1038/onc.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Chen Y, Akirav EM, Chen W, Henegariu O, Moser B, Desai D, Shen JM, Webster JC, Andrews RC, Mjalli AM, Rothlein R, Schmidt AM, Clynes R, Herold KC. RAGE ligation affects T cell activation and controls T cell differentiation. J Immunol. 2008;181:4272–4278. doi: 10.4049/jimmunol.181.6.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JWC, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Fanjul-Fernández M, Folgueras AR, Fueyo A, Balbín M, Suárez MF, Fernández-García MS, Shapiro SD, Freije JMP, López-Otín C. Matrix metalloproteinase mmp-1a is dispensable for normal growth and fertility in mice and promotes lung cancer progression by modulating inflammatory responses. J Biol Chem. 2013;288:14647–14656. doi: 10.1074/jbc.M112.439893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini ME, Plucinska IM, Mayer AS, Gross RH, Brinckerhoff CE. A gene for rabbit synovial cell collagenase: Member of a family of metalloproteinases that degrade the connective tissue matrix. Biochemistry. 1987;26:6156–6165. doi: 10.1021/bi00393a032. [DOI] [PubMed] [Google Scholar]
- Foley CJ, Fanjul-Fernández M, Bohm A, Nguyen N, Agarwal A, Austin K, Koukos G, Covic L, López-Otín C, Kuliopulos A. Matrix metalloprotease 1a deficiency suppresses tumor growth and angiogenesis. Oncogene. 2013 doi: 10.1038/onc.2013.157. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley CJ, Luo C, O’Callaghan K, Hinds PW, Covic L, Kuliopulos A. Matrix metalloprotease-1a promotes tumorigenesis and metastasis. J Biol Chem. 2012;287:24330–24338. doi: 10.1074/jbc.M112.356303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes JL, Enghild JJ, Suzuki K, Nagase H. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J Biol Chem. 1994;269:25742–25746. [PubMed] [Google Scholar]
- Freije JM, Díez-Itza I, Balbín M, Sánchez LM, Blasco R, Tolivia J, López-Otín C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem. 1994;269:16766–16773. [PubMed] [Google Scholar]
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc Natl Acad Sci USA. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Fernández A, Fueyo A, Folgueras AR, Garabaya C, Pennington CJ, Pilgrim S, Edwards DR, Holliday DL, Jones JL, Span PN, Sweep FCGJ, Puente XS, López-Otín C. Matrix metalloproteinase-8 functions as a metastasis suppressor through modulation of tumor cell adhesion and invasion. Cancer Res. 2008;68:2755–2763. doi: 10.1158/0008-5472.CAN-07-5154. [DOI] [PubMed] [Google Scholar]
- Hasty KA, Pourmotabbed TF, Goldberg GI, Thompson JP, Spinella DG, Stevens RM, Mainardi CL. Human neutrophil collagenase. A distinct gene product with homology to other matrix metalloproteinases. J Biol Chem. 1990;265:11421–11424. [PubMed] [Google Scholar]
- Henriet P, Rousseau GG, Eeckhout Y. Cloning and sequencing of mouse collagenase cDNA. Divergence of mouse and rat collagenases from the other mammalian collagenases. FEBS Lett. 1992;310:175–178. doi: 10.1016/0014-5793(92)81323-e. [DOI] [PubMed] [Google Scholar]
- Iwama A, Zhang P, Darlington GJ, McKercher SR, Maki R, Tenen DG. Use of RDA analysis of knockout mice to identify myeloid genes regulated in vivo by PU.1 and C/EBPalpha. Nucleic Acids Res. 1998;26:3034–3043. doi: 10.1093/nar/26.12.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozic D, Bourenkov G, Lim N-H, Visse R, Nagase H, Bode W, Maskos K. X-ray structure of human proMMP-1: New insights into procollagenase activation and collagen binding. J Biol Chem. 2005;280:9578–9585. doi: 10.1074/jbc.M411084200. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Khanna-Gupta A, Berliner N. Isolation and characterization of the cDNA for mouse neutrophil collagenase: Demonstration of shared negative regulatory pathways for neutrophil secondary granule protein gene expression. Blood. 1998;91:2517–2524. [PubMed] [Google Scholar]
- Lederle W, Hartenstein B, Meides A, Kunzelmann H, Werb Z, Angel P, Mueller MM. MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis. 2010;31:1175–1184. doi: 10.1093/carcin/bgp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenglet S, Montecucco F, Mach F, Schaller K, Gasche Y, Copin J-C. Analysis of the expression of nine secreted matrix metalloproteinases and their endogenous inhibitors in the brain of mice subjected to ischaemic stroke. Thromb Haemost. 2014;112 doi: 10.1160/TH14-01-0007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ma Z, Liu Z, Wu R-F, Terada LS. P66(Shc) restrains Ras hyperactivation and suppresses metastatic behavior. Oncogene. 2010;29:5559–5567. doi: 10.1038/onc.2010.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Gong J-H, Wong JP, Wallace JL, Clark-Lewis I, Overall CM. Matrix metalloproteinase processing of monocyte chemoattractant proteins generates CC chemokine receptor antagonists with anti-inflammatory properties in vivo. Blood. 2002;100:1160–1167. [PubMed] [Google Scholar]
- Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massagué J. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montel V, Kleeman J, Agarwal D, Spinella D, Kawai K, Tarin D. Altered metastatic behavior of human breast cancer cells after experimental manipulation of matrix metalloproteinase 8 gene expression. Cancer Res. 2004;64:1687–1694. doi: 10.1158/0008-5472.can-03-2047. [DOI] [PubMed] [Google Scholar]
- Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJ. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987;248:265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Reynolds JJ, Bretz U, Baggiolini M. Collagenase is a component of the specific granules of human neutrophil leucocytes. Biochem J. 1977;162:195–197. doi: 10.1042/bj1620195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 2004;563:129–134. doi: 10.1016/S0014-5793(04)00281-9. [DOI] [PubMed] [Google Scholar]
- Palavalli LH, Prickett TD, Wunderlich JR, Wei X, Burrell AS, Porter-Gill P, Davis S, Wang C, Cronin JC, Agrawal NS, Lin JC, Westbroek W, Hoogstraten-Miller S, Molinolo AA, Fetsch P, Filie AC, O’connell MP, Banister CE, Howard JD, Buckhaults P, Weeraratna AT, Brody LC, Rosenberg SA, Samuels Y. Analysis of the matrix metalloproteinase family reveals that MMP8 is often mutated in melanoma. Nat Genet. 2009;41:518–520. doi: 10.1038/ng.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffen S, Hemmerle T, Weber M, Neri D. Isolation and characterization of human monoclonal antibodies specific to MMP-1A, MMP-2 and MMP-3. Exp Cell Res. 2010;316:836–847. doi: 10.1016/j.yexcr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Quintanilla M, Brown K, Ramsden M, Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986;322:78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Ra H-J, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix biology. J Int Soc Matrix Biol. 2007;26:587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva SM, Jerônimo MS, Silva-Pereira I, Bocca AL, Sousa JB. Expression of metalloproteinases and interleukins on anastomoses in septic rats. J Surg Res. 2013;183:777–782. doi: 10.1016/j.jss.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricklin GP, Jeffrey JJ, Roswit WT, Eisen AZ. Human skin fibroblast procollagenase: Mechanisms of activation by organomercurials and trypsin. Biochemistry. 1983;22:61–68. doi: 10.1021/bi00270a009. [DOI] [PubMed] [Google Scholar]
- Sukhova GK, Schönbeck U, Rabkin E, Schoen FJ, Poole AR, Billinghurst RC, Libby P. Evidence for increased collagenolysis by interstitial collagenases-1 and-3 in vulnerable human atheromatous plaques. Circulation. 1999;99:2503–2509. doi: 10.1161/01.cir.99.19.2503. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin) Biochemistry. 1990;29:10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- Tressel SL, Kaneider NC, Kasuda S, Foley C, Koukos G, Austin K, Agarwal A, Covic L, Opal SM, Kuliopulos A. A matrix metalloprotease-PAR1 system regulates vascular integrity, systemic inflammation and death in sepsis. EMBO Mol Med. 2011;3:370–384. doi: 10.1002/emmm.201100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O’Callaghan K, Covic L, Kuliopulos A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137:332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart HE, Birkedal-Hansen H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti MP, Coon CI, Mengshol JA, Yocum S, Mitchell P, Brinckerhoff CE. Cloning of the gene for interstitial collagenase-3 (matrix metalloproteinase-13) from rabbit synovial fibroblasts: Differential expression with collagenase-1 (matrix metalloproteinase-1) Biochem J. 1998;331:341–346. doi: 10.1042/bj3310341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Shimokado K, Asahara T, Dohi K, Niwa O. Analysis of the c-myc, K-ras and p53 genes in methylcholanthrene-induced mouse sarcomas. Jpn J Cancer Res. 1999;90:40–47. doi: 10.1111/j.1349-7006.1999.tb00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M, Candrian U, Maronpot RR, Stoner GD, Anderson MW. Activation of the Ki-ras protooncogene in spontaneously occurring and chemically induced lung tumors of the strain A mouse. Proc Natl Acad Sci USA. 1989;86:3070–3074. doi: 10.1073/pnas.86.9.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Heniford BW, Ackermann DM, Hawkins BL, Hendler FJ. Harvey ras (H-ras) point mutations are induced by 4-nitroquinoline-1-oxide in murine oral squamous epithelia, while squamous cell carcinomas and loss of heterozygosity occur without additional exposure. Cancer Res. 1994;54:5310–5317. [PubMed] [Google Scholar]
- Zigrino P, Kuhn I, Bäuerle T, Zamek J, Fox JW, Neumann S, Licht A, Schorpp-Kistner M, Angel P, Mauch C. Stromal expression of MMP-13 is required for melanoma invasion and metastasis. J Invest Dermatol. 2009;129:2686–2693. doi: 10.1038/jid.2009.130. [DOI] [PubMed] [Google Scholar]