Abstract
We evaluated urinary isoflavonoid excretion as a biomarker of dietary isoflavone intake during two randomized soy trials (13–24 months) among 256 premenopausal women with a total of 1,385 repeated urine samples. Participants consumed a high-soy diet (2 servings/day) and a low-soy diet (<3 servings/week), completed 7 unannounced 24-hour dietary recalls, and donated repeated urine samples, which were analyzed for isoflavonoid excretion by liquid chromatography methods. We computed correlation coefficients and applied logistic regression to estimate the area under the curve. Median daily dietary isoflavone intakes at baseline, during low- and high-soy diet were 0.5, 0.2, and 67.7 mg aglycone equivalents, respectively. The corresponding urinary isoflavonoid excretion values were 0.9, 1.1, and 43.9 nmol/mg creatinine. Across diets, urinary isoflavonoid excretion was significantly associated with dietary isoflavone intake (rs=0.51, AUC=0.85; p<0.0001) but not within diet periods (rs=0.05–0.06, AUC=0.565–0.573). Urinary isoflavonoid excretion is an excellent biomarker to discriminate between low- and high-soy diets across populations, but the association with dietary isoflavone intake is weak when the range of soy intake is small.
Keywords: Soy foods, isoflavones, biomarker, dietary intervention, epidemiology
INTRODUCTION
Isoflavones, a subclass of flavonoids,1 which are present at high concentrations in soy foods,2 have been researched for many chronic conditions3, 4 and for their potential as therapeutic agents.5 Given the low breast cancer rates observed in Asian countries, high soy consumption in these countries may be protective against breast cancer.6 As a biological mechanism, the anti-carcinogenic and (anti)-estrogen-like effects of isoflavones, have been hypothesized.7, 8 Soy beans are the major source of isoflavones1, 2 and isoflavonoids are excreted in urine within minutes of consuming soy foods, and excretion is completed 24–36 hours after intake.9 Therefore, urinary isoflavonoid excretion (UIE) is considered a biomarker of soy intake and often measured in dietary intervention studies.10 In this secondary analysis of 2 completed trials, our objective was to analyze dietary intake of isoflavones (DII) in relation to UIE during 2 soy trials among premenopausal women, which explored the effects of daily soy consumption on measures of breast cancer.11, 12 In contrast to previous cross-sectional studies,13–16 the current study offered the opportunity to evaluate the efficacy of UIE as a biomarker to discriminate between two groups with well-defined intakes of soy in an intervention setting and with multiple samples per woman.
MATERIALS AND METHODS
Study population
The original Breast, Estrogen, and Nutrition (BEAN1) was a 2-year randomized trial of 220 women with a high- and a low-soy arm conducted in 2000–2003; 174 women, 86 in the intervention (high-soy) group and 88 in the control (low-soy) group, had valid data for the current analysis.11 The BEAN2 trial in 2007–2010 was a cross-over study with 6 months on a high and a low-soy diet each separated by a 1-month washout period.12 Of the 96 randomized women, 82 participants completed both diet periods. Eligibility criteria for both studies included a normal mammogram, no oral contraceptives, not pregnant, no previous cancer diagnosis or breast surgery, regular menstrual periods, low-soy intake, and for BEAN2, the ability to produce nipple aspirate fluid. The same dietary intervention protocol was used; the high-soy diet consisted of 2 servings of soy providing approximately 50 mg of isoflavones/day providing amounts of soy similar to those reported for Asian countries.17 During the low-soy diet, participants continued their regular diet with no more than 3 soy servings/week, a level comparable to that in Western populations.6 Both studies were approved by the Institutional Review Boards of the University of Hawaii and the participating clinics and all women signed an informed consent form. A Data Safety Monitoring Committee conducted annual reviews. The trials were registered at clinicaltrials.gov as NCT00513916.
