Abstract
The mammalian circadian clock is built on a molecular feedback loop in which the PERIOD (PER) proteins, acting in a large, poorly-understood complex, repress CLOCK-BMAL1, the transcription factor driving their expression. We found that mouse PER complexes include the histone methyltransferase HP1γ-Suv39H. PER proteins recruit HP1γ-Suv39H to the Per1 and Per2 promoters, and HP1γ-Suv39H proved important for circadian di- and tri-methylation of histone-3 lysine-9 (H3K9) at the Per1 promoter, feedback transcriptional repression, and circadian clock function. HP1γ-Suv39H was recruited to the Per1 and Per2 promoters ~4 hours after HDAC1, a PER-associated protein we previously implicated in clock function and H3K9 deacetylation at the Per1 promoter. PER complexes containing HDAC1 or HP1γ-Suv39H appeared to be physically separable. Circadian clock negative feedback by the PER complex thus involves dynamic, ordered recruitment of repressive chromatin modifiers to DNA-bound CLOCK-BMAL1.
INTRODUCTION
Circadian clocks are cell-autonomous molecular oscillators that drive daily cycles of physiology, metabolism, and behavior1–3. In mammals, circadian clocks are widely distributed in different cells and tissues4–6, where they locally drive rhythms of tissue-specific processes7–11 and ensure that a multitude of biological activities are properly coordinated with one another and advantageously timed with respect to the behavioral cycle and the daily environmental cycle12.
The mammalian clock is built on a conserved transcriptional negative feedback loop1 in which the three PERIOD (PER) and two CRYPTOCHROME (CRY) proteins repress the activity of CLOCK-BMAL1, the transcription factor driving their own expression. Upon entering the cell nucleus, PER and CRY proteins form one or more large protein complexes13,14 (PER complex) that include at least 25 distinguishable proteins14. The PER complex binds to CLOCK-BMAL1 at E-box sites on Per, Cry, and other circadian target genes, thereby repressing CLOCK-BMAL1 transcriptional activity15–17. Targeted degradation of PER and CRY proteins18–21 enables the re-activation of CLOCK-BMAL1, initiating a new circadian transcriptional cycle with an intrinsic period of ~24 hours. An additional coupled feedback loop involving the transcription factors REV-ERBα and REV-ERBβ supports clock function22,23. CLOCK-BMAL1 target genes encoding products that do not feed back into the clock mechanism serve as circadian outputs, together generating a rhythmic network of cellular functions at the transcriptional and post-transcriptional levels24,25.
Although it is arguably the defining feature of the circadian oscillator, the negative feedback activity of the PER complex is poorly understood. Recent work indicates that the PER complex functions in part by recruiting the SIN3-HDAC complex26, which deacetylates lysine 9 of histone 3 (H3K9) at CLOCK-BMAL1 target sites, contributing to the conversion of local chromatin to a repressive state. In addition, the PER complex recruits SETX and other helicases, thereby inhibiting termination and possibly additional post-initiation transcriptional processes14. The PER complex thus delivers multiple transcriptional regulatory proteins to DNA-bound CLOCK-BMAL1 that together inhibit transcription in an apparently complex, multi-faceted manner.
Accumulating evidence indicates that histone modifications governing transcription are dynamic and cooperative27. In particular, deacetylation and methylation of H3K9 are often coupled in establishing a repressive chromatin state28–30. A study of circadian chromatin modifications of the Dbp gene, a clock output gene, revealed circadian rhythms of H3K9 acetylation and dimethylation that were anti-phase to one another, with the peak of acetylation occurring during the phase of active transcription and the peak of dimethylation occurring during the phase of feedback repression31. These chromatin modifications were dependent on the binding of CLOCK-BMAL1 to the DNA, suggesting that CLOCK-BMAL1 or the PER complex (or both) delivers the relevant chromatin-modifying enzymes to the circadian target gene. Given the precedent for direct recruitment of SIN3-HDAC26, an H3K9 deacetylase, by the PER complex26 and evidence for coupling of H3K9 deacetylation and methylation in transcriptional repression, it seemed plausible that an unrecognized function of the PER complex might be the delivery of one or more H3K9 methyltransferases, contributing to the conversion of local chromatin to a heterochromatin-like repressive state.
RESULTS
PER complexes include the histone methyltransferase HP1γ-Suv39H
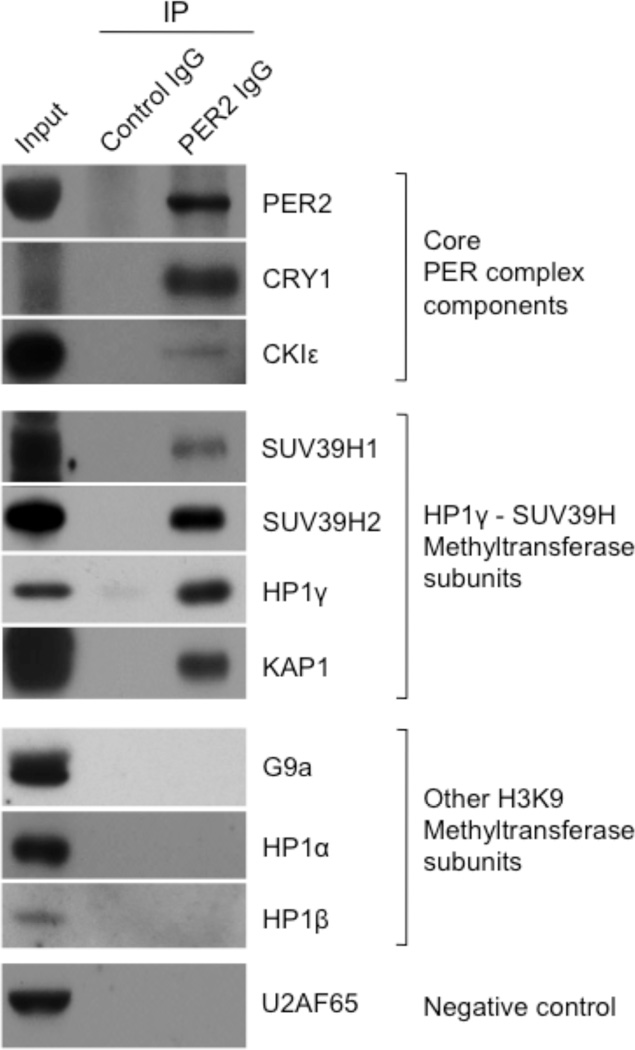
As an initial test of this hypothesis, we immunoprecipitated PER complexes from mouse tissues and looked for the presence of various histone methyltransferase subunits associated with methylation of H3K9, including catalytic subunits G9a, SUV39H1, and SUV39H2 and associated subunits HP1α, HP1β, HP1γ, and KAP132–34. As expected, antibodies to PER2 co-immunopreciptated known core components of the PER complex from nuclear extracts of livers harvested during the negative feedback phase of the circadian cycle, circadian time 18 h (CT 18) (Fig. 1). G9a, HP1α, and HP1β were not detected in the immunoprecipitates, whereas SUV39H1, SUV39H2, HP1γ, and KAP1 co-immunoprecipitated with PER2 (Fig. 1). In addition, antibodies to HP1γ specifically co-immunoprecipitated PER2 and SUV39H1, and antibodies to SUV39H1 specifically co-immunoprecipitated PER2 and HP1γ (Supplementary Figure 1a). These results indicate that an HP1γ-SUV39H histone methyltransferase is a component of one or more PER complexes in vivo. Essentially identical results were obtained from mouse lung nuclear extracts (Supplementary Figure 1b), indicating that the presence of these H3K9 methyltransferase components in a PER complex is not a liver-specific peculiarity, but likely reflects a general or common feature of clock architecture.
