Abstract
Objectives:
DISTINCT (reDefining Intervention with Studies Testing Innovative Nifedipine GITS – Candesartan Therapy) aimed to determine the dose–response and tolerability of nifedipine GITS and/or candesartan cilexetil therapy in participants with hypertension.
Methods:
In this 8-week, multinational, multicentre, randomized, double-blind, placebo-controlled study, adults with mean seated DBP of at least 95 to less than 110 mmHg received combination or monotherapy with nifedipine GITS (N) 20, 30 or 60 mg and candesartan cilexetil (C) 4, 8, 16 or 32 mg, or placebo. The primary endpoint, change in DBP from baseline to Week 8, was analysed using the response surface model (RSM); this analysis was repeated for mean seated SBP.
Results:
Overall, 1381 participants (mean baseline SBP/DBP: 156.5/99.6 mmHg) were randomized. Both N and C contributed independently to SBP/DBP reductions [P < 0.0001 (RSM)]. A positive dose–response was observed, with all combinations providing statistically better blood pressure (BP) reductions from baseline versus respective monotherapies (P < 0.05) and N60C32 achieving the greatest reduction [–23.8/–16.5 mmHg; P < 0.01 versus placebo (–5.3/–6.7 mmHg) and component monotherapies]. Even very low-dose (N20 and C4) therapy provided significant BP-lowering, and combination therapy was similarly effective in different racial groups. N/C combination demonstrated a lower incidence of vasodilatory adverse events than N monotherapy (18.3 versus 23.6%), including headache (5.5 versus 11.0%; P = 0.003, chi-square test) and peripheral oedema over time (3.6 versus 5.8%; n.s.).
Conclusion:
N/C combination was effective in participants with hypertension and showed an improved side effect profile compared with N monotherapy.
Keywords: candesartan cilexetil, combination therapy, DISTINCT study, essential hypertension, nifedipine GITS, vasodilatory side effects
INTRODUCTION
Fast and sustained blood pressure (BP) control in the management of hypertension is paramount [1,2]. To achieve this objective, many guidelines recommend initial combination therapy, as this approach has advantages related to compliance, efficacy and safety [3–5]. To date, two large outcome studies have specifically shown that calcium channel blocker (CCB) angiotensin-converting enzyme (ACE) inhibitor combinations are optimal for preventing major cardiovascular events [6,7]. However, the benefits of using a CCB with an ACE inhibitor rather than an angiotensin receptor blocker (ARB) are less conclusive. The CHIEF study, which is currently ongoing, is exploring the effect of CCB-ARB on cardiovascular outcomes [8]. However, the ONTARGET study showed that ramipril and telmisartan were equivalent in high-risk participants, with telmisartan demonstrating a superior safety profile [9].
On the basis of such safety findings, ARBs may be a better option than ACE inhibitors in fixed-dose combinations (FDCs) with CCBs. However, no FDC contains candesartan cilexetil with a CCB or nifedipine GITS. Nifedipine GITS has an osmotic core that delivers controlled drug release over a 24-h period following once-daily administration, making it suitable for use in a once-daily FDC. Furthermore, few studies have investigated the BP-lowering effects of very low-dose candesartan cilexetil (4 mg) or nifedipine GITS (20 mg), alone or in combination [10–13]. Candesartan cilexetil has been shown to have stronger binding affinity to the AT-1 receptor than other ARBs [14], although the clinical implications of this are not known. In a large observational study [15], candesartan cilexetil was associated with a 14% lower risk for total cardiovascular disease, and a 36% lower risk for heart failure than losartan.
The aim of the present randomized, placebo-controlled study was to determine the dose–response and tolerability of various combinations of nifedipine GITS and candesartan cilexetil compared with respective monotherapies (including very low doses) in patients of different race with mild-to-moderate hypertension. This is the first study to explore this combination.
MATERIALS AND METHODS
Study design
DISTINCT (reDefining Intervention with Studies Testing Innovative Nifedipine GITS – Candesartan Therapy) was an 8-week, multinational, multicentre, randomized, double-blind, placebo-controlled, parallel-group, multifactorial study to determine the dose–response of various combinations of nifedipine GITS and candesartan cilexetil compared with respective monotherapies and placebo in participants with Grade I and II hypertension. Participants were enrolled from 131 study centres in 12 countries (Argentina, Belgium, Canada, Italy, Lithuania, Russia, South Africa, South Korea, Spain, Ukraine, UK and USA) between 28 April 2011 and 28 May 2012. The study protocol was reviewed and approved by each centre's independent ethics committee (IEC) or institutional review board (IRB), and the study was performed in accordance with the Declaration of Helsinki and the International Conference on Harmonization (ICH) guidelines on good clinical practice (GCP). All participants provided written, informed consent.
Following a 2-week (±3 days) screening/washout period, and a 2–4 week, single-blind, placebo run-in period, participants were randomized in equal ratios to one of 16 treatment groups to receive nifedipine GITS (N) 20, 30 or 60 mg or candesartan cilexetil (C) 4, 8, 16 or 32 mg or combination (N/C) 20/4, 20/8, 30/8, 20/16, 30/16, 60/16, 30/32, 60/32 mg or placebo for a double-blind 8-week treatment period. Participants were randomized per country using a computer-generated random code provided by Bayer HealthCare AG (Berlin, Germany). Participants were instructed to take their medication once daily with water at 0800 h (±2 h), except on the day of a clinic visit [Weeks 0 (randomization), 1, 2, 4, 6 and 8], when medication was taken after measurement of BP and heart rate. Participants randomized to the N60C32 treatment arm began treatment with N30C16 and were up-titrated to N60C32 after 1 week.
Participants
Men and women aged at least 18 years with Grade I and II hypertension [16] measured by a calibrated electronic BP device were eligible. Participants had a mean seated DBP at least 90 and less than 110 mmHg at screening, DBP at least 95 and less than 110 mmHg at randomization, and an absolute difference in DBP of less than 10 mmHg between screening and randomization. Major exclusion criteria are described in the additional methods in the supplemental digital content.
Assessments
The primary efficacy endpoint was change in DBP from baseline to Week 8. At the time of study planning, DBP was the gold standard, and the study methodology was approved by the European Medicines Agency Committee for Medicinal Products for Human Use (CHMP) and was in line with comparator 8-week CCB/ARB FDC studies [17–19]. In 2011, after the study was initiated, the CHMP amended their guidelines for the investigation of hypertension treatment to recommend SBP as the primary outcome in antihypertensive trials [20], in line with recent consensus on the importance of elevations in SBP for diagnosis and therapy. Secondary efficacy endpoints incorporated SBP data: change in mean seated SBP from baseline to Week 8; control rate at Week 8, defined as the proportion of participants achieving the predetermined BP target of less than 140/90 mmHg; stratified BP control rate at Week 8, defined as the proportion of participants achieving the predetermined BP target of less than 140/90 or less than 130/80 mmHg for those without and with diabetes mellitus or chronic renal disorder [baseline estimated glomerular filtration rate (GFR) <60 ml/min], respectively; response rate at Week 8, defined as the proportion of participants achieving an SBP response (SBP <140 mmHg or reduction >20 mmHg) or DBP response (DBP <90 mmHg or reduction >10 mmHg); and time to achieve first BP control. Efficacy outcomes in patients from different racial backgrounds and descriptive subgroup analyses according to patient sex, and baseline BMI and hypertension grade were also considered.
