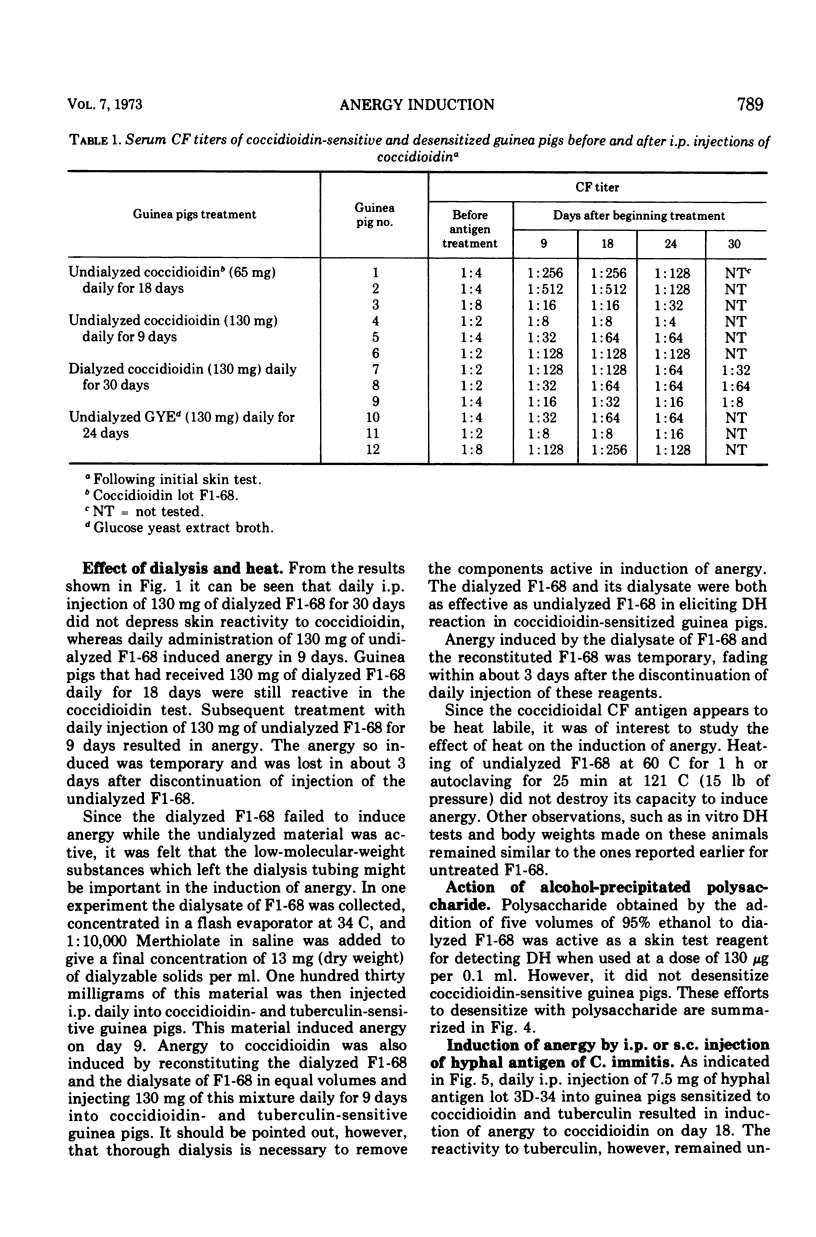
Abstract
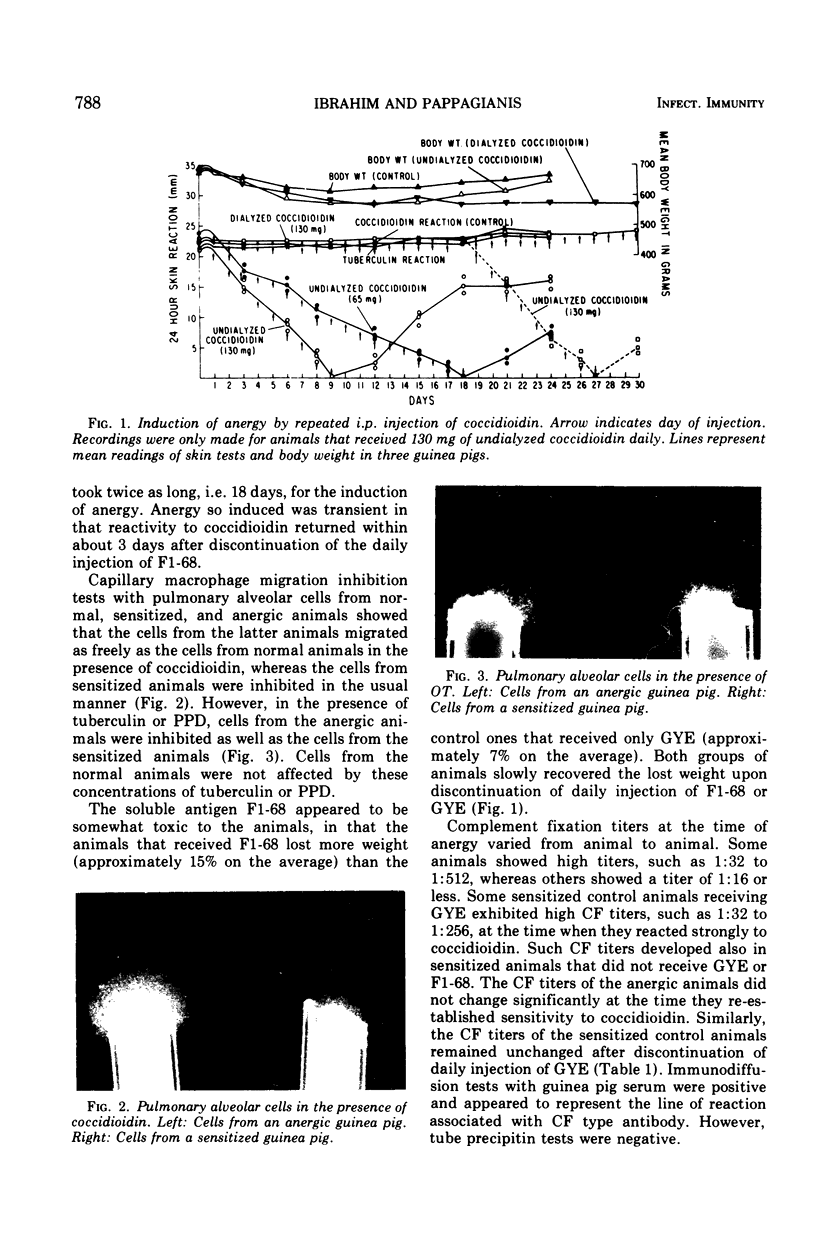
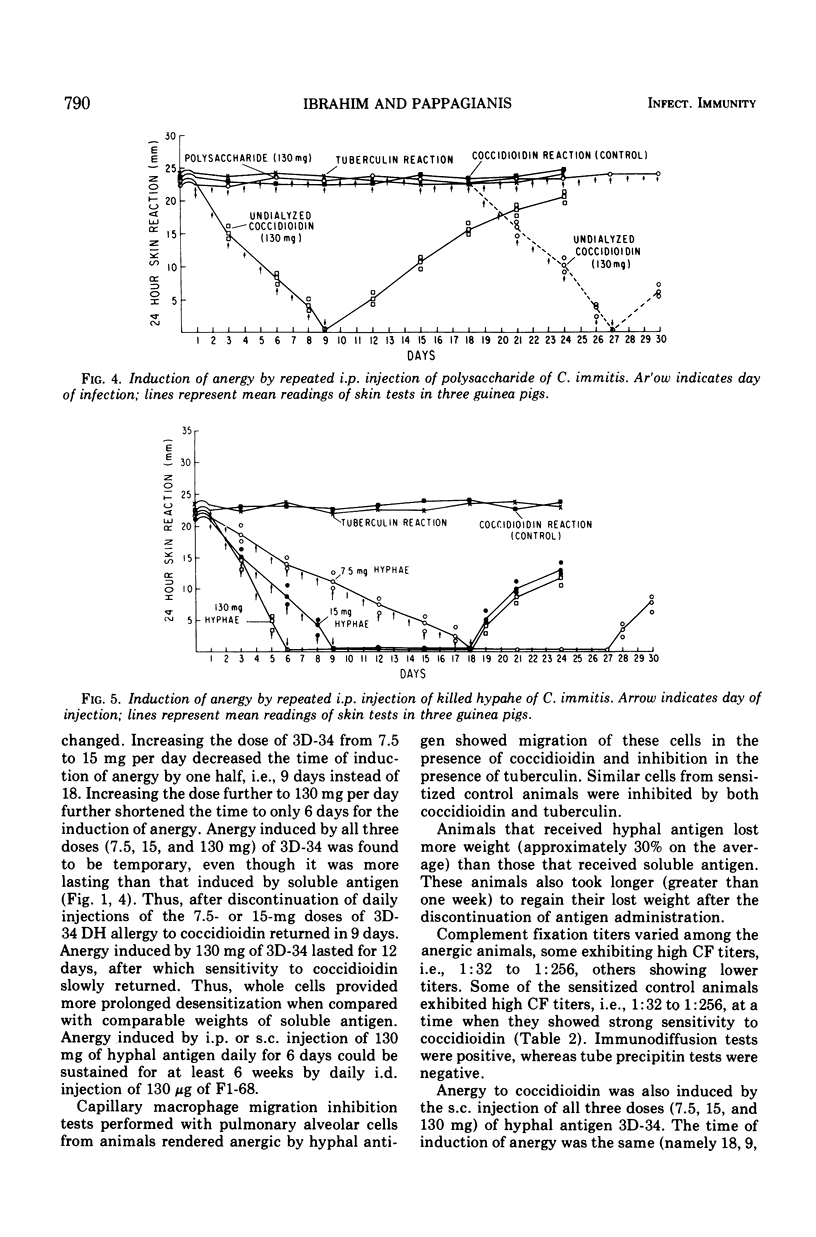
Failure to react to coccidioidin (anergy) often occurs in patients with disseminated coccidioidomycosis. One possible reason may be desensitization by excessive amounts of antigen. This was studied experimentally by injection of soluble and hyphal antigens of Coccidioides immitis into coccidioidin- and tuberculin-sensitive guinea pigs. Guinea pigs sensitized by injection of killed hyphal cells of C. immitis in complete Freund adjuvant were subsequently injected daily either with soluble coccidioidal antigen administered intraperitoneally or with hyphal antigen administered either subcutaneously or intraperitoneally. Gradual loss of cutaneous reactivity to coccidioidin occurred, but the reactivity to tuberculin remained unimpaired. The rapidity of desensitization was roughly proportional to the dose of antigen with desensitization occurring as early as 6 days after beginning injections. This anergic state was temporary, and reactivity returned several days after discontinuing injection of antigen. Injection of coccidioidal antigen led to production of coccidioidal complement-fixing antibody, but there was no consistent relationship between the antibody titer and state of cutaneous reactivity to coccidioidin. Peritoneal exudate or pulmonary alveolar cells from desensitized animals migrated freely in the presence of coccidioidin but were inhibited in the presence of tuberculin. Heat treatment did not impair the capacity of the soluble or hyphal antigen to induce anergy, thus suggesting that the antigen active in complement