Figure 1.
HP1γ-SUV39H histone methyltransferase is a constituent of a PER complex in vivo. Nuclear extract (CT18) of mouse liver (input) and immunoprecipitates (IP) from the extract (antibodies at top) were probed with antibodies to the proteins indicated at right. The abundant nuclear protein U2AF65 (65-kD subunit of U2 small nuclear ribonucleoprotein particle auxiliary factor) served as negative control.
SUV39H catalytic subunits are important for clock transcriptional feedback and function
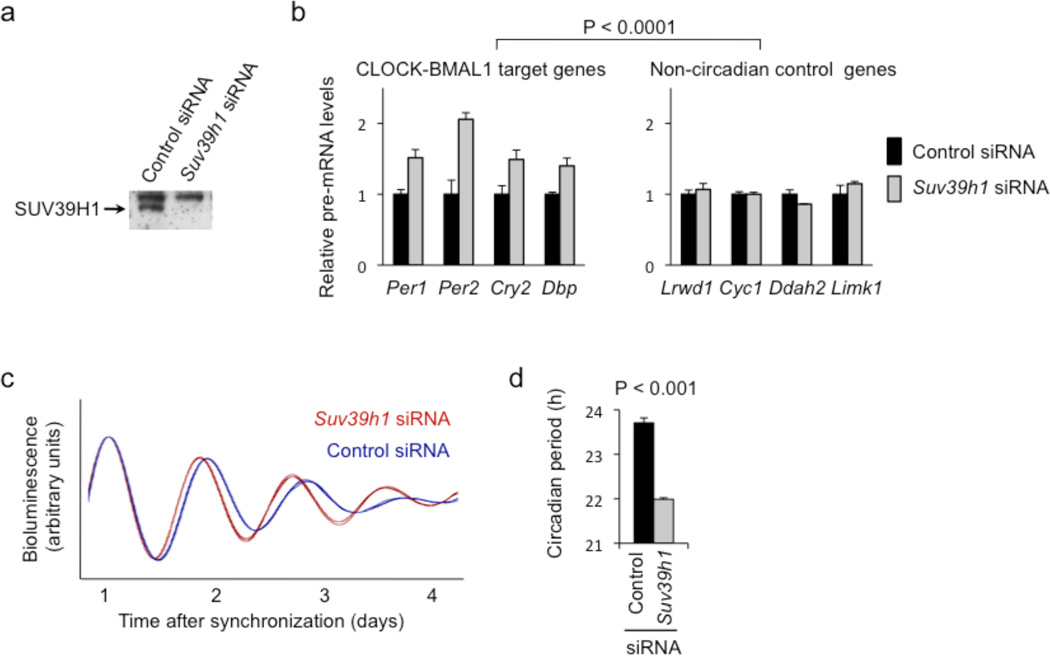
To determine if this histone methyltransferase plays a role in the transcriptional feedback actions of the PER complex, we electroporated small interfering (si) RNAs into unsynchronized cultured mouse fibroblasts (Supplementary Materials and Methods) to deplete the catalytic subunits SUV39H1 (Fig. 2a), SUV39H2 (Supplementary Figure 2a), or both (Supplementary Figure 3). We then monitored the steady-state abundance of pre-mRNAs from four CLOCK-BMAL1 target genes (subject to negative feedback regulation by the PER complex) and four arbitrary control genes. Depletion of endogenous SUV39H1, SUV39H2, or both caused highly-significant increases in the mean steady-state levels of the circadian target gene pre-mRNAs but little or no effect on control pre-mRNAs (Fig. 2b, Supplementary Figure 2b, Supplementary Figure 3, respectively). The double depletion showed an effect only slightly stronger than either single depletion. Thus SUV39H1 and SUV39H2 act selectively to repress the transcriptional activity of CLOCK-BMAL1, indicating an important role in the circadian feedback actions of the PER complex.
Figure 2.
SUV39H1 histone methyltransferase catalytic subunit is important for circadian feedback transcriptional repression and circadian clock function. (a) Western blot showing the effect of point-mutant control Suv39h1 siRNA or matched Suv39h1 siRNA on the steady-state abundance of endogenous SUV39H1 in cultured mouse fibroblasts. Non-specific upper band serves as loading control. (b) Depletion of SUV39H1 from mouse fibroblasts causes a specific increase in transcription of CLOCK-BMAL1 circadian target genes. Quantitative RT-PCR assays showing the steady-state abundance of the indicated pre-mRNAs in mouse fibroblasts after introduction of point-mutant control siRNA (black) or after depletion of SUV39H1 by Suv39h1 siRNA (gray; data normalized to control condition). Shown are mean +/− SEM of triplicate experiment; representative of 3 experiments. Increased transcription of CLOCK-BMAL1 circadian target genes as a class was highly significant compared to control genes as a class after depletion of SUV39H1 (N = 3, t-test, one-tailed). (c) Circadian oscillations of bioluminescence in synchronized reporter fibroblasts after delivery of point-mutant control siRNA (blue) or Suv39h1 siRNA (red). Traces from three independent cultures are shown for each. (d) Circadian periods of fibroblasts in (c) (mean +/− SEM; N = 3 for each condition; t-test, one-tailed).