BP was measured using a calibrated electronic device (Model HEM-705CP; Omron Healthcare, Inc., Bannockburn, Illinois, USA, with a cuff of an appropriate size), which was supplied with instructions for use by Bayer HealthCare AG. BP was measured in the morning (between 8 : 00 and 9 : 00 a.m.), in both arms during the first visit and in the arm with the highest BP reading for all subsequent readings. At each visit, the participant was required to sit for at least 5 min; three BP measurements were then performed 2 min apart, with participants in the seated position, and the arithmetic mean was calculated.
Safety endpoints included the incidence, severity and relation to study drug of adverse events, coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 15.0 (see additional methods, supplemental digital content); vital sign assessments; and routine laboratory evaluations.
Side effects of vasodilation, including oedema, were a particular focus of the adverse event analysis. The occurrence and severity of peripheral oedema was assessed at all scheduled clinic visits, and headache was captured by self-reporting of symptoms by study participants. Enquiries regarding headache and other adverse symptoms were also made at each clinic visit by the investigator. Standardization across investigator sites was maintained through establishment of a detailed clinical protocol and through monitoring for adherence to the protocol by COVANCE Inc., Illinois, USA.
Statistical analyses
On the basis of simulation, a sample size of 1320 randomized participants with 75 evaluable participants per treatment group was required, assuming a 10% dropout rate. This would provide at least 90% power to detect the dose effect of both N and C at a significance level of 0.05 (5%). Efficacy analyses were performed on the full analysis set, which included all randomized participants who received at least one dose of study medication and had baseline and at least one valid post baseline BP measurement. All randomized participants who took at least one dose of study drug were included in safety analyses. For efficacy analyses, missing values were imputed by the last observation carried forward (LOCF) approach. All statistical hypothesis tests were two-sided and conducted at the 5% significance level unless otherwise stated.
The primary efficacy endpoint was analysed using the response surface model (RSM) [21] to build the dose–response of N and C. The mean BP reduction at Week 8 was analysed by fitting the following model: 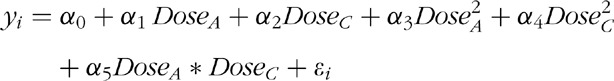
where DoseA: magnitude of the nifedipine GITS dosage (0, 20, 30 and 60 mg), DoseC: magnitude of candesartan cilexetil dosage (0, 4, 8, 16 and 32 mg), y: DBP or SBP change, εi: error term due to model specifications. Each term was tested at a 5% level, and the terms that were not statistically significant were dropped from the model utilizing backward selection. The final model was constructed on the basis of the coefficient tests, and the dose–response relationship was analysed on the basis of the final model. When coefficient α1 and α2 were significant (following testing at the 5% level), it was claimed that nifedipine GITS and candesartan cilexetil treatment each provided a statistically significant contribution to the overall BP-lowering effect of combination therapy. A test for lack of fit (F-test) was used to evaluate whether the RSM fit the data well. A P value of greater than 0.05 indicates no evidence of lack of fit.
For secondary efficacy analyses, changes in DBP and SBP from baseline to Week 8 were analysed using analysis of covariance (ANCOVA), including treatment group, (pooled) centre, and diabetes mellitus status at baseline as fixed effects, and baseline BP and age as covariates. Control and response rates at Week 8 were compared between treatment groups using a logistic regression model with independent variables of treatment group, (pooled) centre, baseline value, age, and diabetes mellitus status at baseline. The time to achieve first BP control was analysed using the Kaplan–Meier method. Statistical evaluations were performed by Bayer HealthCare AG using SAS software 9.2 (SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
Population characteristics
A total of 2817 participants were screened and 1381 (49.0%) were randomized to treatment, with 1362 participants included in the full analysis set. The baseline characteristics of these participants are summarized in Table 1. Overall, 1259 (91.2%) participants completed the study (Fig. 1). The mean (SD) treatment duration was 54.8 (10.0) days, and treatment compliance was 98.9% overall, with no dose-dependent pattern. Concomitant medications were taken by 850 participants (62.4%) during the study, which were most commonly analgesics (25.8%), topical products for joint and muscular pain (23.6%), and serum lipid-reducing agents (18.6%).
TABLE 1.
Baseline characteristics of the randomized population (N = 1381)
| Parameter [mean (SD) unless stated] | Total population (N = 1381) |
| Age (years) | 54.0 (10.3) |
| Age group (years), n (%) | |
| <65 | 1180 (85.4) |
| ≥65–<75 | 175 (12.7) |
| >75 | 26 (1.9) |
| Sex, n (%) | |
| Male | 799 (57.9) |
| Female | 582 (42.1) |
| BMI (kg/m2) | 31.0 (5.7) |
| BMI group, n (%) | |
| <30 kg/m2 | 649 (47.0) |
| ≥30 kg/m2 | 728 (52.7) |
| Missing | 4 (0.3) |
| Race, n (%) | |
| White | 1002 (72.6) |
| Black | 226 (16.4) |
| Asian | 123 (8.9) |
| Other | 30 (2.2) |
| Baseline SBP/DBP (mmHg) | 156.5 (11.3)/99.6 (3.5) |
| Pulse rate (bpm) | 75.3 (10.8) |
| Duration of hypertension,a n (%) | |
| <1 year | 254 (18.6) |
| ≥1–<3 years | 219 (16.1) |
| ≥3 years | 885 (65.0) |
| Missing | 4 (0.3) |
| Hypertension stage, n (%) | |
| Grade I | 536 (38.8) |
| Grade II | 845 (61.2) |
| Prior antihypertensive use, n (%) | 897 (65.0) |
| Diabetes mellitus, n (%) | 205 (14.8) |
| Renal impairment | |
| eGFR <90 ml/min, n (%) | 426 (30.8) |
| eGFR <60 ml/min, n (%) | 50 (3.6) |
bpm, beats per minute; eGFR, estimated glomerular filtration rate.
Full analysis set (n = 1362).
FIGURE 1.