fixation was perhaps not involved in desensitization. Polysaccharide obtained by ethanol precipitation of dialyzed coccidioidin failed to induce anergy. Dialysis of the soluble coccidioidal antigen caused the loss of the desensitizing activity. Thus, specific desensitization could be induced by administration of large doses of coccidioidal antigen but dialyzable components appear important in this desensitization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett B., Bloom B. R. Studies on the migration inhibitory factor associated with delayed-type hypersensitivity: cytodynamics and specificity. Transplantation. 1967 Jul;5(4 Suppl):996–1000. [PubMed] [Google Scholar]
- FEINGOLD B. F., BENJAMINI E. Allergy to flea bites. Clinical and experimental observations. Ann Allergy. 1961 Nov;19:1275–1289. [PubMed] [Google Scholar]
- FREY J. R., GELEICK H. [Desensitization by intravenous injection of dinitrochlorbenzene in contact eczema in guinea pigs]. Dermatologica. 1962;125:132–139. [PubMed] [Google Scholar]
- Ferraresi R. W., Dedrick C. T., Raffel S., Goihman-Yahr M. Studies of the macrophage inhibition test. I. Comparison of the skin and cell migration reactions during the course of development of delayed hypersensitivity. J Immunol. 1969 Apr;102(4):852–858. [PubMed] [Google Scholar]
- GOWANS J. L., McGREGOR D. D., COWEN D. M. Initiation of immune responses by small lymphocytes. Nature. 1962 Nov 17;196:651–655. doi: 10.1038/196651a0. [DOI] [PubMed] [Google Scholar]
- KALISS N. Immunological enhancement of tumor homografts in mice: a review. Cancer Res. 1958 Oct;18(9):992–1003. [PubMed] [Google Scholar]