Because SUV39H1 and SUV39H2 contribute to circadian transcriptional feedback, the two catalytic subunits should be important for clock function. To test this prediction, we depleted endogenous SUV39H1 or SUV39H2 (as above) from circadian reporter fibroblasts35 and monitored real-time circadian rhythms of bioluminescence in the synchronized cell populations. In each case, the depletion caused a shortening of circadian period length that was observed in the individual bioluminescence traces (Fig. 2c, Supplementary Figure 2c) and highly significant in the group data (Fig. 2d, Supplementary Figure 2d). In addition, when different siRNAs were used to deplete SUV39H1 or SUV39H2, in each case a comparable short-period circadian phenotype was observed (Supplementary Figure 4). These results indicate that SUV39H1 and SUV39H2 play a role in the clock mechanism. A short-period circadian phenotype is typical after depletion of negative feedback repressors of CLOCK-BMAL1 activity14,26,35,36.
PER2 and HP1γ-SUV39H show a coordinate circadian rhythm of occupancy at the Per1 promoter
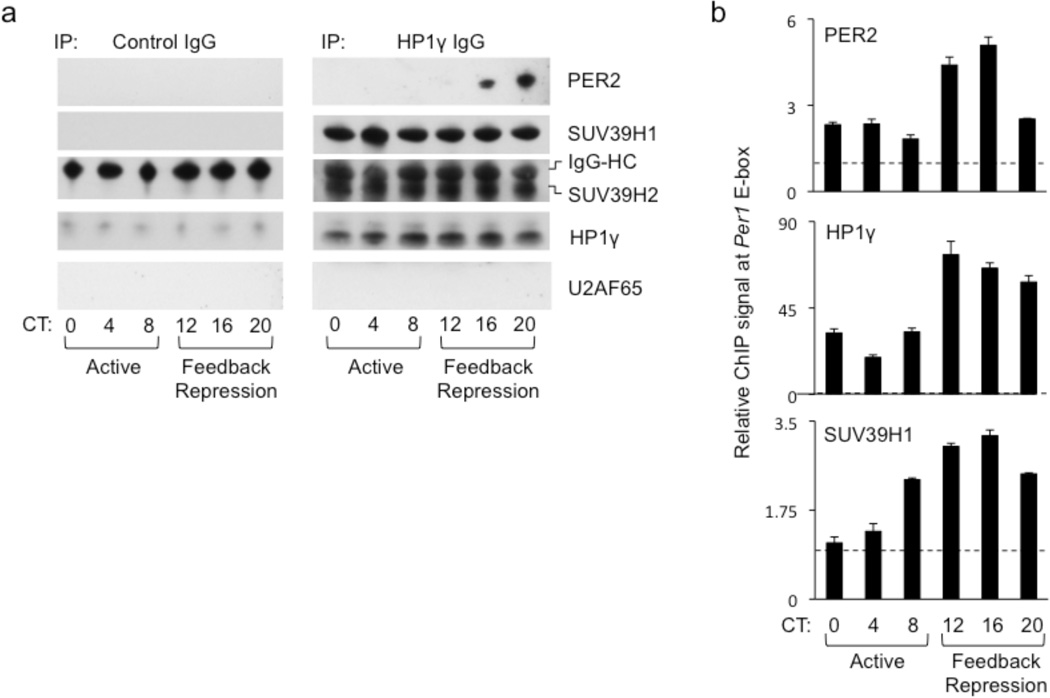
If the HP1γ-SUV39H methyltransferase within a PER complex contributes to clock function by virtue of its known chromatin-modifying activity, then the methyltransferase should be associated with the PER complex when it interacts with CLOCK-BMAL1 on circadian target genes. To test this prediction, we first monitored the formation of this complex every four hours across a circadian cycle by co-immunoprecipitation with HP1γ, the scaffold of the methyltransferase complex, from liver nuclear extracts. SUV39H1 and SUV39H2 specifically co-immunoprecipitated with HP1γ equivalently at all time-points (Fig. 3a). PER2 was detectably co-immunoprecipitated only during the feedback repression phase of the circadian cycle (Fig. 3a), as expected, when PER proteins are maximally expressed. These observations suggest that a core PER complex, assembled from newly-synthesized PER and CRY proteins at the onset of the repression phase, associates with a pre-existing HP1γ-SUV39H methyltransferase complex.
Figure 3.
Coordinate circadian rhythm of PER2 and HP1γ-SUV39H at the Per1 promoter. (a) Circadian profiles of co-immunoprecipitation of the proteins indicated at right with control IgG (left) or HP1γ IgG (right) from mouse liver nuclear extracts. U2AF65 served as negative control, IgG-HC (heavy chain) as positive control. Circadian times (CT) shown at bottom. (b) Circadian cycle of PER2, HP1γ, and SUV39H1 at Per1 proximal E-box site. ChIP assays from mouse livers sampled across a circadian cycle (bottom) performed with antibodies at the top left of each panel. ChIP values are normalized to the signal from a parallel IgG control ChIP (dashed line); data are displayed as mean +/− SEM of triplicate experiment and are representative of three independent experiments. Diagrams showing the sites of primers for ChIP assays of Per gene E-box sites can be found in Supplementary Figure 5b.
We next performed chromatin immunoprecipitation (ChIP) assays on mouse liver nuclear extracts to monitor the occupancy of the proteins on the Per1 promoter across the circadian cycle. HP1γ and SUV39H1 showed a circadian rhythm at the Per1 proximal E-box that was synchronous with the rhythm of PER2, with the peak of the rhythm occurring in the feedback repression phase of the cycle (Fig. 3b). This result is consistent with the interaction of a PER-HP1γ-SUV39H holocomplex with DNA-bound CLOCK-BMAL1, resulting in the targeted delivery of the histone methyltransferase to chromatin of circadian target genes.