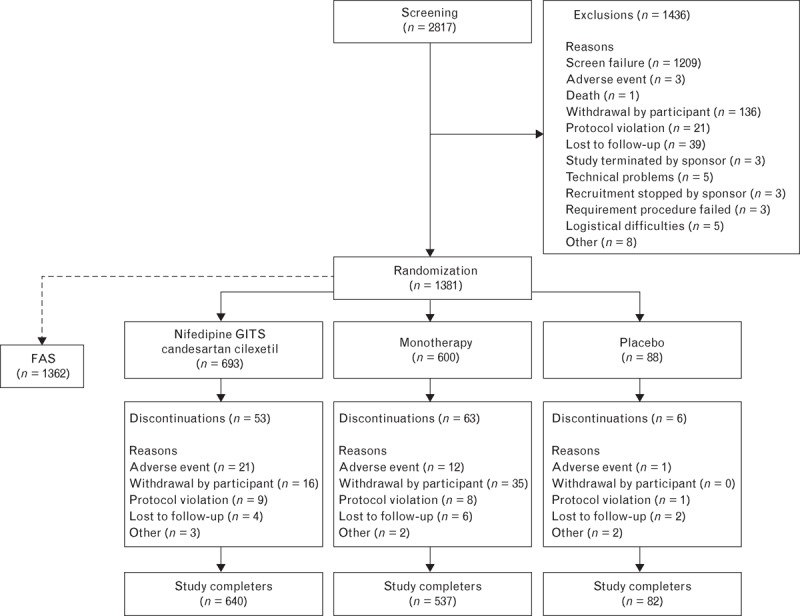
Disposition of study participants. FAS, full analysis set.
Efficacy endpoints
The RSM demonstrated no statistically significant interaction between nifedipine GITS and candesartan cilexetil (P > 0.05). The F-test showed no evidence for lack of fit [lack of fit P values: 0.1401 (DBP) and 0.0872 (SBP)], indicating that RSM was an appropriate model for use in this study. On the basis of the final RSM, both nifedipine GITS and candesartan cilexetil contributed significantly (P < 0.0001) to the BP reduction effect of the combination. A positive dose–response was demonstrated, showing that the higher the dose of each component, the larger the BP reduction effects, within the dose range studied. On the basis of the final RSM, the estimated SBP/DBP reduction from baseline was –8.0/–7.2 mmHg (placebo), –16.3/–11.7 mmHg (N60), –15.1/–11.7 mmHg (C32) and –23.4/–16.2 mmHg (N60C32) (Fig. 2). A signal of plateau effect was observed when the dose of candesartan cilexetil was increased from 16 to 32 mg.
FIGURE 2.
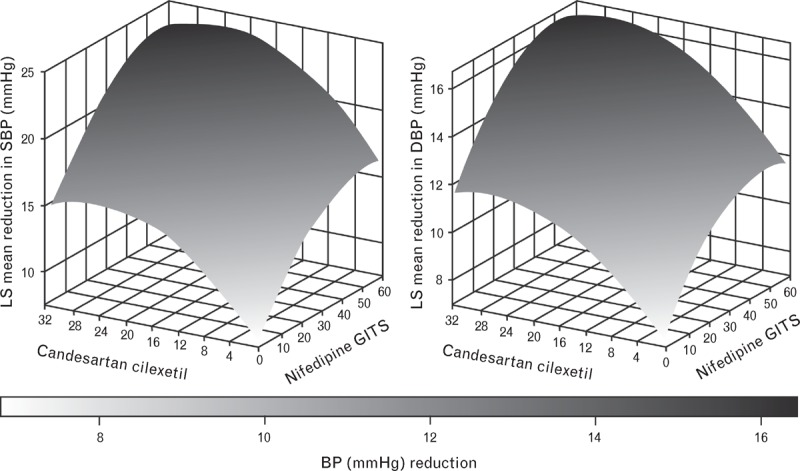
Significant (P < 0.0001) reduction in SBP and DBP (mmHg) from baseline to Week 8 following treatment with nifedipine GITS (0, 20, 30, 60 mg) and/or candesartan cilexetil (0, 4, 8, 16, 32 mg) [LS means from final RSM (n = 1362)]. BP, blood pressure; LS, least squares; RSM, response surface model.
Secondary analyses showed that all nifedipine GITS/candesartan cilexetil combinations were associated with a statistically and clinically significant greater reduction in SBP/DBP than placebo (P < 0.05) and with respective monotherapies (P < 0.05; ANCOVA) at Week 8 (individual comparisons are presented in the results section of supplemental digital content). The greatest reductions in SBP/DBP were observed in the N60C32 group [–23.8/–16.5 mmHg; P < 0.05 versus placebo (–5.3/–6.7 mmHg) and respective monotherapies] and the N30C32 group (–22.1/–16.1 mmHg; P < 0.05 versus placebo/monotherapy). Even the lowest dosing groups showed pronounced least squares mean reductions in SBP/DBP from baseline to Week 8 (C4: –11.8/–9.4 mmHg; N20: –11.9/–9.9 mmHg; P < 0.05), and combination of these doses performed favourably (N20C4: –18.7/–13.7 mmHg; P < 0.05).
The control rate (BP <140/90 mmHg) and response rates at Week 8 were statistically significantly higher than those in the placebo group (P < 0.05) and numerically higher than those in respective monotherapy groups (Fig. 3). Many combination therapy control and response rates were also statistically significantly greater than either or both of the respective monotherapies (Fig. 3, footnote). The greatest control rates were observed in the N30C32 (65.5%, P < 0.05 for combination versus both monotherapy components) and the N60C32 groups (61.9%, P < 0.10 for combination versus both monotherapy components), with the lowest control rate in the placebo group (9.3%).
FIGURE 3.
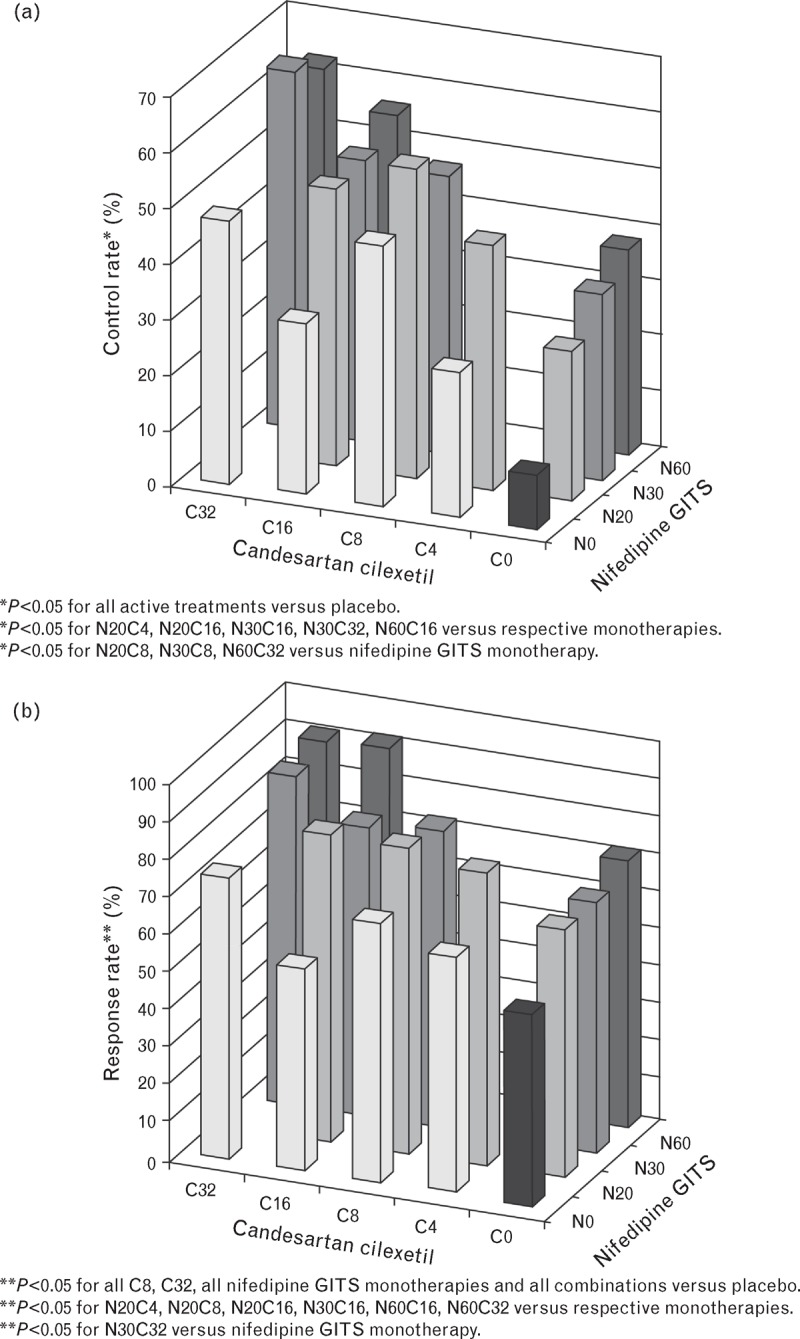
Patients reaching blood pressure targets. Figure showing (a) control rates (%) (BP < 140/90 mmHg) and (b) response rates (%) (SBP <140 mmHg or SBP reduction >20 mmHg or DBP <90 mmHg or DBP reduction >10 mmHg) at Week 8 following treatment with nifedipine GITS (N0, 20, 30, 60 mg) and/or candesartan cilexetil (C0, 4, 8, 16, 32 mg) (n = 1362). BP, blood pressure.
