Abstract
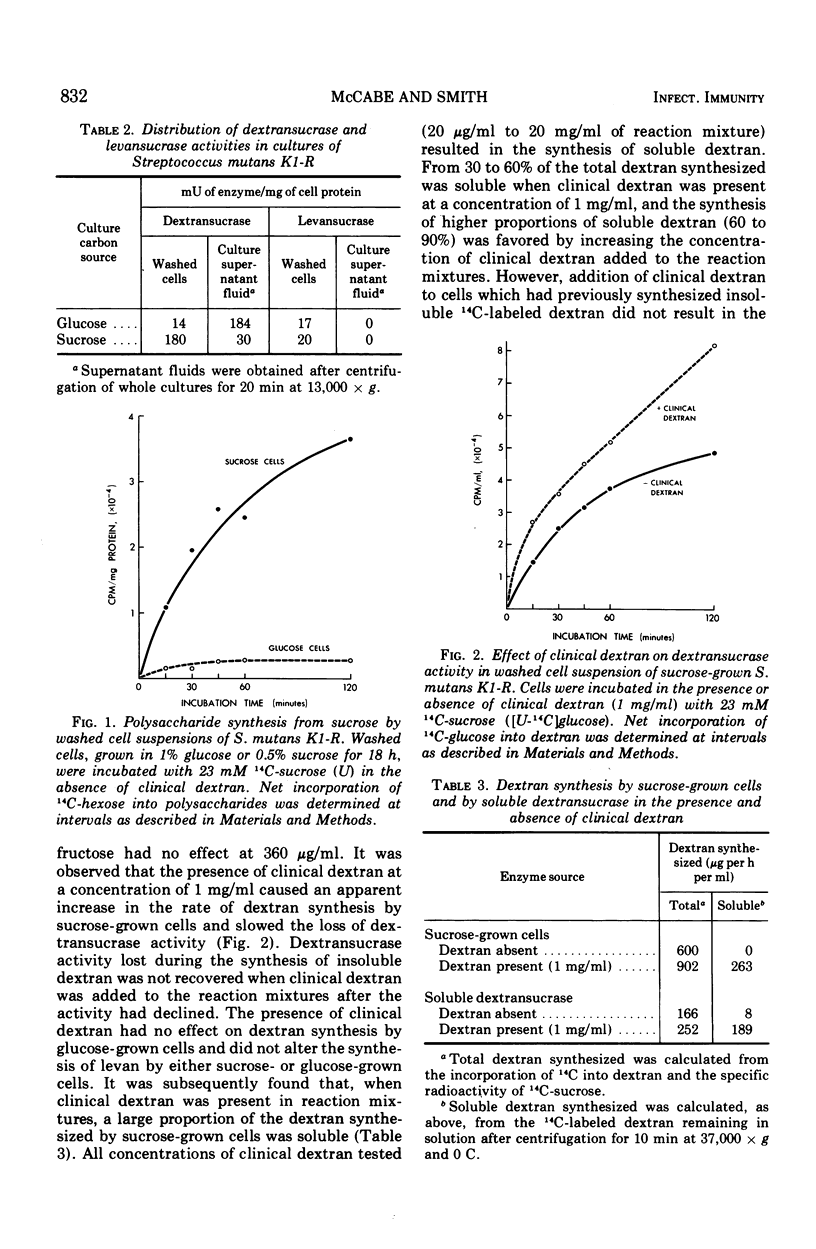
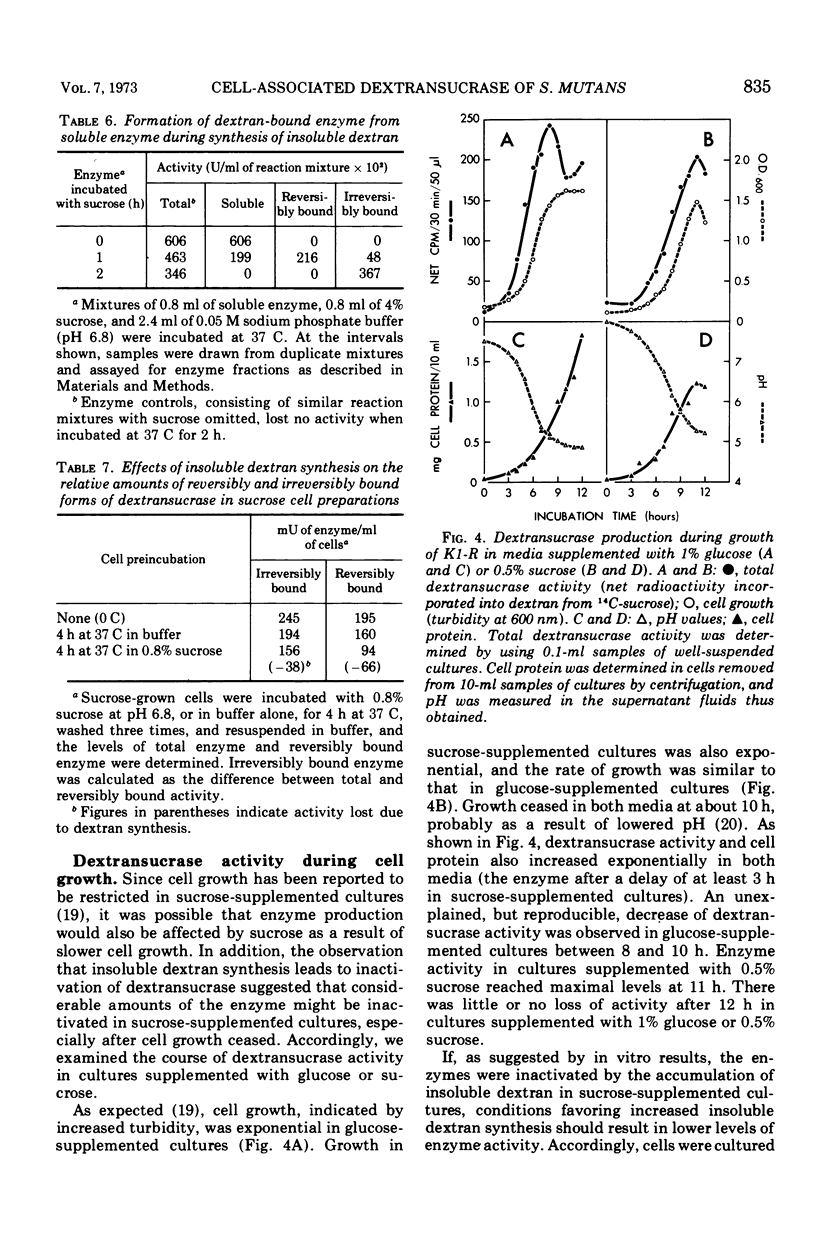
The cell-associated dextransucrase produced by sucrose-grown cells of cariogenic Streptococcus mutans K1-R is derived from soluble dextransucrase. Synthesis of insoluble dextran by soluble dextransucrase gives rise to two dextransucrase fractions bound to the insoluble polysaccharide; a reversibly bound enzyme, which can be eluted in solutions of clinical dextran, and an irreversibly bound enzyme, which cannot be solubilized in this manner. Both of these fractions of dextransucrase are also present on sucrose-grown cells. During the synthesis of insoluble dextran by sucrose-grown cells, dextransucrase is progressively converted from soluble enzyme, first to the reversibly bound fraction and then to the irreversibly bound fraction, and is finally inactivated as insoluble dextran accumulates. The two cell-associated dextransucrase fractions therefore represent two stages in the insolubilization and inactivation of their precursor, soluble dextransucrase. As a result of this process of inactivation, the yield of dextransucrase from cells cultured on sucrose is markedly decreased by high concentrations of sucrose in the culture medium.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY R. W. Transglucosidase activity of rumen strains of Streptococcus bovis. 2. Isolation and properties of dextransucrase. Biochem J. 1959 May;72(1):42–49. doi: 10.1042/bj0720042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Newbrun E., Krasse B. Purification and properties of dextransucrase from Streptococcus sanguis. Arch Oral Biol. 1969 May;14(5):469–478. doi: 10.1016/0003-9969(69)90140-x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Formation and significance of bacterial polysaccharides in caries etiology. Caries Res. 1968;2(2):164–171. doi: 10.1159/000259554. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Keyes P. H. Inhibition of insoluble dextran synthesis, plaque formation and dental caries in hamsters by low molecular weight dextran. Arch Oral Biol. 1969 Jun;14(6):721–724. doi: 10.1016/0003-9969(69)90193-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Kindt T. J., Conrad H. E. The role of primer in glycogen biosynthesis in Aerobacter aerogenes. Biochemistry. 1967 Dec;6(12):3718–3729. doi: 10.1021/bi00864a014. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Putnam S. L. Sensitive biuret method for determination of protein in an impure system such as whole bacteria. Anal Biochem. 1971 Nov;44(1):239–245. doi: 10.1016/0003-2697(71)90366-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd J. B., Whelan W. J. An improved method for enzymic determination of glucose in the presence of maltose. Anal Biochem. 1969 Sep;30(3):467–470. doi: 10.1016/0003-2697(69)90143-2. [DOI] [PubMed] [Google Scholar]
- Newbrun E., Carlsson J. Reaction rate of dextransucrase from Streptococcus sanguis in the presence of various compounds. Arch Oral Biol. 1969 May;14(5):461–468. doi: 10.1016/0003-9969(69)90139-3. [DOI] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Loken A. E., Schmitt M. K. Use of specifically labeled sucrose for comparison of extracellular glucan and fructan metabolism by oral streptococci. Infect Immun. 1972 Feb;5(2):263–266. doi: 10.1128/iai.5.2.263-266.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. E. Biosynthetic relation between the soluble and insoluble dextrans produced by Leuconostoc mesenteroides NRRL B-1299. FEBS Lett. 1970 Dec 23;12(1):33–37. doi: 10.1016/0014-5793(70)80588-9. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Wood W. I., Krichevsky M. I. Linear growth kinetics of plaque-forming streptococci in the presence of sucrose. J Gen Microbiol. 1969 Sep;58(1):125–133. doi: 10.1099/00221287-58-1-125. [DOI] [PubMed] [Google Scholar]
- van Houte J., Saxton C. A. Cell wall thickening and intracellular polysaccharide in microorganisms of the dental plaque. Caries Res. 1971;5(1):30–43. doi: 10.1159/000259730. [DOI] [PubMed] [Google Scholar]