Data Collection
For both studies, the participants completed a self-administered food frequency questionnaire (FFQ) at study entry that asked about dietary intake during the last year, demographic characteristics, anthropometric measures, and reproductive health. To assess adherence to the study protocol, all participants completed 7 unannounced 24-hour dietary recalls conducted by telephone except for the first recall in BEAN2 that was collected in person at the screening visit.11, 12 A standardized, multiple pass method was used to obtain a detailed account of all foods and beverages;18 an interviewer asked several times (i.e., passes through the day) to search his or her memory to increase retrieval of foods consumed. All dietary data were analyzed for soy food and isoflavone intake using an extensive food composition database.19, 20
Urine collection and analysis
Within the same month of the recalls, repeated overnight urine samples were collected in containers with ascorbic and boric acid to control bacterial growth. For BEAN1, 4 urine samples per woman were analyzed by high-pressure liquid chromatography (HPLC) with photo-diode array detection (PDA). In BEAN2, 7 urine samples per woman with corresponding dietary recall data were analyzed by liquid chromatography isotope-dilution tandem mass spectrometry (LCMSMS). Equol was assessed using LCMSMS for women in both studies.21 Ever equol producer status was defined as daidzein excretion ≥2 nmol/mg creatinine plus an equol/daidzein ratio of ≥0.018 in at least one sample.22 Creatinine was measured with a clinical chemistry autoanalyzer (Cobas MiraPlus). To adjust for urine volume, isoflavonoid excretion was expressed as nmol/mg creatinine. Method details including validations were reported in detail previously.23, 24
Statistical analysis
Based on the 24-hour recalls, adherence to the intervention was defined as >40 mg/day of dietary isoflavone intake during the high-soy diet and <10 mg/day during the low-soy diet. Using the SAS statistical software package version 9.3 (SAS Institute, Inc., Cary, NC), we computed Spearman correlation coefficients between self-reported DII and UIE including the results for all individual time points. In addition, we applied mixed general linear models to further evaluate the relation between log-transformed DII and UIE values while taking into account the covariance structure of the repeated measurements within subjects. Subsequently, we applied logistic regression to compare dietary assignment (low- or high-soy) and log-transformed values of all DII and all UIE from each dietary intervention period, and calculated areas under the receiver operating characteristic curves (AUC) to present the summary measure of discriminatory accuracy (low- or high-soy).
RESULTS
The median age (with 25th and 75th percentile) of the 256 women included in this analysis was 42.8 (40.0–45.2) years and the median BMI for both groups was 24.8 (21.9–28.3). The estimated pre-study soy intake was approximately 10 g/day. Only 19% of participants were equol producers with a higher proportion in BEAN2 than BEAN1 (35% vs. 11%). According to 24-hour dietary recalls (Table 1), adherence to the intervention for both groups was 85% during the high-soy diet and 84% during the low-soy diet. The median DIIs were 0.5, 0.2, and 67.7 mg/day, respectively, at baseline, and during low- and high-soy. The respective values for UIE were 0.9, 1.1, and 44.2 nmol/mg creatinine.
Table 1.
Dietary isoflavone intake and urinary isoflavonoid excretion during the study†
| All (N=256) | BEAN1 (N=174) | BEAN2 (N=82) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (all) |
Low soy (control) |
High soy (intervention) |
Baseline (all) |
Low soy (control) |
High soy (intervention) |
Baseline (all) |
Low soy | High soy | |
| Samples, N | 256 | 573 | 556 | 174 | 328 | 313 | 82 | 245 | 243 |
| Dietary isoflavone intake mg/day | 2.3 (0.3–7.4) | 0.2 (0.1–2.3) | 60.4 (49.8–74.6) | 2.7 (1.1–6.6) | 0.2 (0.1–4.3) | 56.9 (49.8–72.4) | 0.5 (0.1–31.3) | 0.2 (0.1–1.1) | 67.7 (48.5–85.3) |
| Urinary isoflavonoids nmol/mg creatinine | 0.4 (0.0–4.2) | 1.0 (0.0–4.4) | 32.4 (11.1–70.3) | 0.0 (0.0–4.1) | 0.9 (0.0–4.6) | 28.6 (8.4–63.6) | 0.9 (0.3–4.7) | 1.1 (0.3–4.2) | 44.2 (18.3–79.4) |
| Adherence to dietary intervention‡ | -- | 84% | 85% | -- | 83% | 87% | -- | 86% | 84% |
| Correlation coefficients between dietary isoflavone intake and urinary isoflavonoid excretion (p value) | |||||||||
| Low or high soy only | -- | 0.05 (p=0.20) | 0.06 (p=0.17) | -- | 0.07 (p=0.21) | 0.06 (p=0.30) | -- | 0.06 (p=0.35) | 0.01 (p=0.84) |
| Low and high soy | -- | 0.51 (p<0.0001) | -- | 0.49 (p<0.0001) | -- | 0.55 (p<0.0001) | |||
| Area under the receiver operating characteristic curve (p value)* | |||||||||
| Low or high soy only | -- | 0.565 (p=0.02) | 0.573 (p=0.03) | -- | 0.555 (p<0.001) | 0.537 (p=0.32) | -- | 0.618 (p=0.06) | 0.627 (p=0.01) |
| Low and high soy | -- | 0.850 (p<0.0001) | -- | 0.822 (p<0.0001) | -- | 0.899 (p<0.0001) | |||
Data are presented as median (lower - upper quartile) unless otherwise noted.