As expected, candesartan cilexetil monotherapy demonstrated a differential treatment effect between black and white patients; however, no substantial racial differences in treatment effect of combination therapy could be detected (Fig. 4). Descriptive subgroup analyses for BP reduction and control rate according to patient sex, BMI and hypertension grade demonstrated a similar trend in treatment effect of the nifedipine GITS/candesartan cilexetil combination versus placebo and the respective monotherapies to the overall analysis (supplemental digital content).
FIGURE 4.
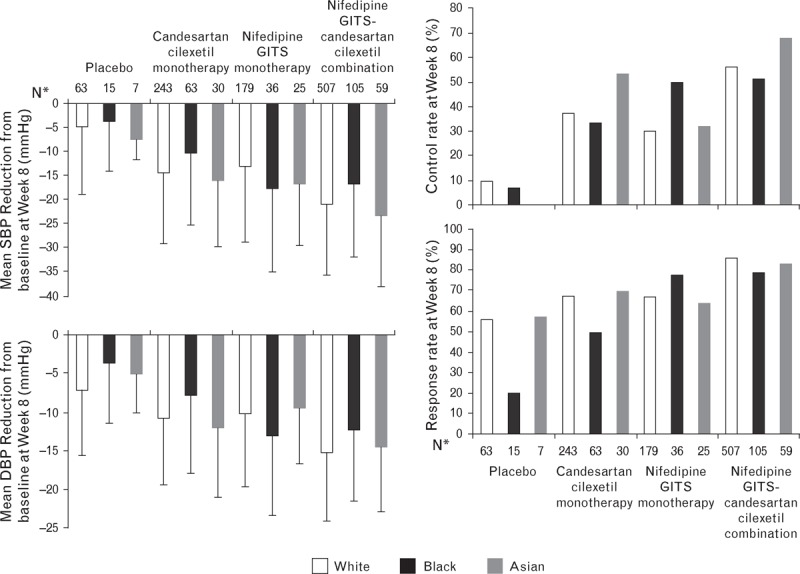
The mean change in SBP and DBP (+SD), and control and response rates from baseline to Week 8 for patients of different race who received treatment with placebo, candesartan cilexetil, nifedipine GITS or nifedipine GITS-candesartan cilexetil combination (n = 1332). SD, standard deviation. aThirty patients were of other racial origins.
In the monotherapy groups, the median time to achieve first BP control (BP < 140/90 mmHg) decreased with increasing dose of nifedipine GITS (20 mg, 68 days; 30 mg, 43 days; 60 mg, 16 days) and candesartan cilexetil (4 mg, 43 days; 8 mg, 30 days; 16 mg, 32 days; 32 mg, 15 days). Combination treatment was associated with a shorter median time to achieve first BP control than monotherapy (supplemental digital content Fig. 1). The shortest median time to achieve first BP control was observed in the N60C16 group (12 days), and the median time to achieve first BP control in all other combination groups was 15 days or less [compared with N60 (16 days) and C16 (32 days)]. The percentage of participants achieving BP control (<140/90 mmHg) over the course of the study is shown in supplemental digital content Fig. 2.
Safety endpoints
A total of 536 participants (38.8%) reported at least one treatment-emergent adverse event (TEAE) (Table 2). The majority of TEAEs were mild or moderate in intensity, with only 21 (1.51%) participants reporting severe TEAEs and seven (0.5%) reporting severe treatment-related adverse events. There were no deaths during the randomized treatment period.
TABLE 2.
Number (%) of participants with treatment-emergent adverse events by pooled treatment with nifedipine GITS (20, 30, 60 mg) monotherapy, candesartan cilexetil (4, 8, 16, 32 mg) monotherapy, combination or placebo (N = 1381)
| Placebo (n = 88) | Candesartan cilexetil monotherapy (n = 346) | Nifedipine GITS monotherapy (n = 254) | Nifedipine GITS-candesartan cilexetil combination (n = 693) | Total (N = 1381) | |
| Any TEAE | 25 (28.4) | 110 (31.8) | 109 (42.9) | 292 (42.1) | 536 (38.8) |
| Any study drug-related TEAE | 9 (10.2) | 36 (10.4) | 52 (20.5) | 127 (18.3) | 224 (16.2) |
| Discontinuation of study drug due to TEAEs | 1 (1.1) | 3 (0.9) | 9 (3.5) | 20 (2.9) | 33 (2.4) |
| Vasodilatory TEAEs | 10 (11.4) | 41 (11.8) | 60 (23.6) | 127 (18.3) | 238 (17.2) |
| Oedema | 4 (4.5) | 30 (8.7) | 36 (14.2) | 89 (12.8) | 159 (11.5) |
| Headache | 6 (6.8) | 12 (3.5) | 28 (11.0) | 38 (5.5)a | 84 (6.1) |
| Flushing | 0 | 0 | 1 (0.4) | 6 (0.9) | 7 (0.5) |
TEAE, treatment-emergent adverse event.
P = 0.003 versus monotherapy.
A total of 33 participants discontinued study drug because of TEAEs. Discontinuation due to treatment-related adverse events was low across all groups [candesartan cilexetil monotherapy (0.9%); nifedipine GITS monotherapy (3.5%); nifedipine GITS/candesartan cilexetil combination (2.9%); and placebo (1.1%)].