PER proteins are required for recruitment of HP1γ -SUV39H to the Per1 promoter
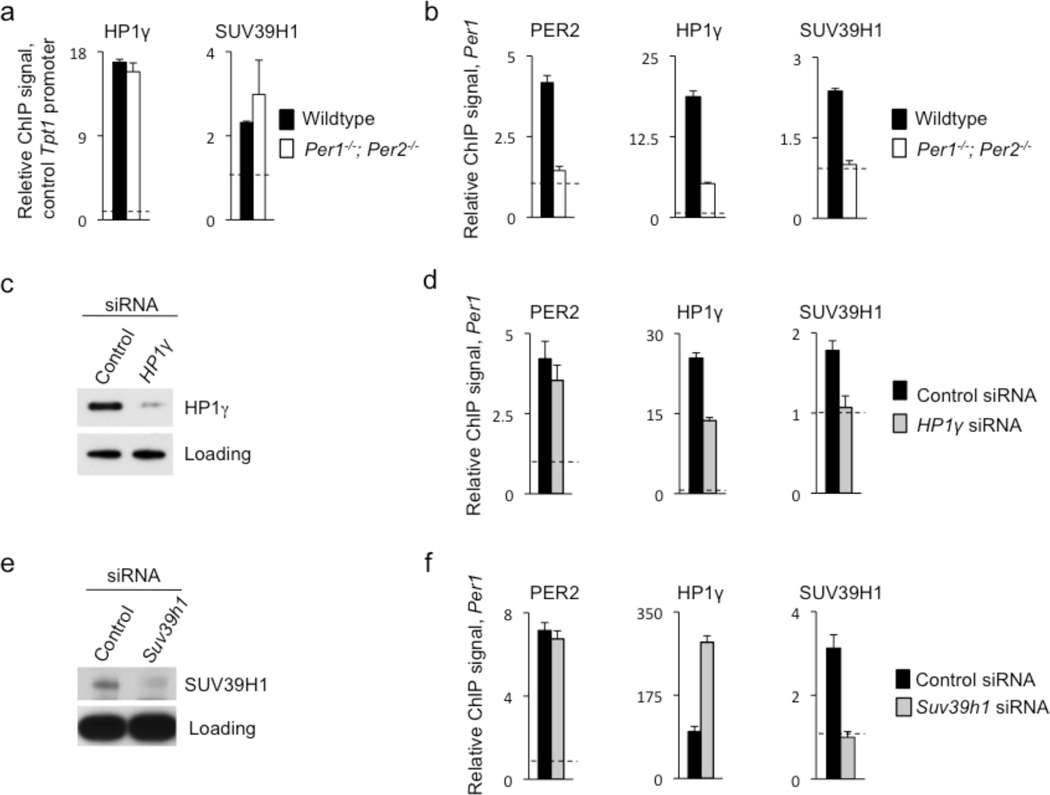
If a PER complex delivers HP1γ-SUV39H to chromatin at CLOCK-BMAL1 circadian target genes, then PER loss-of-function mutations should result in reduced occupancies of HP1γ-SUV39H at circadian target sites but not irrelevant sites. To examine this possibility, we performed ChIP assays on samples from livers of Per1−/−; Per2−/− double mutant mice and wildtype littermates obtained at CT18, during the phase of circadian feedback repression. Chromatin from Per1−/−; Per2−/− mice showed no reduction in the occupancies of HP1γ or SUV39H1 at a non-circadian control promoter (Fig. 4a), indicating that the PER proteins have no general role in the association of HP1γ-SUV39H with chromatin. In contrast, the samples from Per1−/−; Per2−/− mice showed dramatic reductions of both proteins at the Per1 proximal E-box (Fig. 4b). Thus the recruitment of HP1γ-SUV39H to the Per1 E-box site is dependent on PER protein function.
Figure 4.
PER proteins and HP1γ are required for recruitment of SUV39H to the Per1 promoter. (a) Control ChIP assays, displayed as in Fig. 3b, monitoring the occupancies of HP1γ (left) and SUV39H1 (right) at the promoter of non-circadian gene Tpt1 in livers (CT18) of wild-type littermates (black) or Per1−/−; Per2−/− double mutant mice (white). No significant differences were detected. (b) CHIP assays as in (a) showing the occupancies of PER2 (left), HP1γ (middle), and SUV39H1 (right) at the Per1 proximal E-box site in wildtype and Per1−/−; Per2−/− mutant mice. (c) Western blot showing the effect of point-mutant control HP1γ siRNA or matched HP1γ siRNA on the abundance of endogenous HP1γ in cultured unsynchronized mouse fibroblasts. Non-specific band serves as loading control. (d) ChIP assays, as in (b), showing the mean occupancies of PER2, HP1γ, and SUV39H1 at the Per1 proximal E-box site in unsynchronized mouse fibroblasts after introduction of point-mutant control HP1γ siRNA (black) or after depletion of HP1γ by effective HP1γ siRNA (gray). (e) Western blot showing the effect of point-mutant control Suv39h1 siRNA or matched Suv39h1 siRNA on the abundance of endogenous SUV39H1 in cultured unsynchronized mouse fibroblasts. Non-specific band serves as loading control. (f) ChIP assays, as in (d), showing the mean occupancies of PER2, HP1γ, and SUV39H1 at the Per1 proximal E-box site in unsynchronized mouse fibroblasts after introduction of point-mutant control Suv39h1 siRNA (black) or after depletion of SUV39H1 by effective Suv39h1 siRNA (gray). All panels show mean +/− SEM of triplicate experiments and are representative of 3 independent experiments.
HP1γ links chromatin-bound PER complex to SUV39H1
In at least some contexts, HP1γ serves as a scaffold that recruits SUV39H1 to sites of histone methyltransferase action32. If this is the case in the clock mechanism, then depletion of HP1γ should reduce the recruitment of SUV39H1 to circadian target sites but have little or no effect on the occupancy of PER proteins. Furthermore, depletion of SUV39H1 should not cause a reduced occupancy of PER proteins or HP1γ at the site.