- KLIGMAN A. M. Hyposensitization against Rhus dermatitis. AMA Arch Derm. 1958 Jul;78(1):47–72. doi: 10.1001/archderm.1958.01560070051008. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine H. B., Cobb J. M., Scalarone G. M. Spherule coccidioidin in delayed dermal sensitivity reactions of experimental animals. Sabouraudia. 1969 Feb;7(1):20–32. doi: 10.1080/00362177085190051. [DOI] [PubMed] [Google Scholar]
- Lockshin M. D., Bombardieri S. In vitro delayed hypersensitivity in normal and hyporeactive patients. Immunology. 1971 May;20(5):641–648. [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- Marmor M. F., Barnett E. V. Cutaneous anergy without systemic disease. A syndrome associated with mucocutaneous fungal infection. Am J Med. 1968 Jun;44(6):979–989. doi: 10.1016/0002-9343(68)90097-1. [DOI] [PubMed] [Google Scholar]
- OLIVEIRA-LIMA A. Passive transfer of the delayed dermal sensitivity to tuberculin by means of blood leukocytes. Am Rev Tuberc. 1958 Sep;78(3):346–352. doi: 10.1164/artpd.1958.78.3.346. [DOI] [PubMed] [Google Scholar]
- PAPPAGIANIS D., PUTMAN E. W., KOBAYASHI G. S. Polysaccharide of Coccidioides immitis. J Bacteriol. 1961 Nov;82:714–723. doi: 10.1128/jb.82.5.714-723.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPPAGIANIS D., SMITH C. E., KOBAYASHI G. S., SAITO M. T. Studies of antigens from young mycelia of Coccidioides immitis. J Infect Dis. 1961 Jan-Feb;108:35–44. doi: 10.1093/infdis/108.1.35. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Chilgren R. A., Hong R., David J. R. Transfer of cellular hypersensitivity in chronic mucocutaneous candidiasis monitored in vivo and in vitro. Cell Immunol. 1970 Sep;1(3):290–299. doi: 10.1016/0008-8749(70)90050-x. [DOI] [PubMed] [Google Scholar]