Compared with nifedipine GITS monotherapy, combination therapy was associated with a lower incidence of vasodilatory TEAEs [18.3% (n = 127) versus 23.6% (n = 60)], including incidence of headache [5.5% (n = 38) versus 11.0% (n = 28); P = 0.003, chi-square test] (Fig. 5). Combination therapy was associated with fewer reports of oedema as a TEAE compared with nifedipine GITS monotherapy; 12.8 versus 14.2%, respectively (n.s). Peripheral oedema was also assessed as standard at each clinic visit and showed reduced incremental incidence over time for combination versus nifedipine GITS monotherapy (Fig. 6; n.s.). The incidence and severity of oedema showed no clear relationship to dose of nifedipine GITS (see supplemental digital content).
FIGURE 5.
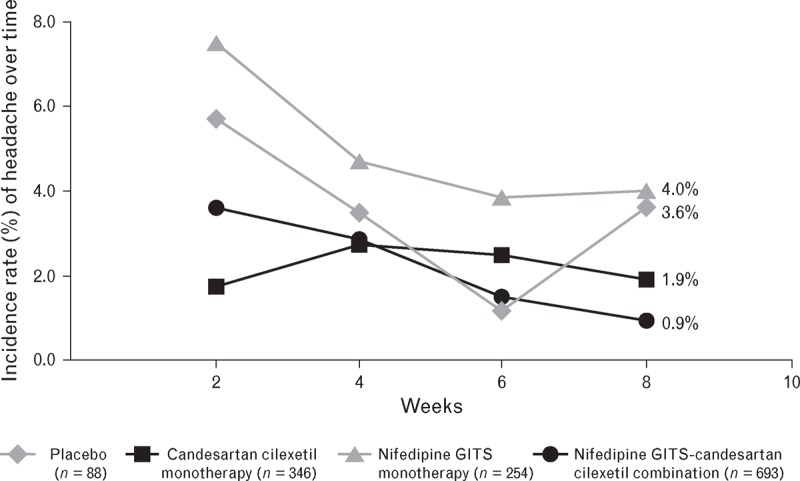
The incidence rate of headache (%) during 8 weeks of treatment with nifedipine GITS monotherapy (20, 30, 60 mg), candesartan cilexetil monotherapy (4, 8, 16, 32 mg), combination or placebo (n = 1381).
FIGURE 6.
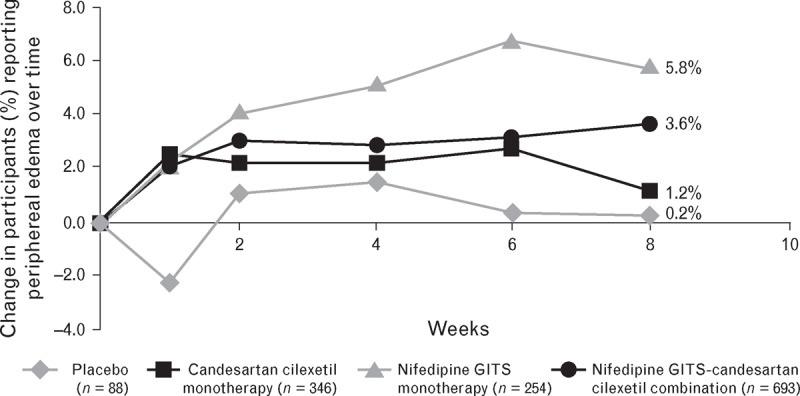
The incremental incidence of peripheral oedema (%) during 8 weeks of treatment with nifedipine GITS monotherapy (20, 30, 60 mg), candesartan cilexetil monotherapy (4, 8, 16, 32 mg), combination or placebo (n = 1381).
A total of four hypotension events occurred in the N20, N20C4, N20C16 and N60C32 groups, respectively (all judged to be related to the study drug but none serious or severe), and no dose-dependent pattern was found.
Further safety results regarding serious TEAEs and laboratory assessments can be found in the supplemental digital content.
DISCUSSION
In DISTINCT, nifedipine GITS-candesartan cilexetil combination produced significantly greater reductions in SBP and DBP than placebo and respective monotherapies at Week 8. Nifedipine GITS and candesartan cilexetil both contributed independently to the significant BP reductions obtained with combination therapy, with a positive dose-dependent effect. The 8-week BP control and response rates were significantly improved for all combinations compared with placebo, and the median time to achieve BP control was shorter than monotherapy, although this measure was linked to visit schedule. A number of combinations (N30C8, N30C16, N60C16 and N60C32) were shown to be of particular clinical interest; however, even very low-dose combination (N20C4) provided significant BP reductions. N60C32 achieved maximal SBP/DBP reduction with a similar safety profile to N60C16 and N60.
Although DISTINCT is the first study to determine the effects of nifedipine GITS-candesartan cilexetil in combination, the efficacy of nifedipine GITS (20–180 mg/day) monotherapy has been described in several randomized studies [11,22,23]. An early study (NICE-Combi) showed that nifedipine controlled release (20 mg) formula and candesartan cilexetil (8 mg) achieved significantly greater BP reduction (P < 0.0001) and superior safety results than up-titrated monotherapy [24]. The current study confirms this efficacy of low-dose nifedipine-candesartan combination (N20C4, N20C8) for significant BP reductions with an improved tolerability profile over CCB monotherapy. Previous research has shown that, for patients not reaching target BP with monotherapy, low-dose combinations of antihypertensive classes with complementary modes of action achieve greater reductions in BP than uptitration of monotherapy, with potentially fewer dose-related side effects [25–27]. Our study supports this, as BP reductions associated with the N20C4 and N20C8 combinations were significantly greater than for the component monotherapies, and greater than the reductions achieved by patients receiving high-dose monotherapy (N60 or C32).
The cardiovascular benefits of ARB therapy have been demonstrated in large-scale outcomes studies [28,29]. In several comparative studies, candesartan was associated with decreases in SBP and DBP similar to telmisartan and valsartan [30], and showed improved cardiovascular risk reduction compared with losartan in a real-life setting, despite similar reductions in BP [15]. Candesartan may also have benefits over other ARBs in terms of its AT-1 receptor binding affinity [14], although the clinical implications of this are unknown. In the current study, a signal of plateau antihypertensive effect was observed when increasing the dose of candesartan cilexetil from 16 to 32 mg. This has not been observed in previous dosing studies of candesartan in a range of hypertensive patients [12,31], but a similar plateau effect in response rate was demonstrated during pharmacodynamic studies of the drug [32]. We can draw no firm conclusion from our data, due to the relatively low numbers of patients in each dose group.