To test the first prediction, we depleted HP1γ from mouse fibroblasts (Fig. 4c) and performed ChIP assays to monitor the occupancies of PER2, HP1γ, and SUV39H1 at the proximal Per1 E-box. As predicted, depletion of HP1γ caused a dramatic reduction in the occupancy of SUV39H1, but not PER2 (Fig. 4d). A similar result was observed at two additional CLOCK-BMAL1 circadian target sites, a Per2 E-box and the Per1 distal E-box (Supplementary Figure 5a, b).
We next depleted SUV39H1 (Fig. 4e) and performed ChIP assays as above. As predicted, depletion of SUV39H1 did not reduce the occupancy of either PER2 or HP1γ at the Per1 promoter (Fig. 4f). Unexpectedly, HP1γ occupancy was increased, apparently as a consequence of a compensatory increase in total nuclear HP1γ following SUV39H1 depletion (Supplementary Figure 5c). These results indicate that a PER complex delivers HP1γ-SUV39H to circadian target genes and that HP1γ serves to link SUV39H to the chromatin-associated PER complex.
PER proteins and SUV39H1 are important for di- and tri-methylation of H3K9 at the Per1 promoter
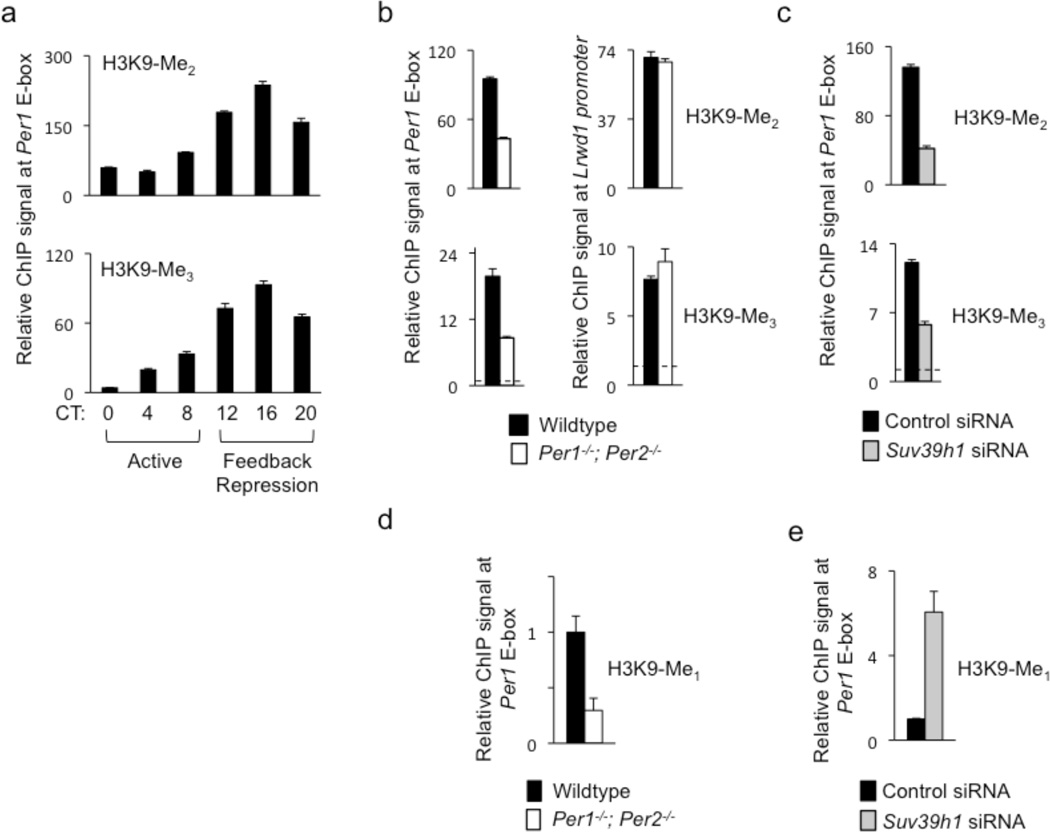
SUV39H proteins prefer mono- and di-methylated H3K9 as substrates in vitro and appear to be involved primarily in di- and tri-methylation of H3K9 in vivo37,38. If the transcriptional repression activity of HP1γ-SUV39H delivered by a PER complex results from its histone methyltransferase activity, then there should be a circadian rhythm of H3K9 di- and tri-methylation (H3K9-Me2 and H3K9-Me3, respectively) on the Per1 promoter that mirrors the rhythm of occupancy of the catalytic subunit SUV39H1. To test this prediction, we performed ChIP assays on mouse liver samples collected every four hours across a circadian cycle. We observed a robust circadian rhythm of both H3K9-Me2 and H3K9-Me3 at the Per1 promoter, peaking at approximately CT16 (Fig. 5a), essentially identical to the rhythm of SUV39H1 occupancy (Fig. 3b). This rhythm resembles the cycle of H3K9-Me2 previously observed on the Dbp gene31.
Figure 5.
PER proteins and SUV39H1 are important for di- and tri-methylation of H3K9 at the Per1 promoter. (a) Circadian cycle of di-methylated (top) and tri-methylated (bottom) H3K9 at Per1 proximal E-box site. ChIP assays are from mouse livers sampled across a circadian cycle (bottom). ChIP values are relative to the signal from parallel IgG control. (b) ChIP assays showing di-methylated (top) and tri-methylated (bottom) H3K9 at Per1 proximal E-box site in livers (CT18) of wild-type littermates (black) or Per1−/−; Per2−/− double mutant mice (white). (c) ChIP assays showing di-methylated (top) and tri-methylated (bottom) H3K9 at Per1 proximal E-box site in unsynchronized mouse fibroblasts after introduction of point-mutant control Suv39h1 siRNA (black) or after depletion of SUV39H1 by effective Suv39h1 siRNA (gray). (d) ChIP assays showing mono-methylated H3K9 at Per1 proximal E-box site in livers (CT18) of wild-type littermates (black) or Per1−/−; Per2−/−- mutants (white). (e) ChIP assays showing mono-methylated H3K9 at Per1 proximal E-box site in unsynchronized mouse fibroblasts after introduction of point-mutant control Suv39h1 siRNA (black) or after depletion of SUV39H1 by effective Suv39h1 siRNA (gray; normalized to control condition). All data are displayed as mean +/− SEM of triplicate experiments; panels (a)–(c) are representative of three independent experiments and (d) and (e) are representative of two independent experiments.