Adherence to dietary intervention was defined using estimated dietary isoflavone intake (DII) from 24-hour dietary recalls as >40 mg/day during the high soy diet and <10 mg/day during the low soy diet.
P values and areas under the receiver operating characteristic curve were calculated in logistic regression models with log-transformed urinary isoflavonoid excretion as a continuous independent variable and dietary intervention (high-soy or low-soy diet) as a binary outcome variable.
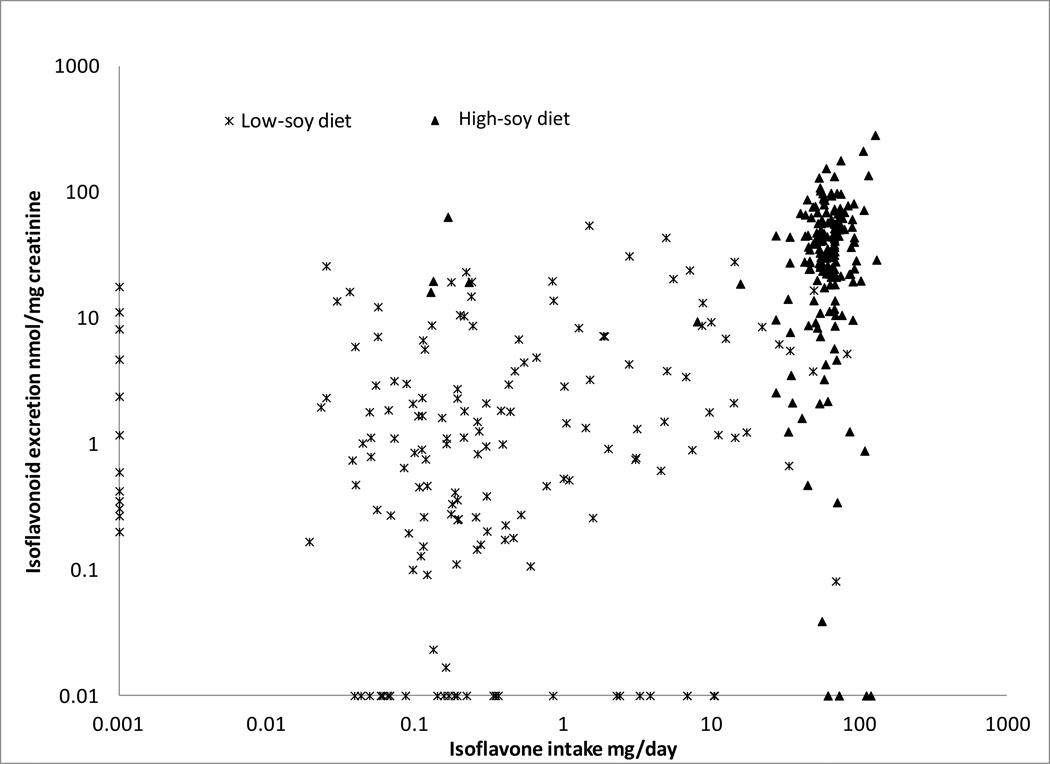
The strongly-correlated median values for DII and UIE were clustered by diet period (Figure 1). Correlations between DII and UIE were weak within diet period (Table 1) but highly significant when combined (rs=0.51; p<0.0001). Similar patterns of associations were suggested by the mixed models; the respective parameter estimates between DII and UIE for both diet periods in combination and either low-soy or high-soy alone were 0.44 (p<0.0001), 0.03 (p=0.37), and 0.07 (p=0.56). DII and UIE were also strongly associated in a logistic model (AUC=0.85; p<0.0001), but after stratification by dietary assignment the AUCs were only 0.565 and 0.573 during the low- and high-soy period, respectively. The values were greater (>0.6) for BEAN2 than BEAN1. If women were equol excretors or not was not a significant predictor in logistic models (p=0.96); equol excretors and non-excretors showed the same correlation (rs=0.50) between DII and UIE.
Figure 1.