Differences in response to antihypertensive therapies according to race have been noted in the literature for many years, with consistent small differences in responses between blacks and whites, especially for ACE inhibitors, ARBs (including candesartan cilexetil) and β-blockers [33]. Black patients generally respond more favourably to CCBs and diuretics, and this is reflected in hypertension management guidelines [34,35]. In accordance with this, our study demonstrated a difference in treatment effect of candesartan monotherapy in the black versus white populations; however, the treatment effect of nifedipine-candesartan combination therapy was similar in black and white patients. These data are supportive of previous studies of CCB/ARB combination therapy in different racial groups [36,37]. Descriptive subgroup analyses (sex, BMI, hypertension grade) demonstrated a similar treatment effect of combination therapy versus placebo and respective monotherapies, across patient groups. However, there was a consistently greater BP-lowering effect of study drug in patients with Grade II versus Grade I hypertension at baseline. These data suggest that nifedipine-candesartan therapy may be an effective CCB-ARB combination in a wide range of patients.
In the current study, nifedipine GITS/candesartan cilexetil combination improved the CCB-related side effect profile compared with nifedipine GITS monotherapy. This included a significant reduction in headache and a nonsignificant decrease in the incremental incidence of peripheral oedema. In addition to the recording of peripheral oedema as an adverse event, oedema was measured at each clinic visit. Data from these repeated measurements of oedema support that combination therapy was associated with reduced oedema compared with nifedipine monotherapy. Furthermore, no clear relationship between oedema incidence/severity and dose of nifedipine was observed; low doses of nifedipine were associated with oedema rates similar to those for candesartan monotherapy, although a trend for a greater incidence of moderate oedema with 60 mg doses of nifedipine (monotherapy or in combination) was indicated. Such an increase in incidence of peripheral oedema has previously been noted when up-titrating from 30 to 60 mg nifedipine GITS [38]; however, this side effect appears to be largely ameliorated when nifedipine GITS is taken at bedtime rather than in the morning [39]. As the data from this study indicate similar efficacy in BP control with N30C32 and N60C32, further research is warranted into the clinical potential of these dose combinations and their tolerability profiles.
The incidence of headache tended to decrease over the course of the study in the placebo, nifedipine GITS monotherapy and combination groups. Although the occurrence of headache for nifedipine GITS monotherapy was higher than for placebo over the whole trial (11.0 versus 6.8%, respectively), the incidence of headache at Week 8 was similar in these two groups due to increased incidence in the placebo population from Week 6 to Week 8 (Fig. 5). Previous pooled data from participants using nifedipine GITS show that adverse drug reactions occur with a low incidence (<3%), except for oedema (9.9%) and headache (3.9%) [40]. In the current study, it is possible that active reporting and increased focus on vasodilatory side effects could have contributed to the higher rate of headache (11.0%) and oedema (14.2%) than previous data on nifedipine GITS. CCBs are known to cause arteriolar vasodilation, but the addition of a RAS blocker may mitigate this by increasing venous vasodilation, resulting in normalization of intra-capillary pressure [41]. However, ARB therapy has been associated with a substantial reduction in the incidence of headache (of around one-third) compared with placebo, and is being highlighted as a potential prophylactic treatment for patients with migraine [42,43]. CCB-ARB combinations have been associated with a significantly lower incidence of headache and oedema than CCB monotherapy, which may improve compliance and BP goal attainment [44]. Similarly, this study demonstrated a 50% reduction in headache when candesartan was combined with nifedipine GITS, which may lead to improvements in adherence.
A limitation in our study was the selection of reduction in DBP as the primary endpoint, rather than SBP, which is now recognized as a more important prognostic factor of cardiovascular outcomes than is DBP [45]. Nonetheless, this choice is in line with similar CCB/ARB studies, making our data directly comparable. Furthermore, analysis of SBP outcomes and BP control and response rates was prespecified in the trial protocol. A further limitation to our study may be that we did not employ 24-h ambulatory BP as an outcome. Since this study protocol was designed and approved, the prognostic value of 24-h BP measurements and variability has been more widely recognised [46].
In conclusion, nifedipine GITS and candesartan cilexetil combination therapy provided statistically significant BP lowering, with efficacy superior to respective monotherapies and each drug contributing independently in combination. A positive dose–response was obtained for all combinations tested, and even very low-dose therapy provided significant BP reductions. The 8-week BP control rates were significantly higher for most of the combinations of clinical interest. Combination treatment was associated with a shorter median time to achieve first BP control than monotherapy, and the treatment effect of combination therapy was similar in black and white patients. Importantly, combination therapy was well tolerated compared with the respective monotherapies, with a low rate of discontinuation, no increased risk of hypotension and a reduced risk of CCB-associated (vasodilatory) side effects.
ACKNOWLEDGEMENTS
DISTINCT Programme Investigators and Advisory Board Members: Agaiby J, Aggarwal N, Ainsworth P, Akhras R, Amaluan V, Ballarin A, Bardauskiene L, Berra FC, Blagden M, Bodalia B, Borghi C, Bundy C, Burgess L, Buynak R, Cafferata A, Cahill T, Capiau L, Capuano V, Casanova R, Cecil J, Cha G, Chapman J, Chilvers M, Christensen S, Cho Y-H, Chung W-B, Cipollone F, Coca A, Colombo H, Contreras EM, Crowley D, Cusco-Prieto B, Decarlini F, Doh J-H, Dzongowski P, Dzyak G, Ellery A, Extremera BG, Farias E, Farrington C, Fidelholtz J, Fouche L, Gabito A, Gainza M, Gani M, Gaunt R, Gelersztein E, Giuliano M, Glazunov A, Glorioso N, Goloschekin B, Gumbley M, Gupta A, Guzman L, Ha J-W, Hart R, Harvey P, Haworth D, Henein S, Henry D, Her S-H, Heyvaert F, Hollanders G, Hominal M, Hong B-K, Hong T-J, Hwang K-K, Jacovides A, Jacqmein J, Jeon HK, Jones N, Kanani S, Kang H, Karpenko O, Kenton D, Kimzey N, Kjeldsen SE, Kovalenko V, Kushnir M, Lasko B, Lee KJ, Lee N, Lewin A, Litvak M, Luksiene D, Majul C, Mannarino E, Manuale O, Marcadis A, Miller D, Mills R, Misik K, Mortelmans J, O’Mahony M, O’Mahony W, Park C, Pedrinelli R, Petrulioniene Z, Pettyjohn F, Piskorz D, Poss G, Pudi K, Pyun WB, Raad G, Raila G, Ramirez Espinosa MF, Ramlachan P, Rhee M, Rudenko L, Ruiz TS, Ryan J, Schacter G, Shin J-H, Short D, Sica D, Sirenko Y, Slapikas R, Somani R, Stanislavchuk M, Stewart R, Svishchenko Y, Sychov O, Teitelbaum I, Tseluyko V, Van Rensburg DJ, Vaquer Perez JV, Via LM, Vico M, Villa G, Vizir V, Vogel D, Wellmann H, Yoo BS.
Editorial assistance was provided by Samantha Phillips and Claire Price of PAREXEL International, which was contracted by the study sponsor (Bayer HealthCare AG).
All authors contributed to the design of the study, acted as principal investigators at study sites, recruited patients, collected data, actively reviewed the draft manuscript and approved the final version for submission.
Data collection, analysis and manuscript preparation were sponsored by Bayer HealthCare AG.
ClinicalTrials.gov identifier: NCT01303783.