Since PER proteins are required for the recruitment of HP1γ-SUV39H to the Per1 promoter, loss of PER proteins should result in a decrease of H3K9-Me2, H3K9-Me3, or both at the site. We therefore performed ChIP to compare the levels of H3K9-Me2 and H3K9-Me3 at the Per1 promoter during the negative feedback phase in livers of wildtype mice and littermate Per1−/−; Per2−/− double mutants. Both H3K9-Me2 and H3K9-Me3 were substantially reduced in the Per1−/−; Per2−/− mutants at the Per1 promoter, but there was no significant difference between genotypes for either modification at a non-circadian control promoter (Fig. 5b). Depletion of SUV39H1 from mouse fibroblasts caused a similar reduction of both H3K9-Me2 and H3K9-Me3 at the Per1 promoter (Fig. 5c). This depletion had no detectable effect on H3K9-Ac levels at Per1 E-box sites (Supplementary Figure 6), indicating that regulation of H3K9 acetylation at these sites is not coupled to di- or tri-methylation.
If PER-associated HP1γ-SUV39H acts specifically to catalyze the formation of H3K9-Me2 and H3K9-Me3 (but not H3K9-Me1), then depletion of SUV39H1 should cause an increase of H3K9-Me137 at the Per1 promoter, representing accumulation of unconverted substrate. Alternatively, if it also catalyzes H3K9 mono-methylation, then SUV39H1 depletion should cause a decrease of H3K9-Me1 at the Per1 promoter. Liver samples from Per1−/−; Per2−/− double mutants showed a substantial reduction in H3K9-Me1 at the Per1 promoter (Fig. 5d), indicating that all three H3K9 methylation reactions depend on PER function. In contrast, depletion of SUV39H1 from mouse fibroblasts caused a dramatic increase in H3K9-Me1 at the Per1 promoter (Fig. 5e). Thus HP1γ-SUV39H recruited by a PER complex preferentially di-and tri-methylates H3K9 at the Per1 promoter, exhibiting little or no activity on an unmethylated substrate.
Temporally distinct recruitment of HDAC1 and SUV39H1 to Per promoters
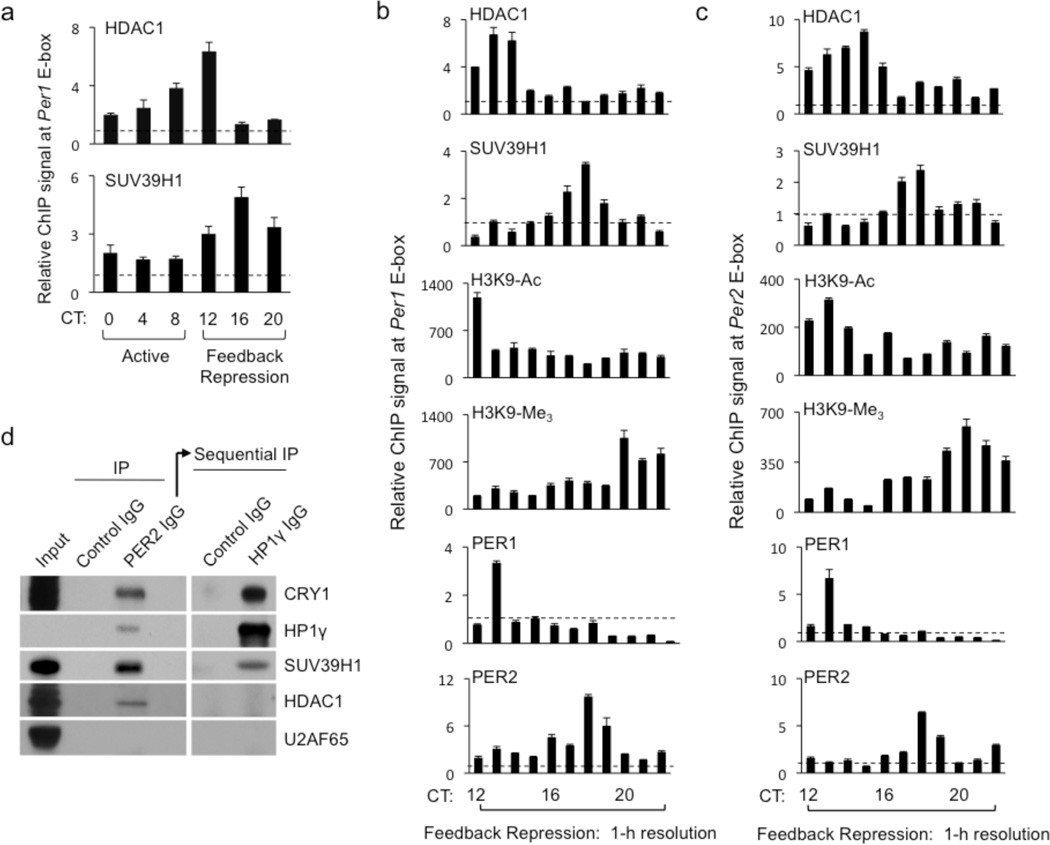
Because many examples of transcriptional repression suggest that de-acetylation and methylation of H3K9 are tightly coupled28,30,39, we initially expected that a chromatin-associated PER complex would include both HDAC126 and HP1γ-SUV39H, which would act together in a concerted process, seemingly simultaneous on a circadian time-scale. However, we observed circadian recruitment of SUV39H1 to the Per1 promoter a few hours after HDAC1 (Fig. 6a). To examine this phenomenon more closely, we obtained livers from mice at 1-h intervals throughout the circadian negative feedback phase (CT12–22) and performed ChIP assays to monitor the two enzymes on the Per1 and Per2 promoters. For both genes, the circadian profile of SUV39H1 binding lagged behind that of HDAC1 binding by approximately 4 h, with little or no overlap between the two peaks (Fig. 6b, c). As expected, a decrease in H3K9-Ac followed the early recruitment of HDAC1, and an increase in H3K9-Me3 followed the late recruitment of SUV39H1 (Fig. 6b, c). Unexpectedly, at both sites the peak binding of PER2 lagged behind that of PER1 by about 5 hours, with the PER2 profile resembling that of SUV39H1 and the PER1 profile resembling that of HDAC1 (Fig. 6b, c). Similar results were obtained at a third E-box site (Supplementary Figure 7).