Dietary isoflavone intake in relation to urinary isoflavonoid excretion during 2 soy trials based on median values during each soy diet period and using a logarithmic scale
DISCUSSION
The current results compare well to previous population-based investigations with cross-sectional designs.13–16 For example, in a sample of 363 Washington State women, isoflavone intake and excretion correlated significantly (r=0.39; p=0.07) among women who consumed soy at either of the two dietary recalls or at the FFQ.10 In a Chinese population with a median intake of 100 g/day of soy foods,14 the urinary excretion rate of total isoflavonoids was correlated with dietary soy food intake (r=0.5; p<0.001). Among 333 women from a large United Kingdom prospective study, significant relations (p<0.02) between both urinary and serum concentrations of isoflavones across increasing tertiles of dietary intakes were observed.15 The frequency of soy intake was also significantly associated with UIE in Singapore Chinese.16 Among 102 women of Caucasian, Native Hawaiian, Chinese, Japanese, and Filipino, the correlation was twice as high for DII during the previous 24 hours than the previous year (rs=0.31 and 0.62; p<0.01).13
The major weakness of UIE for epidemiologic research is that it only represents the previous 24–36 hours due to the isoflavones’ short half-lives of 8–10 hours.9 In addition, dietary recalls and urine collection did not occur on the same day, although the time gap was usually less than a month and the women were on the same dietary regimen. This probably has attenuated the associations within diet periods. However, the lower correlations between DII and UIE as compared to a previous study10 (rs=0.06 vs. Pearson’s correlations of 0.39) were primarily due to the narrow DII within diet group. The overall correlation when soy consumers and non-consumers were combined were comparable between the two studies (rs=0.51 vs. Pearson’s correlations of 0.45–0.56). Timing of soy consumption in relation to overnight urine collection, e.g., morning vs. evening, may also affect the isoflavonoid measures in urine samples that would capture a greater proportion of isoflavones consumed during dinner than breakfast of the previous day. Although non-compliance to the dietary intervention strategy may have influenced our findings, it appears that the adherence was greater than 85%. The fact that HPLC with lower sensitivity was applied for BEAN111 as opposed to LCMSMS for BEAN212 was responsible for a larger proportion of values below the detection level; however, given the large range of soy intake, this was not a major problem. The repeated measurement design with multiple UIE and DII assessments is a considerable strength of the current analysis and provides confidence that high adherence to dietary intervention was achieved throughout the study periods even when DII and UIE are not assessed on the same day but within an assigned treatment. This study did not compare UIE with blood levels, but significant correlations between UIE and plasma levels are well known from previous studies,25 as well as a similar correlation of plasma isoflavone levels with dietary intake to that of UIE.24 Use of urine samples has advantages over blood samples; the collection is non-invasive and easier for study participants.
These findings support the use of UIE as an excellent biomarker to discriminate between populations with low- and high-soy intakes. Given the high specificity of isoflavones to soy foods, as they are primarily found in soy beans and only present in trace amounts in other foods, urinary excretion levels provide a useful biomarker of soy food consumption, especially if repeated measurements are collected. When the range of soy intake is small, the correlation of UIE with self-reported DII is weak. However, in many epidemiologic studies, it is of primary interest to distinguish high- from low-soy consumers, such as those differences previously seen in Asian vs. non-Asian populations.6, 17
ACKNOWLEDGEMENTS
Support for this study was obtained by grants from the National Cancer Institute R01 CA 80843 and P30 CA71789 and from the National Center for Research Resources S10 RR020890.
Footnotes
Yukiko Morimoto, Planned analysis, implemented statistical programming, drafted paper and conducted literature review
Fanchon Beckford, Implemented statistical programming and drafted paper
Adrian A. Franke, Designed studies, obtained funding, provided and interpreted laboratory results
Gertraud Maskarinec, Designed studies, obtained funding, and planned analyses.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Contributor Information
Yukiko Morimoto, Email: Morimoto@cc.hawaii.edu.
Fanchon Beckford, Email: Fbeckford@cc.hawaii.edu.
Adrian A. Franke, Email: Adrian@cc.hawaii.edu.
Gertraud Maskarinec, Email: Gertraud@cc.hawaii.edu.
REFERENCES
- 1.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 2.United States Department of Agriculture. USDA-Iowa State University Database on the Isoflavone Content of Foods. [Accessed on 6-23-2005];2002 http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav/isoflav.html.