Conflicts of interest
S.E.K. has received lecture honoraria from AZ, Bayer, Medtronic, MSD and Takeda, honoraria for consulting from Bayer, Medtronic, Serodus and Takeda, and unrestricted research grants from AstraZeneca and Pronova.
A.J.L. has received research grants in the last 2 years from Abbott Laboratories, AbbVie, ActivX Biosciences, Inc., Akros Pharma, Inc., Amarin Pharma, Inc., Amgen, Inc., Amylin Pharmaceuticals, AstraZeneca, Bavarian Nordic, Bayer, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Catabasis, Daiichi Sankyo, Elcelyx Therapeutics, Eli Lilly & Company, Esperion, Forest Research Institute, Gilead Sciences, Inc., GlaxoSmithKline, F. Hoffmann-La Roche, Incyte Corporation, Idenix, Isis, InteKrin Therapeutics, Inc., Kowa Research Institute, Merck & Co., Inc., Metabolic Solutions Development Co., N-Gene Pharmaceuticals, Novartis Pharmaceuticals Corp., Novo Nordisk, Omthera Pharmaceuticals Inc., Pfizer Inc, Pharmacopeia, Inc., PhaseBio, Phenomix Corporation, Pronova, Sanofi-Aventis, Sanofi Pasteur, Schering-Plough, Shionogi USA, Inc., Surface Logix, Takeda Pharmaceuticals America, Theracos, Inc., TransTech, TWI Biotechnology, VIVUS, Inc., XOMA, LLC.
P.H. has received research grants from AZ, Merck, Pfizer, Boerhinger Ingelheim, Bayer, Ipsen, Sanofi Aventis, Novo Nordisk, Novartis, Takeda, Mitsubushi, Solvay, GSK, Lilly, Lundbeck, Mundi Pharma, Proctor&Gamble and Jannsen.
D.S., H.H., G.C., B.G.-E., F.H., G.V., G.M. declare they have no conflicts of interest.
Supplementary Material
Reviewers’ Summary Evaluations
Reviewer 1
This well conducted placebo-controlled trial of combination therapy with nifedipine and candesartan in various doses in middle-aged moderately overweight mild-to-moderate hypertensive patients show additive effects on blood pressure by the drug combination, with a favourable profile on side effects. This study supports the use of drugs that block the renin-angiotensin system and calcium channel blockers as first-line combination therapy. Unfortunately, this study only lasted 8 weeks, did not assess 24 h ambulatory blood pressure monitoring, and provided no information on prognosis and outcome.
Reviewer 2
These results are concordant with those of other studies that combine dihydropiridine calcium antagonists with angiotensin AT1 receptor blockers but, such as the authors underline, this is the first multicenter study evaluating the efficacy and tolerability of the combination candesartan plus nifedipine GITS, at different doses, in a wide range of hypertensive patients.
Besides the limitations noted by the authors, it is possible that a larger extensión of the treatment period (3–6 months) would have offered a better perspective on the efficay and tolerability profile of this antihypertensive combination.
Footnotes
Abbreviations: ACE, angiotensin-converting enzyme; ANCOVA, analysis of covariance; ARB, angiotensin receptor blocker; C, candesartan cilexetil; CCB, calcium channel blocker; DISTINCT, reDefining Intervention with Studies Testing Innovative Nifedipine GITS – Candesartan Therapy; FDC, fixed-dose combination; GCP, good clinical practice; GFR, glomerular filtration rate; GITS, gastrointestinal therapeutic system; ICH, International Conference on Harmonization; IEC, independent ethics committee; IRB, institutional review board; LOCF, last observation carried forward; MedDRA, Medical Dictionary for Regulatory Activities; N, nifedipine GITS; RSM, response surface model; TEAE, treatment-emergent adverse event
REFERENCES
- 1.Parati G, Bilo G, Ochoa JE. Benefits of tight blood pressure control in diabetic patients with hypertension: importance of early and sustained implementation of effective treatment strategies. Diabetes Care 2011; 34 Suppl 2:S297–S303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weir MR, Zappe D, Orloski LA, Sowers JR. How early should blood pressure control be achieved for optimal cardiovascular outcomes? J Hum Hypertens 2011; 25:211–217. [DOI] [PubMed] [Google Scholar]
- 3.Kjeldsen SE, Messerli FH, Chiang CE, Meredith PA, Liu L. Are fixed-dose combination antihypertensives suitable as first-line therapy? Curr Med Res Opin 2012; 28:1685–1697. [DOI] [PubMed] [Google Scholar]
- 4.Law MR, Wald NJ, Morris JK, Jordan RE. Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 2003; 326:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makani H, Bangalore S, Romero J, Wever-Pinzon O, Messerli FH. Effect of renin-angiotensin system blockade on calcium channel blocker-associated peripheral edema. Am J Med 2011; 124:128–135. [DOI] [PubMed] [Google Scholar]
- 6.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005; 366:895–906. [DOI] [PubMed] [Google Scholar]
- 7.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 8.Ma L, Wang W, Zhao Y, Zhang Y, Deng Q, Liu M, et al. Combination of amlodipine plus angiotensin receptor blocker or diuretics in high-risk hypertensive patients: a 96-week efficacy and safety study. Am J Cardiovasc Drugs 2012; 12:137–142. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 10.Karlson BW, Zetterstrand S, Olofsson B, Elmfeldt D. A dose-response analysis of candesartan-hydrochlorothiazide combination therapy in patients with hypertension. Blood Press 2009; 18:149–156. [DOI] [PubMed] [Google Scholar]
- 11.Kuschnir E, Bendersky M, Resk J, Panart MS, Guzman L, Plotquin Y, et al. Effects of the combination of low-dose nifedipine GITS 20 mg and losartan 50 mg in patients with mild to moderate hypertension. J Cardiovasc Pharmacol 2004; 43:300–305. [DOI] [PubMed] [Google Scholar]
- 12.Reif M, White WB, Fagan TC, Oparil S, Flanagan TL, Edwards DT, et al. Effects of candesartan cilexetil in patients with systemic hypertension. Candesartan Cilexetil Study Investigators. Am J Cardiol 1998; 82:961–965. [DOI] [PubMed] [Google Scholar]
- 13.Ruzicka M, Coletta E, Floras J, Leenen FH. Effects of low-dose nifedipine GITS on sympathetic activity in young and older patients with hypertension. J Hypertens 2004; 22:1039–1044. [DOI] [PubMed] [Google Scholar]
- 14.Van Liefde I, Vauquelin G. Sartan-AT1 receptor interactions: in vitro evidence for insurmountable antagonism and inverse agonism. Mol Cell Endocrinol 2009; 302:237–243. [DOI] [PubMed] [Google Scholar]
- 15.Kjeldsen SE, Stalhammar J, Hasvold P, Bodegard J, Olsson U, Russell D. Effects of losartan vs candesartan in reducing cardiovascular events in the primary treatment of hypertension. J Hum Hypertens 2010; 24:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitworth JA. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens 2003; 21:1983–1992. [DOI] [PubMed] [Google Scholar]