Figure 6.
Distinct and ordered recruitment of HDAC1 and SUV39H1 to Per promoters. (a) Circadian cycle of occupancies of HDAC1 (top) and SUV39H1 (bottom) at Per1 proximal E-box site. ChIP assays are from mouse livers sampled across a circadian cycle (bottom). Shown are mean +/− SEM of triplicate experiments. (b) ChIP assays showing temporal profiles of HDAC1, SUV39H1, H3K9-Ac, H3K9-Me3, PER1, and PER2 (as indicated at top left of each panel) at Per1 E-box site during the phase of circadian feedback transcriptional repression at 1-h time resolution. Shown are mean +/− SEM of triplicate experiments; representative of three independent ChIP assays from the 11 mice. (c) ChIP assay as in (b) but showing temporal profiles at Per2 E-box site. Shown are mean +/− SEM of triplicate experiments; representative of two independent ChIP assays from the 11 mice. (d) PER complexes containing HP1γ-SUV39H do not detectably include HDAC1. Nuclear extracts (Input) of mouse livers (CT18) were first immunoprecipitated with control IgG or PER2 IgG and probed for the indicated proteins (left panels). PER complexes were then eluted from PER2 IgG by competing epitpope peptide, immunoprecipitated with control IgG or HP1γ IgG, and probed for the same indicted proteins (right panels). U2AF65, negative control.
The temporally distinct profiles suggested that the two H3K9-modifying enzymes might be components of different PER complexes. To test this prediction, we performed sequential immunoprecipitations from nuclear extracts of mouse livers obtained at CT18, a phase when HDAC1 binding at Per promoters has returned to baseline and SUV39H1 binding has reached its maximum. First, we immunoprecipitated PER complexes with an antibody against PER2, then eluted the complexes with a peptide corresponding to the PER2 epitope. The eluted material included core PER complex component CRY1, HP1γ-SUV39H1, and HDAC1, but not an irrelevant nuclear protein (Fig. 6d, left panels). Thus a PER complex containing HDAC1 is present in the nucleus at CT18, even though it no longer detectably associates with chromatin at this time. Because both enzymes co-immunoprecipitated with PER2, both must be in PER complexes that include PER2, so the temporally-distinct ChIP profiles for PER1 and PER2 (Fig. 6b, c) presumably reflect PER complexes of differing PER protein stoichiometry or post-translational modification, not complexes mutually exclusive for PER1 or PER2, as might be initially imagined.
Next, we immunoprecipitated the eluted material with an antibody against HP1γ and monitored the presence of the same proteins by immunoblot. We detected CRY1 and HP1γ-SUV39H1, but no signal was detected for HDAC1 (Fig. 6d, right panels) even though PER complexes containing HDAC1 were present in the sample. Thus HP1γ-SUV39H1 is a component of PER complexes that do not detectably include HDAC1, suggesting that physically distinct PER complexes deliver HDAC1 and HP1γ-SUV39H to catalyze sequential modifications of H3K9 at distinct circadian phases.
DISCUSSION
The results described here reveal new aspects of PER complex action in the core circadian clock feedback loop. Circadian rhythms of histone modification have been observed at the promoters of core clock genes in multiple organisms25,31,40–42, but their mechanistic basis and relationship to components of the core clock machinery was unclear. Recent work and the results described here indicate that in mammals at least three of these rhythmic epigentic modifications, H3K9 deacetylation26 and H3K9 di- and tri-methylation, result from direct negative feedback actions of the PER complex. Our analysis indicates that a PER complex, by virtue of association with HP1γ, recruits an HP1γ-SUV39H1/H2 histone methyltransferase to DNA-bound CLOCK-BMAL1 to di- and tri-methylate H3K9, thereby establishing a local repressive chromatin state important for circadian clock negative feedback and clock function.
An unrelated histone methyltransferase subunit, WDR5, was previously identified as a PER-associated protein and shown to be important for H3K4 tri-methylation and H3K9 dimethylation at the Rev-Erbα promoter, although it did not appear to be important for clock function13. That study did not assess chromatin modifications at Per genes, making a direct comparison with our findings difficult. Our data show that PER complexes act in the sequential modification of H3K9 at Per1 and Per2 genes of the core feedback loop. We do not yet know if the same PER complexes act broadly at circadian target genes or if there are PER complex variants selectively recruited to CLOCK-BMAL1 at different target genes.
The finding of HP1γ rather than HP1α or HP1β in a PER-associated SUV39H histone methyltransferase is at first glance somewhat unexpected. It is the HP1α and HP1β isoforms, working in conjunction with H3K9 methylation, that are most commonly associated with transcriptional repression, albeit characteristically in the setting of heterochromatic gene silencing33. These actions are mediated by the dual abilities of HP1α and HP1β to bind to methylated H3K9 and to recruit histone methyltransferases to methylate H3K9 on neighboring nucleosomes, thereby leading to additional HP1α or HP1β binding, H3K9 methylation, and propagation of a repressive chromatin state along the chromosome32,33.
In contrast, HP1γ is known in at least some contexts to function as a euchromatic transcriptional activator by virtue of enhancing transcriptional elongation and possibly pre-mRNA splicing and termination43–45. However, HP1γ is also known to function in association with SUV39H1 as a gene-specific euchromatic transcriptional repressor46,47, acting in a manner that requires interaction with a transcription factor for specific gene targeting but not with methylated histones47. The transcriptional repression in these cases is highly labile or readily reversible46,47. Our data indicate a similar repressive role for PER-associated HP1γ SUV39H in the clock feedback loop, a network that depends on reversal of feedback transcriptional repression each circadian cycle. These observations do not exclude a possible role for HP1α accumulation at methylated H3K9 sites in circadian transcriptional repression, as observed at the Dbp locus31.
Consistent with previous studies of SUV39H1, we found that PER-associated SUV39H specifically catalyzed H3K9 di- and tri-methylation at the Per1 promoter, with little or no activity on an unmethylated H3K9 substrate. The PER-dependence of all three methylation steps strongly suggests that a PER complex recruits an as-yet unidentified histone methyltransferase that catalyzes the addition of a single methyl group to unmethylated H3K9 after the de-acetylation action of PER-associated HDAC1 and prior to the action of PER-associated HP1γ-SUV39H.