- 3.Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr. 2002;76:1191–1201. doi: 10.1093/ajcn/76.6.1191. [DOI] [PubMed] [Google Scholar]
- 4.Adlercreutz H. Phyto-oestrogens and cancer. Lancet Oncol. 2002;3:364–373. doi: 10.1016/s1470-2045(02)00777-5. [DOI] [PubMed] [Google Scholar]
- 5.Virk-Baker MK, Nagy TR, Barnes S. Role of phytoestrogens in cancer therapy. Planta Med. 2010;76:1132–1142. doi: 10.1055/s-0030-1250074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68:1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 8.Hertrampf T, Schmidt S, Seibel J, Laudenbach-Leschowsky U, Degen GH, Diel P. Effects of genistein on the mammary gland proliferation of adult ovariectomised Wistar rats. Planta Med. 2006;72:304–310. doi: 10.1055/s-2005-916229. [DOI] [PubMed] [Google Scholar]
- 9.Franke AA, Custer L, Hundahl S. Determinants for urinary and plasma isoflavones in humans after soy intake. Nutr Cancer. 2004;50:141–154. doi: 10.1207/s15327914nc5002_3. [DOI] [PubMed] [Google Scholar]
- 10.Atkinson C, Skor HE, Fitzgibbons ED, Scholes D, Chen C, Wahala K, et al. Overnight urinary isoflavone excretion in a population of women living in the United States, and its relationship to isoflavone intake. Cancer Epidemiol Biomarkers Prev. 2002;11:253–260. [PubMed] [Google Scholar]
- 11.Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy SP, et al. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13:1736–1744. [PubMed] [Google Scholar]
- 12.Maskarinec G, Morimoto Y, Conroy SM, Pagano IS, Franke AA. The volume of nipple aspirate fluid is not affected by 6 months of treatment with soy foods in premenopausal women. J Nutr. 2011;141:626–630. doi: 10.3945/jn.110.133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maskarinec G, Singh S, Meng L, Franke AA. Dietary soy intake and urinary isoflavone excretion among women from a multiethnic population. Cancer Epidemiol Biomarkers Prev. 1998;7:613–619. [PubMed] [Google Scholar]
- 14.Chen Z, Zheng W, Custer LJ, Dai Q, Shu XO, Jin F, et al. Usual dietary consumption of soy foods and its correlation with the excretion rate of isoflavonoids in overnight urine samples among Chinese women in Shanghai. Nutr Cancer. 1999;33:82–87. doi: 10.1080/01635589909514752. [DOI] [PubMed] [Google Scholar]
- 15.Grace PB, Taylor JI, Low YL, Luben RN, Mulligan AA, Botting NP, et al. Phytoestrogen concentrations in serum and spot urine as biomarkers for dietary phytoestrogen intake and their relation to breast cancer risk in European prospective investigation of cancer and nutrition-norfolk. Cancer Epidemiol Biomarkers Prev. 2004;13:698–708. [PubMed] [Google Scholar]
- 16.Seow A, Shi CH, Franke AA, Hankin H, Lee HP, Yu MC. Isoflavonoid levels in spot urine predict frequency of dietary soy intake in a population-based sample of middle-aged Chinese in Singapore. Cancer Epidemiol Biomarkers Prev. 1998;7:135–140. [PubMed] [Google Scholar]
- 17.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 18.Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, et al. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 19.Maskarinec G, Morimoto Y, Takata Y, Murphy SP, Stanczyk FZ. Alcohol and dietary fibre intakes affect circulating sex hormones among premenopausal women. Public Health Nutr. 2006;9:875–881. doi: 10.1017/phn2005923. [DOI] [PubMed] [Google Scholar]
- 20.Park SY, Murphy SP, Wilkens LR, Yamamoto JF, Sharma S, Hankin JH, et al. Dietary patterns using the Food Guide Pyramid groups are associated with sociodemographic and lifestyle factors: the multiethnic cohort study. J Nutr. 2005;135:843–849. doi: 10.1093/jn/135.4.843. [DOI] [PubMed] [Google Scholar]
- 21.Maskarinec G, Morimoto Y, Heak S, Isaki M, Steinbrecher A, Custer L, et al. Urinary estrogen metabolites in two soy trials with premenopausal women. Eur J Clin Nutr. 2012;66:1044–1049. doi: 10.1038/ejcn.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 23.Franke AA, Custer LJ, Cerna CM, Narala K. Rapid HPLC analysis of dietary phytoestrogens from legumes and from human urine. Proc Soc Exp Biol Med. 1995;208:18–26. doi: 10.3181/00379727-208-43826. [DOI] [PubMed] [Google Scholar]
- 24.Franke AA, Halm BM, Kakazu K, Li X, Custer LJ. Phytoestrogenic isoflavonoids in epidemiologic and clinical research. Drug Test Anal. 2009;1:14–21. doi: 10.1002/dta.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arai Y, Uehara M, Sato Y, Kimira M, Eboshida A, Adlercreutz H, et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol. 2000;10:127–135. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]