- 17.Philipp T, Smith TR, Glazer R, Wernsing M, Yen J, Jin J, et al. Two multicenter, 8-week, randomized, double-blind, placebo-controlled, parallel-group studies evaluating the efficacy and tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertension. Clin Ther 2007; 29:563–580. [DOI] [PubMed] [Google Scholar]
- 18.Chrysant SG, Melino M, Karki S, Lee J, Heyrman R. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double-blind, placebo-controlled, 8-week factorial efficacy and safety study. Clin Ther 2008; 30:587–604. [DOI] [PubMed] [Google Scholar]
- 19.Littlejohn TW, III, Majul CR, Olvera R, Seeber M, Kobe M, Guthrie R, Oigman W. Results of treatment with telmisartan-amlodipine in hypertensive patients. J Clin Hypertens (Greenwich) 2009; 11:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Medicines Agency Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products in the treatment of hypertension. EMAdocument library; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/12/WC500100191.pdf [Accessed 12 May 2014]. [Google Scholar]
- 21.Hung HMJ, Ng TH, Chi GYH. Response surface and factorial designs for combination antihypertensive drugs. Drug Information J 1990; 24:371–378. [Google Scholar]
- 22.Mancia G, Parati G, Bilo G, Choi J, Kilama MO, Ruilope LM. Blood pressure control by the nifedipine GITS-telmisartan combination in patients at high cardiovascular risk: the TALENT study. J Hypertens 2011; 29:600–609. [DOI] [PubMed] [Google Scholar]
- 23.Taddei S, Omboni S, Ghiadoni L, Caiazza A, Fogari R, Innocenti P, et al. Combination of lisinopril and nifedipine GITS increases blood pressure control compared with single drugs in essential hypertensive patients. J Cardiovasc Pharmacol 2003; 41:579–585. [DOI] [PubMed] [Google Scholar]
- 24.Hasebe N, Kikuchi K. Controlled-release nifedipine and candesartan low-dose combination therapy in patients with essential hypertension: the NICE Combi (Nifedipine and Candesartan Combination) Study. J Hypertens 2005; 23:445–453. [DOI] [PubMed] [Google Scholar]
- 25.Andreadis EA, Tsourous GI, Marakomichelakis GE, Katsanou PM, Fotia ME, Vassilopoulos CV, Diamantopoulos EJ. High-dose monotherapy vs low-dose combination therapy of calcium channel blockers and angiotensin receptor blockers in mild to moderate hypertension. J Hum Hypertens 2005; 19:491–496. [DOI] [PubMed] [Google Scholar]
- 26.Neutel JM, Smith DH, Weber MA. Low dose combination therapy vs. high dose monotherapy in the management of hypertension. J Clin Hypertens (Greenwich) 1999; 1:79–86. [PubMed] [Google Scholar]
- 27.Neutel JM. The use of combination drug therapy in the treatment of hypertension. Prog Cardiovasc Nurs 2002; 17:81–88. [DOI] [PubMed] [Google Scholar]
- 28.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003. [DOI] [PubMed] [Google Scholar]
- 29.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004; 363:2022–2031. [DOI] [PubMed] [Google Scholar]
- 30.Ozaki N, Nomura Y, Sobajima H, Kondo K, Oiso Y. Comparison of the effects of three angiotensin II receptor type 1 blockers on metabolic parameters in hypertensive patients with type 2 diabetes mellitus. Eur J Intern Med 2010; 21:236–239. [DOI] [PubMed] [Google Scholar]
- 31.Bravo E, Weir MR, Neutel JM, Llewellyn M, Harris S, Michelson EL, Wang R. A047: dose response of candesartan cilexetil in essential hypertension: a clinical experience trial. Am J Hypertens 2000; 13:128A.10701811 [Google Scholar]
- 32.Gleiter CH, Morike KE. Clinical pharmacokinetics of candesartan. Clin Pharmacokinet 2002; 41:7–17. [DOI] [PubMed] [Google Scholar]
- 33.Johnson JA. Ethnic differences in cardiovascular drug response: potential contribution of pharmacogenetics. Circulation 2008; 118:1383–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH, Jr, et al. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800. [DOI] [PubMed] [Google Scholar]
- 35.Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community a statement by the american society of hypertension and the international society of hypertension. J Hypertens 2014; 32:3–15. [DOI] [PubMed] [Google Scholar]
- 36.Chrysant SG, Lee J, Melino M, Karki S, Heyrman R. Efficacy and tolerability of amlodipine plus olmesartan medoxomil in patients with difficult-to-treat hypertension. J Hum Hypertens 2010; 24:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith TR, Philipp T, Vaisse B, Bakris GL, Wernsing M, Yen J, Glazer R. Amlodipine and valsartan combined and as monotherapy in stage 2, elderly, and black hypertensive patients: subgroup analyses of 2 randomized,;1; placebo-controlled studies. J Clin Hypertens (Greenwich) 2007; 9:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toal CB, Mahon WA, Barnes C, Burelle D. Nifedipine gastrointestinal therapeutic system (GITS) for hypertensive patients in a primary care setting: results of the Extended Release Adalat Canadian Trial (EXACT). Clin Ther 1997; 19:924–935. [DOI] [PubMed] [Google Scholar]
- 39.Hermida RC, Calvo C, Ayala DE, Lopez JE, Rodriguez M, Chayan L, et al. Dose- and administration time-dependent effects of nifedipine gits on ambulatory blood pressure in hypertensive subjects. Chronobiol Int 2007; 24:471–493. [DOI] [PubMed] [Google Scholar]
- 40.Bayer Core Data Sheet, Nifedipine (20 mg, 30 mg, 60 mg) prolonged-release tablets, Version 16; 1 December 2009. Bayer HealthCare AG, Berlin, Germany: http://www.medicines.org.uk/emc/medicine/20531 [Accessed 15 July 2014]. [Google Scholar]
- 41.de la Sierra A. Mitigation of calcium channel blocker-related oedema in hypertension by antagonists of the renin-angiotensin system. J Hum Hypertens 2009; 23:503–511. [DOI] [PubMed] [Google Scholar]
- 42.Etminan M, Levine MA, Tomlinson G, Rochon PA. Efficacy of angiotensin II receptor antagonists in preventing headache: a systematic overview and meta-analysis. Am J Med 2002; 112:642–646. [DOI] [PubMed] [Google Scholar]
- 43.Tronvik E, Stovner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with an angiotensin II receptor blocker: a randomized controlled trial. JAMA 2003; 289:65–69. [DOI] [PubMed] [Google Scholar]
- 44.Neutel JM. Prescribing patterns in hypertension: the emerging role of fixed-dose combinations for attaining BP goals in hypertensive patients. Curr Med Res Opin 2008; 24:2389–2401. [DOI] [PubMed] [Google Scholar]
- 45.Mancia G, Seravalle G, Grassi G. Systolic blood pressure: an underestimated cardiovascular risk factor. J Hypertens Suppl 2002; 20:S21–S27. [PubMed] [Google Scholar]
- 46.O’Brien E, Parati G, Stergiou G, Asmar R, Beilin L, Bilo G, et al. European society of hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31:1731–1768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