- SMITH C. E., SAITO M. T., BEARD R. R., KEPP R. M., CLARK R. W., EDDIE B. U. Serological tests in the diagnosis and prognosis of coccidioidomycosis. Am J Hyg. 1950 Jul;52(1):1–21. doi: 10.1093/oxfordjournals.aje.a119404. [DOI] [PubMed] [Google Scholar]
- Schlossman S. F., Levin H. A., Rocklin R. E., David J. R. The compartmentalization of antigen-reactive lymphocytes in desensitized guinea pigs. J Exp Med. 1971 Sep 1;134(3 Pt 1):741–750. doi: 10.1084/jem.134.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein A. M., Borek F. Desensitization studies of delayed hypersensitivity, with special reference to the possible role of high-affinity antibodies. J Immunol. 1966 Jun;96(6):953–959. [PubMed] [Google Scholar]
- Stulbarg M., Schlossman S. F. The specificity of antigen-induced thymidine-2-14C incorporation into lymph node cells frrom sensitized animals. J Immunol. 1968 Oct;101(4):764–769. [PubMed] [Google Scholar]
- Thor D. E., Dray S. A correlate of human delayed hypersensitivity: specific inhibition of capillary tube migration of sensitized human lymph node cells by tuberculin and histoplasmin. J Immunol. 1968 Jul;101(1):51–61. [PubMed] [Google Scholar]
- UHR J. W., PAPPENHEIMER A. M., Jr Delayed hypersensitivity. III. Specific desensitization of guinea pigs sensitized to protein antigens. J Exp Med. 1958 Dec 1;108(6):891–904. doi: 10.1084/jem.108.6.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URBACH F., SONES M., ISRAEL H. L. Passive transfer of tuberculin sensitivity to patients with sarcoidosis. N Engl J Med. 1952 Nov 30;247(21):794–797. doi: 10.1056/NEJM195211202472103. [DOI] [PubMed] [Google Scholar]