The unexpectedly large 4-hour time difference between PER complex recruitment of HDAC1 and SUV39H1 to Per promoters indicates that the assembly or stability of particular PER complexes or their interaction with DNA-bound CLOCK-BMAL1 is under precise temporal control on the time-scale of a few hours. This regulation appears to insure that repressive chromatin modifying enzymes acting successively on H3K9 are recruited in the appropriate sequence. We do not know the mechanisms underlying this process. It seems plausible that clock-dependent rhythms of post-translational modifications of CLOCK-BMAL1, PER proteins, or components of PER complexes, if set at appropriate phases, could generate an interval timer governing the temporal orchestration of chromatin modifiers within the circadian clock feedback loop.
ONLINE METHODS
Methods and associated references are available in Supplementary Information.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. Jenuwein for valuable discussion. This work was supported by an award from the G. Harold & Leila Y. Mathers Charitable Foundation (C.J.W.) and an NIH Training Grant in Fundamental Neuorbiology (H.A.D.).
References
- 1.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 4.Schibler U, Ripperger J, Brown SA. Peripheral circadian oscillators in mammals: time and food. J Biol Rhythms. 2003;18:250–260. doi: 10.1177/0748730403018003007. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 6.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 7.Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durgan DJ, et al. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–24269. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 9.Jeyaraj D, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcheva B, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. doi: 10.1007/s00125-010-1920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci U S A. 1998;95:8660–8664. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown SA, et al. PERIOD1-associated proteins modulate the negative limb of the mammalian circadian oscillator. Science. 2005;308:693–696. doi: 10.1126/science.1107373. [DOI] [PubMed] [Google Scholar]
- 14.Padmanabhan K, Robles MS, Westerling T, Weitz CJ. Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science. 2012;337:599–602. doi: 10.1126/science.1221592. [DOI] [PubMed] [Google Scholar]
- 15.Kume K, et al. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- 16.Sangoram AM, et al. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–1113. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- 17.Griffin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- 18.Siepka SM, et al. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129:1011–1023. doi: 10.1016/j.cell.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godinho SI, et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- 20.Asher G, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 21.Busino L, et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316:900–904. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- 22.Solt LA, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho H, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rey G, et al. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011;9:e1000595. doi: 10.1371/journal.pbio.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koike N, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science. 2011;332:1436–1439. doi: 10.1126/science.1196766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eberharter A, Becker PB. Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002;3:224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen SJ, et al. Rb targets histone H3 methylation and HP1 to promoters. Nature. 2001;412:561–565. doi: 10.1038/35087620. [DOI] [PubMed] [Google Scholar]
- 30.Czermin B, et al. Physical and functional association of SU(VAR)3-9 and HDAC1 in Drosophila. EMBO Rep. 2001;2:915–919. doi: 10.1093/embo-reports/kve210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38:369–374. doi: 10.1038/ng1738. [DOI] [PubMed] [Google Scholar]
- 32.Kwon SH, Workman JL. The changing faces of HP1: From heterochromatin formation and gene silencing to euchromatic gene expression: HP1 acts as a positive regulator of transcription. Bioessays. 2011;33:280–289. doi: 10.1002/bies.201000138. [DOI] [PubMed] [Google Scholar]
- 33.Hiragami K, Festenstein R. Heterochromatin protein 1: a pervasive controlling influence. Cell Mol Life Sci. 2005;62:2711–2726. doi: 10.1007/s00018-005-5287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinkai Y, Tachibana M. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev. 2011;25:781–788. doi: 10.1101/gad.2027411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robles MS, Boyault C, Knutti D, Padmanabhan K, Weitz CJ. Identification of RACK1 and protein kinase Calpha as integral components of the mammalian circadian clock. Science. 2010;327:463–466. doi: 10.1126/science.1180067. [DOI] [PubMed] [Google Scholar]
- 36.Zhao WN, et al. CIPC is a mammalian circadian clock protein without invertebrate homologues. Nat Cell Biol. 2007;9:268–275. doi: 10.1038/ncb1539. [DOI] [PubMed] [Google Scholar]
- 37.Peters AH, et al. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell. 2003;12:1577–1589. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 38.Rea S, et al. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406:593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 39.Vaute O, Nicolas E, Vandel L, Trouche D. Functional and physical interaction between the histone methyl transferase Suv39H1 and histone deacetylases. Nucleic Acids Res. 2002;30:475–481. doi: 10.1093/nar/30.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421:177–182. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- 41.Taylor P, Hardin PE. Rhythmic E-box binding by CLK-CYC controls daily cycles in per and tim transcription and chromatin modifications. Mol Cell Biol. 2008;28:4642–4652. doi: 10.1128/MCB.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belden WJ, Loros JJ, Dunlap JC. Execution of the circadian negative feedback loop in Neurospora requires the ATP-dependent chromatin-remodeling enzyme CLOCKSWITCH. Mol Cell. 2007;25:587–600. doi: 10.1016/j.molcel.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Kwon SH, Workman JL. HP1c casts light on dark matter. Cell Cycle. 2011;10:625–630. doi: 10.4161/cc.10.4.14796. [DOI] [PubMed] [Google Scholar]
- 44.Saint-Andre V, Batsche E, Rachez C, Muchardt C. Histone H3 lysine 9 trimethylation and HP1gamma favor inclusion of alternative exons. Nat Struct Mol Biol. 2011;18:337–344. doi: 10.1038/nsmb.1995. [DOI] [PubMed] [Google Scholar]
- 45.Vakoc CR, Mandat SA, Olenchock BA, Blobel GA. Histone H3 lysine 9 methylation and HP1gamma are associated with transcription elongation through mammalian chromatin. Mol Cell. 2005;19:381–391. doi: 10.1016/j.molcel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 46.du Chene I, et al. Suv39H1 and HP1gamma are responsible for chromatin-mediated HIV-1 transcriptional silencing and post-integration latency. EMBO J. 2007;26:424–435. doi: 10.1038/sj.emboj.7601517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi JD, Park MA, Lee JS. Suppression and recovery of BRCA1-mediated transcription by HP1gamma via modulation of promoter occupancy. Nucleic Acids Res. 2012;40:11321–11338. doi: 10.1093/nar/gks947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.